Abstract
Objective
To determine the mechanism by which hypoxia increases expression of iNOS in human normal peritoneal and adhesion fibroblasts.
Design
Prospective experimental study.
Setting
University medical center.
Patient(s)
Primary cultures of fibroblasts from normal peritoneum and adhesion tissues.
Intervention(s)
Hypoxia treated cells.
Main Outcome Measure(s)
We utilized real-time RT-PCR to quantify mRNA levels of iNOS and NF-κB. Western blots were used to determine iNOS, NF-κB, IκB-α and phospho-IκB expression levels in normal peritoneal and adhesion fibroblasts in response to hypoxia.
Result(s)
Hypoxia resulted in a significant increase in iNOS and NF-κB expression in normal and adhesion fibroblasts. Furthermore, both cell types manifested lower levels of NF-κB, cytoplasmic phospho-IκB-α, and iNOS proteins. In contrast, they manifested higher levels of cytoplasmic IκB-α and IκB-α/NF-κB ratios as well as phosphorylated-IκB-α/NF-κB ratio. Under hypoxic conditions, both cell types exhibited significantly decreased cytoplasmic NF-κB, IκB-α levels, and significantly increased cytoplasmic phospho-IκB-α, iNOS, and NF-κB protein levels.
Conclusions
Hypoxia increases iNOS expression by a mechanism involving activation of NF-κB. The ratio of IκB-α/NF-κB or IκB-α/p-IκB-α can be used to monitor activation.
Keywords: Fibroblasts, adhesion, peritoneum, hypoxia, iNOS, real-time RT-PCR, NF-κB, IκB-α
INTRODUCTION
Postoperative adhesion development and fibrosis are the major complications of surgical procedures. They are the major causes of infertility, abdominopelvic pain, and small bowel obstruction (1–3). Despite their overbearing influence on surgical care, the mechanisms by which injury to the peritoneum triggers the inflammatory response which leads to postoperative adhesion development remain poorly understood.
Previously, we characterized differences between fibroblasts isolated from normal peritoneum and adhesions of the same patients and have identified phenotypic differences between these two cell types (4–6). Under hypoxic conditions, both normal peritoneal and adhesion fibroblasts have a significant increase in mRNA and protein levels for iNOS. The main isoform of NO synthases expressed in normal peritoneal and adhesion fibroblasts is iNOS (7, 8). Its expression is regulated at the transcriptional level by cytokines and the exposure of cells to other inflammatory stimuli such as hypoxia or reactive oxygen species (ROS) (9).
Many studies have shown that inflammatory lesions are characterized by hypoxia (10–13). Experimental studies further indicated that hypoxia directly activates the transcription of NF-κB and the expression of iNOS (14). In fact, the induction of iNOS by activated normal fibroblasts and adhesions under hypoxic conditions has been proposed as an important cause of development of adhesion following abdominal surgery (7, 15, 16).
Nuclear factor kappa B (NF-κB) is a key transcriptional factor of the Rel family and is induced by a number of pathogens, cytokines, and hypoxia (17, 18). The active DNA-binding forms are either homodimers (p65/p65) or heterodimers (p50/p65). In most cells, NF-κB is present within the cytoplasm as inactive complexes with inhibitory IκB-α protein (19–21). Activation of NF-κB in response to stimuli, such as hypoxia, cytokines, lipopolysaccharide (LPS), and oxidants involves activation of IκB kinase (IKK), phosphorylation, and proteolytic degradation of IκB-α, with a release of activated NF-κB. Activated NF-κB then translocates to the nucleus, activates transcription by binding to the DNA sequences in the promoter region of its target genes that play key roles for the inflammatory responses, including hypoxia, cytokines, cell adhesion molecules, and inflammatory enzymes (22–28). The promoters of the human gene encoding iNOS contain a consensus sequence for the binding of NF-κB, which is necessary to confer inducibility by hypoxia, cytokines, and LPS (29).
Because of the relevance to hypoxia and inflammation and its ubiquitous role in the pathogenesis of inflammatory gene expression, NF-κB and its inhibitor, IκB, can be used as a marker for iNOS gene expression in human peritoneal fibroblasts in response to hypoxia. Here, we tested whether IκB/NF-κB or IκB/p-IκB ratios can mirror the mechanism by which hypoxia induces iNOS expression in human normal peritoneal and adhesion fibroblasts.
MATERIALS AND METHODS
Source and Culture of Human Fibroblasts
Fibroblasts were isolated from normal peritoneum and adhesions as previously described (4). Briefly, at initiation of the surgery, normal parietal peritoneal tissues from the anterior abdominal wall lateral to the midline incision and adherent tissues were excised from different patients undergoing laparotomy for pelvic pain. Normal peritoneum was ≥ 3 inches (7.6 cm) from any adhesions. All five patients did not have an active pelvic or abdominal infection and were not pregnant. The patients gave informed written consent to tissue collection, which was conducted under a protocol approved by the Wayne State University Institutional Review Board. Harvested tissue samples were immediately placed in standard Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen Corp, Carlsbaad, CA) containing 10% fetal bovine serum (FBS, Invitrogen Corp, Carlsbaad, CA), and 2% penicillin and streptomycin (Invitrogen Corp, Carlsbaad, CA). The tissues were cut into small pieces in a sterile culture dish and transferred into another fresh T-25 flask with 3 mL of dispase solution (2.4 U/mL; Invitrogen, Inc.). The flasks were incubated 4 hours at 37°C. The samples were then centrifuged for 5 minutes at 1400g, transferred into a fresh T-25 flask with pre-warmed DMEM medium, and put in a 37°C incubator (95% air and 5% CO2). The outgrowth of fibroblasts generally took 2 weeks. When confluence was reached, the cells were transferred to 100-mm tissue culture dishes and cultured in standard media with 10% FBS. Thereafter, the confluent dishes were subcultured by trypsinization (1:5 split ratios). Studies were conducted using cells from passage 3 to 5 to maintain comparability.
Hypoxic Culture Conditions
All hypoxic experiments were performed in an airtight modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA). The chamber was deoxygenated by a positive infusion of 2% O2 in a CO2-nitrogen gas mixture. Cultures were then placed in a standard humidified tissue incubator. There were no statistically significant differences in viability by crystal violet or trypan blue exclusion (data not shown). Parallel cultures were placed in normoxia for all time points. Cells were harvested after 24 and 48-hours. All experiments were performed in triplicate.
Reverse Transcription Real-time Polymerase Chain Reaction (RT-PCR) for iNOS and NF-κB
RNA isolation
Total RNA was extracted from human normal peritoneal fibroblasts and adhesions using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the protocol provided by the manufacturer.
Reverse transcription
A 20 μL cDNA reaction volume was prepared with the use of QuantiTect Reverse Transcription Kit (Qiagen), as described by the manufacturer’s protocol.
Real-time RT-PCR primer design and controls
Optimal oligonucleotide primer pairs for real-time RT-PCR amplification of reverse-transcribed cDNA were selected with the aid of software program Beacon Designer (Premier Biosoft Int., Palo Alto, CA). Human oligonucleotide primers, which amplify variable portions of the protein coding regions were used. β-actin primers which amplified a 559 base pair fragment are as follows: sense primer AAGCAGGAGTATGACGAGTCCG, and antisense primer GCCTTCATACATCTCAAGTTGG. iNOS primers which amplified a 127 base pair fragment are as follows: sense primer GTTCTCAAGGCACAGGTCTC, and antisense primer GCAGGTCACTTATGTCACTTATC. NF-κB primers which amplified a 128 base pair fragment are as follows: sense primer ATGGCTTCTATGAGGCTGAG, and antisense primer GTTGTTGTTGGTCTGGATGC.
Real-time RT-PCR was performed with a QuantiTect SYBR Green RT-PCR kit (Qiagen) and a Cepheid 1.2f Detection System (Cepheid, Sunnyvale, CA). Each reaction was 25-μL including 12.5 μL of 2 X QuantiTect SYBR Green RT-PCR master mix, 1 μL of cDNA template, and 0.2 μmol/L each of target-specific primer that was designed to amplify a part of the gene of interest. To quantify each target transcript, a standard curve was constructed with a tenfold dilution series of β-actin standard plasmid (Invitrogen Corp, Carlsbaad, CA). The three-step polymerase chain reaction protocol applied for iNOS reaction consisted of 40 cycles of 95 °C for 15s, 55 °C (iNOS) or 62 °C (NF-κB) for 30s, and 72 °C for 30s. After real-time RT-PCR, a melting curve analysis was performed to demonstrate the specificity of the PCR product as a single peak. A control, which contained all the reaction components except for the template, was included in all experiments. The amount of mRNAs was then normalized to a housekeeping gene, β-actin.
Detection of iNOS, NF-κB and IκB-α/phospho-IκB-α Protein levels in normal and adhesion fibroblasts
Western blots were used for analysis of the effects of hypoxia on the expression of iNOS, NF-κB, and IκB. Human peritoneal and adhesion fibroblasts (5 × 106) treated with hypoxia for 24 and 48 hours were lysed with cell lysis buffer and cleared by centrifugation (for 10 minutes at 1,000g at 4°C). Nuclear proteins were extracted with nuclear extract kit (Active Motif, Carlsbad, CA) according to the protocol provided by the manufacturer. Cell proteins were separated and analyzed by SDS-PAGE/Western blot. Protein bands were scanned and analyzed by NIH image J 3.0.
Statistical Analysis
Real-time RT-PCR data were analyzed using a Kruskall Wallis test followed by Dunn’s multiple comparison tests. Correlation analysis was performed by calculating the Pearson R2 coefficient, followed by calculation of a two-tailed P-value. Statistically significant differences for comparison of means were established at p<0.05. All statistical analyses were performed using prism 4.0 for Windows and Mac (GraphPad Software, Inc.).
RESULTS
We have utilized real-time RT-PCR to measure mRNA levels of iNOS in human normal peritoneal and adhesion fibroblasts under normoxic and hypoxic conditions. Consistent with our previous findings (8), under normoxic conditions, iNOS mRNA levels were 3.13 × 104/ug RNA and 3.30 × 104/ug RNA in normal peritoneal and adhesion fibroblasts respectively. There is no significant difference for iNOS expression between normal peritoneal fibroblasts and adhesion fibroblasts (P=0.1367). When cells were cultured under hypoxic conditions, iNOS expression levels increased 2 fold in both normal (96%, P<0.001) and adhesion fibroblasts (94%, P<0.001) as compared to normoxic conditions. There is no significant difference in iNOS mRNA levels in normal peritoneal and adhesion fibroblasts under hypoxic conditions (6.13 × 104/ug RNA vs 6.39 × 104/ug RNA, P=0.3061) (8).
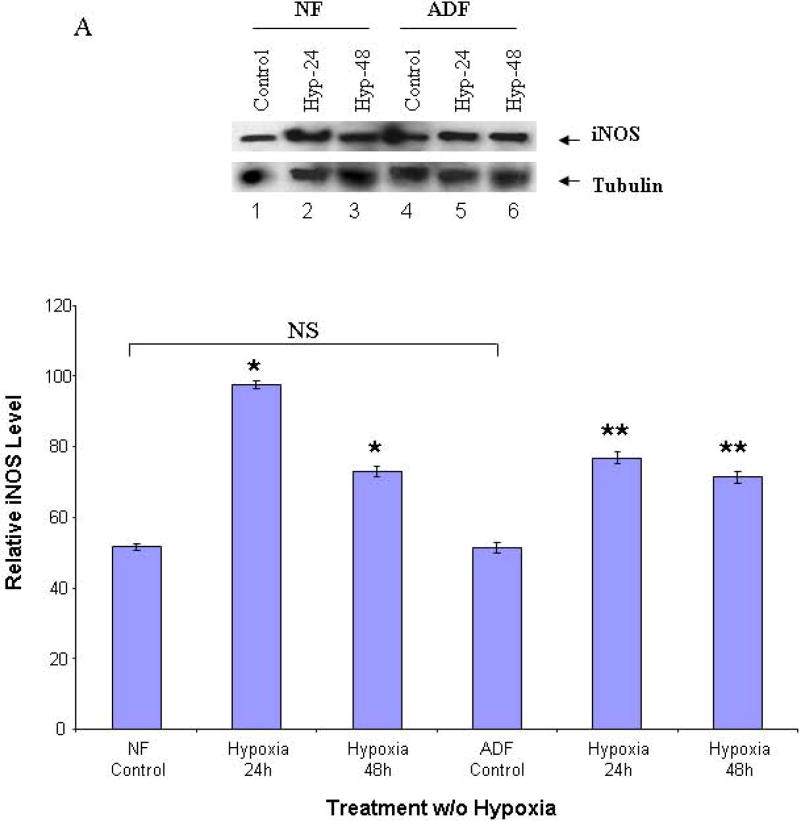
To determine the effects of hypoxia on iNOS protein levels in human normal peritoneal and adhesion fibroblasts, Western blots were performed with cell lysates in 24 and 48 hours as described in the Materials and Methods section. As compared to the baseline in normoxia, hypoxia increased iNOS protein levels by 88% in normal and 47% in adhesion in 24 hours (P<0.0001), and by 40% in normal and 37% in adhesion in 48 hours (P<0.0001) in normal peritoneal and adhesion fibroblasts, respectively (Fig. 1A, 1B).
FIGURE 1. Western blots of iNOS.
Cell lysates from normal peritoneal and adhesion fibroblasts before and after hypoxia (2% O2) were fractionated with SDS-PAGE. Membrane was probed with anti-iNOS and anti-tubulin antibodies. A: normal fibroblasts (Lane 1–3), and adhesion fibroblasts (Lane 4–6); B: results were analyzed by NIH image J 3.0. *P<0.0001 compared to normal peritoneal fibroblasts cultured under normoxic conditions. ** P<0.0001 compared to adhesion fibroblasts cultured under normoxic conditions. NF/AF, normal/adhesion fibroblast. Hyp-24/Hyp-48, normal/adhesion fibroblast exposed to hypoxia for 24h and 48h.
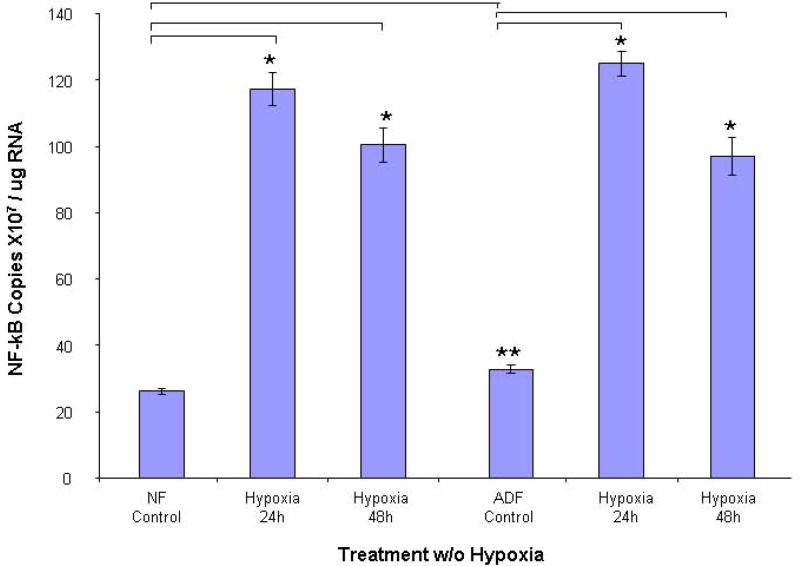
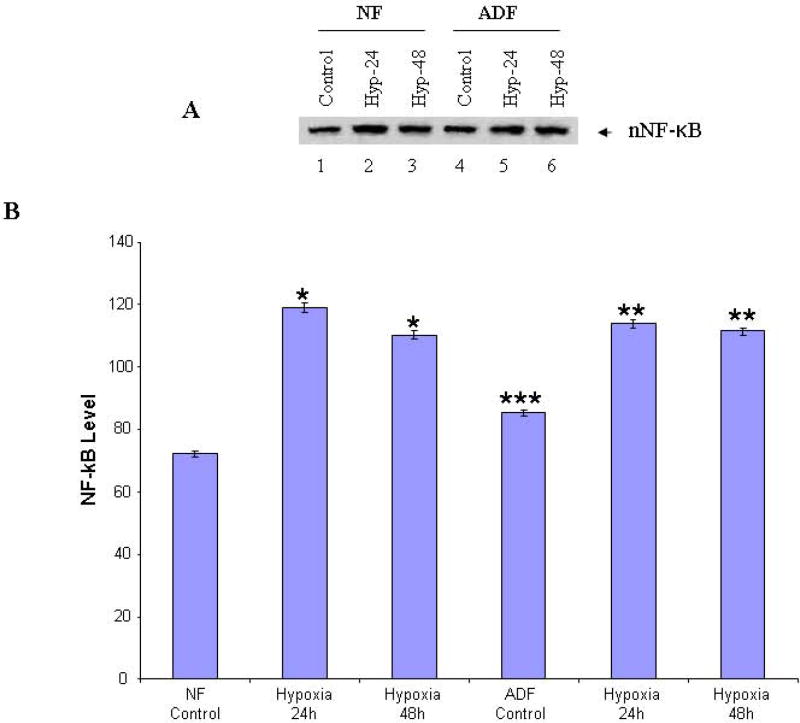
Hypoxia activates the transcription of NF-κB and the expression of iNOS by releasing NF-κB from the complex, following signal-mediated phosphorylation and degradation of the inhibitory protein, IκB. To test the activation of this pathway in human normal peritoneal and adhesion fibroblasts subjected to hypoxia, the cellular RNA and nuclear protein extracts were analyzed for activation by real-time RT-PCR and Western blot using anti-NF-κB p65 antibody. As shown in Fig. 2, NF-κB mRNA levels were significantly increased after exposure to hypoxia for 24h in normal peritoneal (5.5 fold, P<0.001) and adhesion fibroblasts (5.2 fold, P<0.001). However, it starts to decrease at 48h. The trends of nuclear NF-κB protein levels are the same as that of mRNA. When cells were subjected to 24h hypoxia, NF-κB reaches its highest level, which is a 1.65 fold increase from baseline in normal peritoneal (P<0.0001), and a 1.48 fold increase in adhesion fibroblasts (P<0.0001, Fig. 3A, 3B).
FIGURE 2. NF-κB Real-time RT-PCR.
Real-time RT-PCR comparison of NF-κB mRNA levels in human normal peritoneal (n=5) and adhesion fibroblasts (n=5) before and after hypoxia (2% O2) treatment. *P<0.00001 compared to normal peritoneal fibroblasts cultured under normoxic conditions. ** P<0.00002 compared to adhesion fibroblasts cultured under normoxic conditions. Results are representative of the mean of three independent experiments.
FIGURE 3. Western blot of nuclear NF-κB.
Nuclear extracts from normal peritoneal and adhesion fibroblasts before and after hypoxia (2% O2) were fractionated with SDS-PAGE. Membrane was probed with anti-NF-κB antibody. A: normal fibroblasts (Lane 1–3), and adhesion fibroblasts (Lane 4–6); B: results were analyzed by NIH image J 3.0. *P<0.0001 compared to normal peritoneal fibroblasts cultured under normoxic conditions. ** P<0.0001 compared to adhesion fibroblasts cultured under normoxic conditions. *** P<0.0001 compared to normal fibroblasts cultured under normoxic conditions. NF/AF, normal/adhesion fibroblast. Hyp-24/Hyp-48, normal/adhesion fibroblast exposed to hypoxia for 24h and 48h.
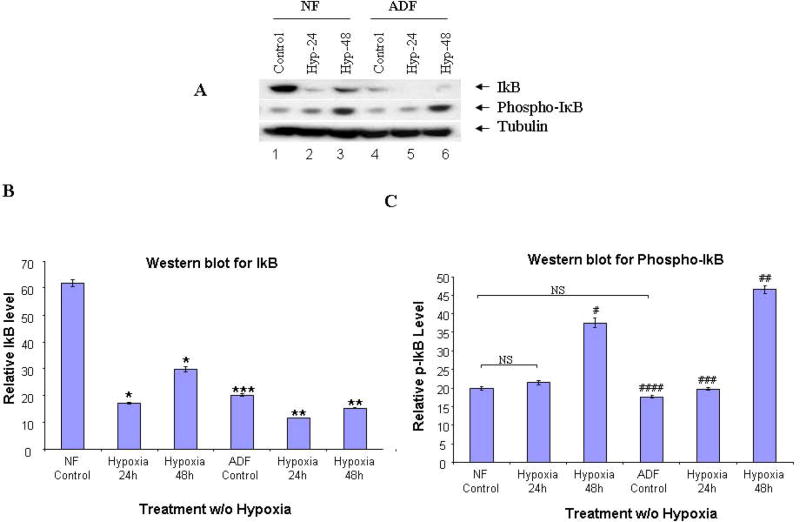
We further determined IκB phosphorylation and degradation by Western blot analysis using anti-IκB and antiphospho-IκB antibodies (Cell Signaling Technology, Danvers, MA), respectively. IκB degradation and phosphorylation occurred in both cells (Fig. 4). After 24h exposure to hypoxia, IκB was markedly reduced in both normal peritoneal (72%) and adhesion fibroblasts (81%) (P<0.0001, Fig. 4A upper panel, 4B). In contrast, phospho-IκB was significantly increased in normal peritoneal (88%) and adhesion fibroblasts (133%) after 48h exposure to hypoxia (P<0.0001, Fig. 4A middle panel, 4C). There was no change in IκB mRNA levels before and after hypoxia in both cell types (Data not shown).
FIGURE 4. Western blot of IκB-α, p-IκB-α.
Cytoplasmic fractions from normal peritoneal and adhesion fibroblasts before and after hypoxia (2% O2) were fractionated with SDS-PAGE. Membranes were probed with anti-IκB, antiphospho-IκB and anti-tubulin antibodies. A: normal fibroblasts (Lane 1–3), and adhesion fibroblasts (Lane 4–6); B/C: IκB/p-IκB results were analyzed by NIH image J 3.0. *P, #P<0.0001 compared to normal peritoneal fibroblasts cultured under normoxic conditions. ** P, ##P<0.0001, ###P<0.0034 compared to adhesion fibroblasts cultured under normoxic conditions. *** P<0.0001, ####P=0.0033 compared to normal fibroblasts cultured under normoxic conditions. NF/AF, normal/adhesion fibroblast. Hyp-24/Hyp-48, normal/adhesion fibroblast exposed to hypoxia for 24h and 48h.
To find the relationship between the ratio of IκB-α/NF-κB or IκB-α/p-IκB-α and the expression of iNOS in human normal peritoneal and adhesion fibroblasts, we compared IκB-α and NF-κB or IκB-α and p-IκB-α levels in both cell types under hypoxic condition with controls. There was no detectable difference in iNOS mRNA or protein levels in both cell lines. After the 24h hypoxia treatment, the IκB-α/NF-κB ratio increased by 30% and 19% in normal and adhesion fibroblasts respectively. The IκB-α/p-IκB-α ratio increased by 81% and 59% in normal and adhesion fibroblasts respectively, after 24h hypoxia treatment. After the 24h hypoxia treatment, iNOS expression increased by 88% and 47% in normal and adhesion fibroblasts respectively. After the 48h hypoxia treatment, the IκB-α/NF-κB ratio increased by 57% and 24% in normal and adhesion fibroblasts respectively. In addition, the IκB-α/p-IκB-α ratio increased by 79% and 33% in normal and adhesion fibroblasts respectively after 48h hypoxia treatment. After the 48h hypoxia treatment, iNOS expression increased by 40% and 37% in normal and adhesion fibroblasts respectively. This relationship indicates that the ratio of IκB-α/NF-κB or IκB-α/p-IκB-α can be used as a marker of iNOS expression in human postoperative adhesions.
DISCUSSION
The principle of this work was to investigate the molecular mechanism of iNOS expression triggered by hypoxic stimulation of human normal peritoneal and adhesion fibroblasts. Secondly, we sought to find a marker for the induction of iNOS gene expression in relation to an activation of the intracellular signaling pathway. Our present results showed that both cell types respond to hypoxia in their induction of iNOS expression at mRNA levels, with no difference between these two cell types. Western blot studies further showed that iNOS protein expression was markedly increased in both cell types exposed to hypoxia, again with no difference between these two cell types. Our observation of increased expression of iNOS in response to hypoxia in human peritoneal and adhesion fibroblasts is in agreement with the recent study showing that oxygen and glucose deprivation in rat forebrain results in a significant increase the neuronal expression of iNOS (30).
The activation of NF-κB can be regulated by three mechanisms (31, 32). In the first two pathways, the p50/p65 or unprocessed p105/p65 heterodimer constitutes the inactive cytoplasmic tertiary complex with IκB-α. In both pathways, phosphorylation of IκB-α and p105 by a protein kinase(s) is essential for the subsequent degradation of IκB-α and the processing of p105, respectively. An alternative mechanism for NF-κB activation involves IκB tyrosine phosphorylation and translocation of NF-κB to the nucleus but without IκB degradation (32).
Cellular responses to IL-1β, TNF-α, and LPS signaling, such as activation of several MAPK kinases, including p42/p44 MAPK, p38 MAPK, and JNK MAPK indicate their diverse biological effects (33). However, little information is yet available as to how hypoxia causes NF-κB activation and iNOS expression in human normal peritoneal and adhesion fibroblasts. The present analysis of hypoxic activation of NF-κB and iNOS expression in human normal peritoneal and adhesion fibroblasts clearly demonstrates that stimulation with hypoxia caused longer-lasting activation profiles of the NF-κB; a steady increase in phosphorylation of IκB and a subsequent steady degradation of IκB-α, followed by NF-κB activation and its nuclear translocation in both cell types. IκB degradation peaked at 24h, compared to the cells of rat dorsocaudal brain stem (34) and rat microglia (14). The hypoxic activation of NF-κB we observed is consistent with the previous findings (34–37). The mechanisms underlying this subtle difference are likely to be complex and the implications are thus far unclear.
Our results showed that iNOS expression is parallel to the activation of NF-κB, which is consistent with previous findings (38, 39). The ratio of IκB-α/NF-κB or IκB-α/p-IκB-α is inversely proportional to the expression of iNOS in both cell types exposed to hypoxia, as indicated in Table 2. Interestingly, IκB-α phosphorylation increased at 48h. One explanation for this is NF-κB activation being longer-lasting in both cells in response to hypoxia.
Although human peritoneal and adhesion fibroblasts share the same signal pathway in activation of NF-κB, NF-κB and IκB-α levels are different between these two cell types. The higher NF-κB (19%) and lower IκB-α (67%) levels in adhesion fibroblasts may be responsible for the adhesion phenotype.
Taken together, hypoxia increases iNOS expression in human peritoneal and adhesion fibroblasts through IκB-α phosphorylation and degradation, which in turn activates NF-κB. The ratio of IκB-α/NF-κB or IκB-α/p-IκB-α is inversely proportional to iNOS expression. Therefore, the ratios of IκB-α/NF-κB or IκB-α/p-IκB-α can be used as a marker of iNOS expression in the development of the adhesion phenotype.
Acknowledgments
This study was supported in part by NIH grant number NIH RO1 GM069941 to G.M. Saed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diamond MP, Daniell JF, Feste J, Surrey MW, McLaughlin DS, Friedman S. Adhesion reformation and de novo adhesion formation after reproductive pelvic surgery. Fertil Steril. 1987;47:864–6. doi: 10.1016/s0015-0282(16)59181-x. [DOI] [PubMed] [Google Scholar]
- 2.Diamond MP, Hershlag A. Adhesion formation/reformation. Prog Clin Biol Res. 1990;358:23–33. [PubMed] [Google Scholar]
- 3.Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;577:5–9. [PubMed] [Google Scholar]
- 4.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–768. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 5.Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertil Steril. 2002;78:144–147. doi: 10.1016/s0015-0282(02)03146-1. [DOI] [PubMed] [Google Scholar]
- 6.Saed GM, Diamond MP. Apoptosis and proliferation of human peritoneal fibroblasts in response to hypoxia. Fertil Steril. 2002;78:137–143. doi: 10.1016/s0015-0282(02)03145-x. [DOI] [PubMed] [Google Scholar]
- 7.Saed GM, Abu-Soud HM, Diamond MP. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil Steril. 2004;82 (Suppl 3):1198–205. doi: 10.1016/j.fertnstert.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z, Zhu XP, Diamond MP, Abu-Soud HM, Saed GM. Nitric oxide synthase isoforms expression in fibroblasts isolated from human normal peritoneum and adhesion tissues. doi: 10.1016/j.fertnstert.2007.07.1313. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol. 1999;21(7):435–443. doi: 10.1016/s0192-0561(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 10.Kokura S, Yoshida N, Yoshikawa T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic Biol Med. 2002;33(4):427–32. doi: 10.1016/s0891-5849(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 11.Haddad JJ. Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for hypoxia-inducible factor-1alpha. Crit Care. 2003;7(1):47–54. doi: 10.1186/cc1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadi S, Wrenshall LE, Platt JL. Regional manifestations and control of the immune system. FASEB J. 2002;16:849–856. doi: 10.1096/fj.01-0690hyp. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukocyte Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 14.Guo G, Bhat NR. Hypoxia/reoxygenation differentially modulates NF-kappaB activation and iNOS expression in astrocytes and microglia. Antioxid Redox Signal. 2006;8(5–6):911–918. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- 15.Saed GM, Zhao M, Diamond MP, Abu-Soud HM. Regulation of inducible nitric oxide synthase in post-operative adhesions. Hum Reprod. 2006;21:1605–11. doi: 10.1093/humrep/dei500. [DOI] [PubMed] [Google Scholar]
- 16.Saed GM, Lu H, Jiang ZL, Abuolba S, Abu-Soud HM, Diamond MP. Cross talk between inducible nitric oxide syntheses (iNOS) and myeloperoxidase (MPO) in fibroblasts isolated from normal peritoneal and adhesion tissues. (submitted) [Google Scholar]
- 17.Fujimori K, Fujitani Y, Kadoyama K, Kumanogoh H, Ishikawa K, Urade Y. Regulation of lipocalin-type prostaglandin D synthase gene expression by Hes-1 through E-box and interleukin 1-beta via two NF-kappa B elements in rat leptomeningeal cells. J Biol Chem. 2003;278:6018–6026. doi: 10.1074/jbc.M208288200. [DOI] [PubMed] [Google Scholar]
- 18.Jiang B, Xu S, Brecher P, Cohen RA. Growth factors enhance interleukin 1-beta induced persistent activation of nuclear factor kappa B in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1811–1816. doi: 10.1161/01.atv.0000037679.60584.3f. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs MD, Harrison SC. Structure of an I kappa B alpha/NF-kappa B complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 20.Huguet C, Crepieux P, Laudet V. Rel/NF-kappa B transcription factors and I kappa B inhibitors: evolution from a unique common ancestor. Oncogene. 1997;15:2965–2974. doi: 10.1038/sj.onc.1201471. [DOI] [PubMed] [Google Scholar]
- 21.Whiteside ST, Israel A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Gaynor RB. I kappa B kinases: key regulators of the NF-kappa B pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Brockman JA, Scherer DC, Mckinsey TA, Hall SM, Qi X, Lee WY, et al. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Chen H, Qwarnstrom E. Degradation of I kappa B alpha is limited by a postphosphorylation/ubiquitination event. Biochem Biophys Res Commun. 2001;285:603–608. doi: 10.1006/bbrc.2001.5205. [DOI] [PubMed] [Google Scholar]
- 25.Dejardin E, Deregowski V, Chapelier M, Jacobs N, Gielen J, Merville MP, et al. Regulation of NF-kappaB activity by I kappa B-related proteins in adenocarcinoma cells. Oncogene. 1999;18:2567–2577. doi: 10.1038/sj.onc.1202599. [DOI] [PubMed] [Google Scholar]
- 26.Wulczyn FG, Krappmann D, Scheidereit C. Signal-dependent degradation of IκBα is mediated by an inducible destruction box that can be transferred to NF-κB, Bcl-3 or p53. Nucleic Acids Res. 1998;26:1724–1730. doi: 10.1093/nar/26.7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renard P, Percherancier Y, Kroll M, Thomas D, Virelizier JL, Arenzana-Seisdedos F, Bacheleriel F. Inducible NF-kappa B activation is permitted by simultaneous degradation of nuclear I kappa B alpha. J Biol Chem. 2000;275:15193–15199. doi: 10.1074/jbc.275.20.15193. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 30.Moro MA, De Alba J, Leza JC, Lorenzo P, Fernandez AP, Bentura ML, Bosca L, Rodrigo J, Lizasoain I. Neuronal expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices. Eur J Neurosci. 1998;10:445–456. doi: 10.1046/j.1460-9568.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- 31.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 32.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86(5):787–98. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87(3):565–76. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 34.Gozal E, Sinakajornboon N, Gozal D. NF-kappaB induction during in vivo hypoxia in dorsocaudal brain stem of rat: Effect of MK-801 and L-NAME. J Appl Physiol. 1998;85:372–376. doi: 10.1152/jappl.1998.85.1.372. [DOI] [PubMed] [Google Scholar]
- 35.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Gabriel C, Justicia C, Camins A, Planas AM. Activation of nuclear factor-kappaB in the rat brain after transient focal ischemia. Brain Res Mol Brain Res. 1999;65:61–69. doi: 10.1016/s0169-328x(98)00330-1. [DOI] [PubMed] [Google Scholar]
- 37.Stanimirovic D, Zhang W, Howlett C, Lemieux P, Smith C. Inflammatory gene transcription in human astrocytes exposed to hypoxia: roles of the nuclear factor-B and autocrine stimulation. J Neuroimmunol. 2001;119:365–376. doi: 10.1016/s0165-5728(01)00402-7. [DOI] [PubMed] [Google Scholar]
- 38.Goldring CE, Narayanan R, Lagadec P, Jeannin JF. Transcriptional inhibition of the inducible nitric oxide synthase gene by competitive binding of NF-kappa B/Rel proteins. Biochem Biophys Res Commun. 1995;209:73–79. doi: 10.1006/bbrc.1995.1472. [DOI] [PubMed] [Google Scholar]
- 39.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]