Abstract
Opioids containing the Dmt-Tic pharmacophore, especially the δ agonists H-Dmt-Tic-Gly-NH-Ph 1 and H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid 4 (UFP-512) were evaluated for the influence of the substitution of Gly with aspartic acid, its chirality, and the importance of the – NH-Ph and N1H-Bid hydrogens relative to δ agonism. The results provide the following conclusions: (i) Asp increases δ selectivity by lowering μ affinity; (ii) -NH-Ph and N1H-Bid nitrogen methylation transforms δ agonists into δ antagonists; (iii) substitution of Gly with L-Asp/D-Asp in the δ agonist H-Dmt-Tic-Gly-NH-Ph resulted in δ antagonists, while the same substitution in the δ agonist H-Dmt-Tic-NH-CH2-Bid yielded more selective δ agonists, H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid and H-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid; (iv) L-Asp seems important only for functional bioactivity, not receptor affinity; (v) H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid(N1-Me) (10) revealed analgesia similar to 4, which was reversed by naltrindole only in the tail-flick test. Compounds 4 and 10 had opposite behaviours in mice: 4 caused agitation, while 10 gave sedation and convulsions.
Introduction
The prototype opioid pharmacophore Dmt-Tic,α, 1 which evolved from H-Tyr-Tic-OH2 as a simplified form of TIP(P),3 represents the minimum sequence that selectively interacts with δ opioid receptors as a potent δ antagonist. Extensive structure-activity studies on this prototype revealed that even minor chemical modifications changed its pharmacological profile, including: a wide range of properties such as: enhanced δ antagonism,4 appearance of mixed μ agonism/δ agonism5 as well as mixed μ agonism/δ antagonism,5 μ agonism,6 μ antagonism,6 δ inverse agonism,7 and δ agonism.5, 8–10 Among all synthesized analogues, some lead compounds were obtained; for example, the potent and selective δ antagonist N,N(Me)2-Dmt-Tic-OH11 and the δ inverse agonist N,N(Me)2-Dmt-Tic-NH2 12, 13 as useful tools for pharmacological studies. Some other lead compounds endowed with potential utility as therapeutic agents are represented by: the μ agonist/δ antagonist H-Dmt-Tic-Gly-NH-Bzl (2); the μ agonist/δ agonist H-Dmt-Tic-Gly-NH-Ph (1); and the potent δ agonists H-Dmt-Tic-NH-CH2-Bid 3 (UFP-502)5 and H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid 4 (UFP-512)8. On the basis of different pharmacological studies, μ agonists/δ antagonists14 and μ agonists/δ agonists15 may provide new classes of analgesics with low propensity to induce tolerance, physical dependence, constipation and other side effects. δ Opioid receptor agonists are known to produce many pharmacological effects in rodents, including analgesia,16 antidepressant,17, 10 neuroprotection/neurogenesis18 and anti-Parkinson19 activities. Moreover, peripheral δ-opioid receptors seem to be involved in cancer,20 cardiovascular disease,21 gastrointestinal disorders,22 and newer paradigms for pain relief that use peripherally restricted opioids.23 Starting from our selected lead compounds, here we report some new attempts to gain a better understanding of their biological profiles, especially in the light of the fact that even minor modifications can change their pharmacological characteristics.24, 25 In particular, we focused our attention once again on the importance of the C-terminal Bid (1H-benzimidazole-2-yl) and the anilide function (considered as an open ring surrogate of Bid); in fact, both groups are important for δ agonist activity. From previous studies, hydrogen linked to the N1 of Bid24, 25 (and probably the corresponding hydrogen linked to the anilide function) seem to be important for the induction of δ agonism. To ascertain this hypothesis we synthesized some N1-Bid and N-anilide methylated analogues of our selected lead compounds. Moreover, to verify the importance of the negative charge in the induction of δ selectivity and to confirm the ineffectiveness of the C-terminal chiral center,8 we substituted Gly with L-Asp or D-Asp residues. Furthermore, this modification seems to be capable of influencing the blood-brain barrier (BBB) penetration of opioids; in fact, both 326, 27 and 410 show antidepressant activities when administered icv., but only 4 expresses these properties after intraperitoneal administration.
Chemistry
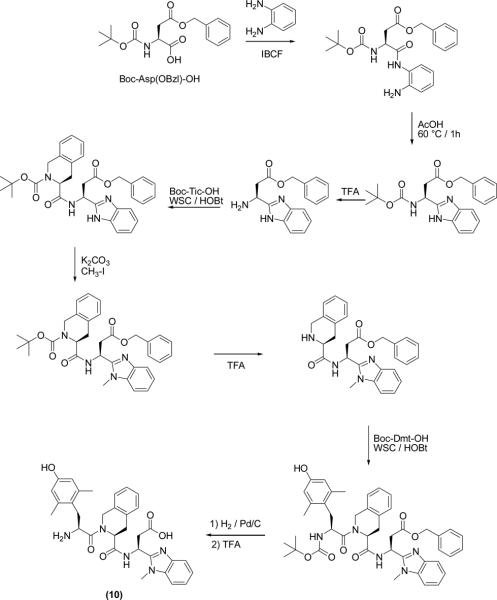
All peptides and pseudopeptides (5–13) were prepared stepwise in solution using conventional synthetic methods as outlined in Scheme 1 for the more representative compound (10). The intermediates containing Bid at their C-termini, Boc-NH-(S)CH(CH2-COOBzl)-Bid8 and Boc-NH-(R)CH(CH2-COOBzl)-Bid were prepared according to the procedure of Nestor et al.28 Briefly, mixed carbonic anhydride coupling of Boc-Asp(OBzl)-OH or Boc-D-Asp(OBzl)-OH with o-phenylendiamine gave the crude intermediate amides, which were converted without purification to the desired benzimidazole heterocycles (Bid) by cyclization / dehydration in acetic acid at 60 °C for 1 h. The corresponding Boc-protected intermediate anilides, or N(Me)-anilides, or benzylamides were prepared by condensation of Boc-Gly-OH, or Boc-Asp(OBzl)-OH, or Boc-D-Asp(OBzl)-OH with aniline, or N-methyl-aniline, or benzylamine, via mixed carbonic anhydride. Each intermediate, after Boc deprotection with TFA, was condensed with Boc-Tic-OH via WSC/HOBt. N1-Bid methylation was accomplished in DMF using K2CO3 as a base, followed after 1 h by the reaction with iodomethane.24 Subsequently, Boc deprotection (TFA) and the final condensation (WSC/HOBt) with Boc-Dmt-OH gave the fully protected compounds. Final deprotection of Asp or D-Asp side chains (by catalytic hydrogenation) and N-terminal amine functions (by TFA treatment) gave the crude final compounds (5–13), which were purified by preparative reverse phase HPLC.
Scheme 1.
Synthesis of Compound 10.
Results and Discussion
Receptor Affinity Analysis
Receptor binding data for δ- and μ-receptors and δ-selectivity (Kiμ/Kiδ) are reported in Table 1. All new compounds (5–13) had subnanomolar affinities for δ-opioid receptors (Kiδ = 0.036–0.186 nM) which is in good accordance with the reference compounds. As expected, the introduction of a negative charge, derived from the substitution of Gly with L- or D-Asp, increased δ-selectivity essentially by decreasing μ-affinity. In fact, compounds 6–13 with a Kiμ = 7.49–364.3 nM exhibited a δ-selectivity ranging from 101 to 5730 in comparison with the reference compounds and 5 lacking the negative charge (δ-selectivity = 4–14). The largest increase in δ-selectivity derived from the substitution of Gly with aspartic acid was seen in H-Dmt-Tic-Gly-NH-Bzl (2); in fact, its selectivity (Kiμ/Kiδ = 5) rose over 3 orders of magnitude to 5730. The same substitution gave lower increases in selectivity when applied to the reference compounds 1 and 3. On the basis of the C-terminal aromatic substituents, the highest selectivity appeared in the following series: –NH-Bzl > -N(Me)-Ph > -NH-Ph > -Bid ≥ -Bid(N1-Me). With regard to the aspartic acid chirality, no final conclusions can be drawn about its influence on receptor selectivity.
Table 1.
Receptor Binding Affinities and Functional Bioactivities of Compounds 1–13.
| Structure | Receptor affinityb (nM) | Selectivity | Functional bioactivity | ||||
|---|---|---|---|---|---|---|---|
| Ref. | Kiδ | Kiμ | Kiμ/Kiδ | MVD pA2d | MVD IC50c (nm) | GPI I C50c (nM) | |
| 1 | H-Dmt-Tic-Gly-NH-Phe | 0.042 | 0.16 | 4 | - | 3.02 | 2.57 |
| 2 | H-Dmt-Tic-Gly-NH-Bzle | 0.031 | 0.16 | 5 | 9.25 | - | 2.69 |
| 3 | H-Dmt-Tic-NH-CH2-Bide | 0.035 | 0.50 | 14 | - | 0.13 | 26.92 |
| 4 | H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bidf | 0.443 | 53.9 | 122 | - | 0.12 | 1724 |
| Cpd. | |||||||
| 5 | H-Dmt-Tic-Gly-N(Me)-Ph | 0.143 ± 0.024 (4) | 1.75 ± 0.067 (3) | 12 | 8.80 | - | 1466 ± 629 |
| 6 | H-Dmt-Tic-Asp-NH-Ph | 0.036 ± 0.002 (3) | 10.8 ± 1.2 (5) | 300 | 9.40 | - | 265 ± 10.4 |
| 7 | H-Dmt-Tic-D-Asp-NH-Ph | 0.058 ± 0.003 (3) | 7.49 ± 0.57 (4) | 129 | 8.62 | - | 2655 ± 127.8 |
| 8 | H-Dmt-Tic-Asp-N(Me)-Ph | 0.186 ± 0.008 (3) | 364.3 ± 37 (3) | 1958 | 8.75 | - | >10000 |
| 9 | H-Dmt-Tic-D-Asp-N(Me)-Ph | 0.084 ± 0.008 (4) | 45.0 ± 4.3 (5) | 536 | 8.06 | - | >10000 |
| 10 | H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid(N1-Me) | 0.059 ± 0.008 (4) | 5.93 ± 0.82 (5) | 101 | 9.90 | - | 2886 ± 983 |
| 11 | H-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid(N1-Me) | 0.070 ± 0.007 (4) | 11.8 ± 1.4 (5) | 169 | 9.65 | - | >10000 |
| 12 | H-Dmt-Tic- NH-(R)CH(CH2-COOH)-Bid | 0.067 ± 0.008 (5) | 16.1 ± 1.9 (6) | 240 | - | 1.21 | 179 |
| 13 | H-Dmt-Tic-Asp-NH-Bzl | 0.054 ± 0.006 (4) | 309.4 ± 24 (3) | 5730 | 8.53 | - | >10000 |
The Ki values (nM) were determined according to Cheng and Prusoff.43 The mean ± SE with n repetitions in parenthesis is based on independent duplicate binding assays with five to eight peptide doses using several different synaptosomal preparations.
Agonist activity was expressed as IC50 obtained from dose-response curves. These values represent the mean ± SE for at least four tissue samples. DPDPE and PL017 were the internal standards for MVD (δ-opioid receptor bioactivity) and GPI (μ-opioid receptor bioactivity) tissue preparation, respectively.
The pA2 values of opioid antagonists against the δ and μ agonists (deltorphin II and endomorphin-2, respectively) were determined by the method of Kosterlitz and Watt.49
Data taken from Balboni et al.5
Data taken from Balboni et al.8
Functional Bioactivity
Compounds 5–13 were tested in the electrically stimulated MVD and GPI pharmacological assays for intrinsic functional bioactivity (Table 1). As quite usually observed with compounds containing the Dmt-Tic pharmacophore, a close correlation between binding and functional bioactivity data is often lacking. Recently, a similar lack of correlation was observed by Hruby et al. with 4-anilidopiperidine analogues.29 As expected and partially demonstrated for 3,24, 25 all N-methylated analogues of anilides and N1-Bid (5, 8–11) revealed potent and selective δ-opioid antagonist activity (MVD, pA2 = 8.06–9.90), confirming the importance of the hydrogen of –NH-Ph and N1H-Bid on the induction of δ agonism. Surprisingly, the substitution of Gly with L-Asp (6) or D-Asp (7) in reference compound 1, gave two potent and quite selective δ antagonists (MVD, pA2 = 9.40 and 8.62, respectively) despite of the presence of the –NH-Ph hydrogen. Compound 12, the diastereoisomer containing the D-Asp side chain of δ agonist 4, indicated for the first time that better results can be obtained using L-amino acids in the synthesis of compounds containing a C-terminal Bid. In fact, it shows a δ agonist activity of one order of magnitude lower than 4, and a μ agonist activity of almost one order of magnitude higher. Interestingly, compound 10, the N1-Bid methylated analogue of 4, yielded the highest δ antagonism (pA2 = 9.90) in this series of compounds, and associated with a μ agonism 1.7 fold greater than 4. The substitution of Gly with Asp (13) in the μ agonist/δ antagonist 2 was detrimental in its activity profile; in fact, 13 had a selective δ antagonist activity (5-fold lower than 2) and associated with a very weak μ antagonist activity (GPI, pA2 = 6.26, not reported in Table 1). Finally, in the 3 pairs of compounds (6, 7; 8, 9; and 10, 11) and in the pair consisting of 4 and 12, the best δ activities were consistently seen with the analogues containing L-aspartic acid; however, this trend is not supported by the corresponding affinity data.
In Vivo Biological Activity
In recallling the data reported by Codd et al.,30 they demonstrated the in vivo biotrasformation of a δ opioid agonist into a μ agonist by N deethylation. A close look at our new compounds (5–13), a similar behaviour might be theoretically expected from all N-methylated analogues (5, 8–11). On the basis of this hypothesis,30 we chose a potent and selective δ antagonist (10) as a potential protodrug of the potent and selective δ agonist 4. However, preliminary enzymatic degradation studies (Supporting Information) failed to demonstrate and support this assumption; in fact, both compounds 4 and 10 appeared to be fully stable to enzymic degradation for 4 h and 2 h in plasma and brain homogenate, respectively. Notwithstanding the preceding negative results, we further tested 10 for in vivo analgesia in comparison with 4; a positive result might be tentatively considered as indirect evidence of the N-demethylation of 10 (δ antagonist) to the corresponding 4 (δ agonist) based on the analgesic effects of the tail-flick and hot-plate tests. Results reported in Figure 1 indicated a similar dose dependent analgesic effect for both compounds after intracerebroventricular injection: analgesia of both compounds was reversed by the δ selective antagonist naltrindole and the non-selective antagonist naloxone in the tail-flick test, but not in the hot-plate test (Figures 2 and 3). Interestingly, at the same dose 4 and 10 provided opposite behavioural effects; namely 4 caused excessive grooming and agitation (constant, fast moving in the cage, burrowing in the nesting material), while with 10 the mice appeared sedated, quiet, easily handled, and moving slowly if at all. Furthermore, 4 did not induce convulsions even at the highest doses, confirming our previous data on its antidepressant and anxiolytic studies,9, 10 which are in accord with observations about the higher convulsive effects of the nonpeptidic δ agonists in comparison to opioid peptides.26, 31, 32
Figure 1.
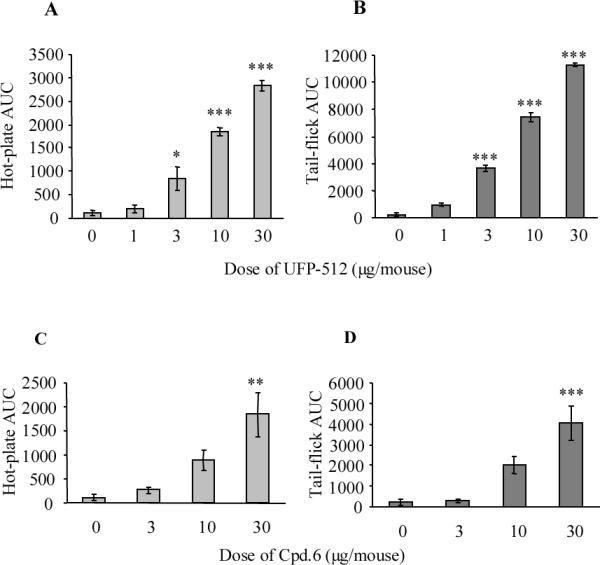
Dose dependent effect of icv injected 4 (A, B) and 10 (C, D) in the hot-plate (A, C) and tail-flick (B, D) tests. Each point represents the mean ± SEM (n = 5 mice). The asterisks denote AUC values that are significantly different from saline treated mice by Dunnett's test (*, p < 0.05; **, p < 0.01; ***, p < 0.001) following ANOVA (panel A: P < 0.0001: F = 71.49, d.f. 4; panel B: P < 0.0001: F = 251.7, d.f. 4; panel C: P < 0.001, F = 9.356, d.f. 3; panel D: p < 0.0001: F = 15.14, d.f. 3).
Figure 2.
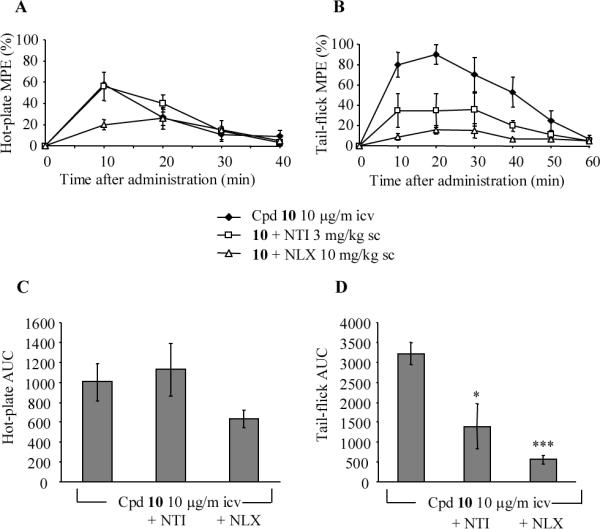
Effect of sc injected naltrindole (3 mg/kg) and naloxone (10 mg/kg) on 10 induced antinociception in hot-plate (A, C) and tail-flick (B, D) tests. Compound 10 was administered icv (10 μg/mouse) 20 min after administration of antagonists. (A, B) Time course, (C, D) Area Under the Curve (AUC). Each point represents the mean ± SEM (n = 5 mice). The asterisks denote AUC values that are significantly different from saline treated mice by Dunett's test (*, p < 0.05; ***, p < 0.001) following ANOVA (panel C: p = 0.2138: F = 1.759, d.f. 2; panel D: p <0.001: F = 13.61, d.f. 2).
Figure 3.
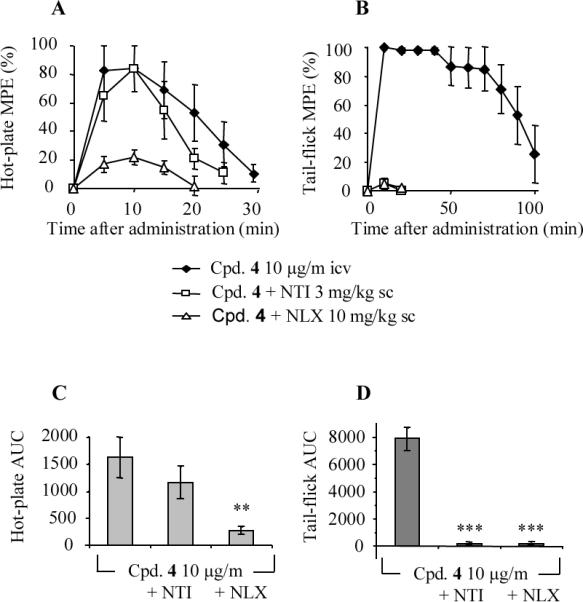
Effect of sc injected naltrindole (3 mg/kg) and naloxone (10 mg/kg) on 4 induced antinociception in hot-plate (A, C) and tail-flick (B, D) tests. 4 was administered icv (10 μg/mouse) 20 min after administration of antagonists. (A, B) Time course, (C, D) Area Under the Curve (AUC). Each point represents the mean ± SEM (n = 5 mice). The asterisks denote AUC values that are significantly different from saline treated mice by Dunnett's test (**, p < 0.01; ***, p < 0.001) following ANOVA (panel C: p = 0.0136: F = 6.277, d.f. 2; panel D: p < 0.0001: F = 87.13, d.f. 2).
Conclusions
Starting from the assumption that even minor chemical modifications can change the pharmacological profile of opioids, such as peptides and pseudopeptides containing the Dmt-Tic pharmacophore or nonpeptide derivatives related to morphine,33 we selected some reference compounds, especially our known δ agonists 1 and 4, and evaluated the influence of aspartic acid and its chirality, and the importance of the –NH-Ph and N1H-Bid hydrogens in the induction of δ agonism. The results obtained confirmed two expectations based on prior experimental data: (i) Asp increased the δ selectivity by lowering μ affinity; and (ii) methylation of -NH-Ph and N1H- Bid nitrogen transformed potent δ agonists into potent δ antagonists. On the other hand, several observations were quite unexpected: (i) the substitution of Gly with L-Asp or D-Asp in the δ agonist 1 gave potent and selective δ antagonists, while the same substitution in the δ agonist 3 produced the more selective δ agonists 4 and 12; (ii) Asp chirality seems to be important only in terms of functional bioactivity, since analogues 4, 6, 8, and 10, containing L-Asp are more active than the corresponding diastereoisomers 12, 7, 9, and 11, but the same is not true for receptor affinity; (iii) finally and totally unexpected— and in our opinion of potential interest—the potent and selective δ antagonist 10 yielded an analgesic effect similar to 4 that was reversed by naltrindole only when it was tested by the tail-flick test, and not in the hot-plate test. Furthermore, 4 and 10 resulted in opposite behavioural effects in mice; namely, 4 caused grooming and agitation, while with 10 mice appeared nearly sedated; convulsions were observed only in animals treated with the δ antagonist at very high doses icv. Considering the novelty of the pharmacological profile of this compound, more detailed pharmacological studies are in progress both in vivo (as an analgesic for neuropatic and inflammatory pain, antidepressant and anxiolytic) and in vitro, considering also its potential interaction with different receptors,34 or with heterodimers involving δ receptors.35 Preliminary enzymatic degradation studies that failed to demonstrate the N-demethylation of 10 to the corresponding 4 necessitates a more detailed study involving the use of different methods36 and/or other tissue preparations, such as kidney and liver30 which are planned in collaboration with other laboratories. If enzymatic N-demethylation occurs under these conditions, 6 could be considered a new lead compound of potential pharmacological utility for in vivo studies relative to δ opioid receptors trafficking at the membrane level.37–39
Experimental Section
Chemistry
Boc-Gly-N(Me)-Ph
A solution of Boc-Gly-OH (1.00 g, 5.71 mmol) and NMM (0.62 mL, 5.71 mmol) in DMF (10 mL) was treated at −20 °C with IBCF (0.74 mL, 5.71 mmol). After 10 min. at −20 °C, N-methylaniline (0.62 mL, 5.71 mmol) was added. The reaction mixture was allowed to stir while slowly warming to room temperature (1 h) and was then stirred for an additional 3 h. The solvent was evaporated and the residue was partitioned between EtOAc and H2O. The EtOAc layer was washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine and dried over Na2SO4. The solution was filtered, the solvent evaporated, and the residual oil was precipitated from Et2O/Pe (1:9, v/v): yield 1.39 g (92%); Rf(B) 0.52; HPLC K′ 6.01; mp 99–101 °C; m/z 265 (M+H)+; 1H-NMR (DMSO-d6) δ 1.40 (s, 9H), 2.78 (s, 3H), 3.88–3.92 (m, 2H), 7.10–7.31 (m, 5H).
TFȦH-Gly-N(Me)-Ph
Boc-Gly-N(Me)-Ph (1.36 g, 5.15 mmol) was treated with TFA (2.5 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 1.36 g (95%); Rf(A) 0.49; HPLC K′ 5.12; mp 115–117 °C; m/z 165 (M+H)+.
Boc-Tic-Gly-N(Me)-Ph
To a solution of Boc-Tic-OH (0.20 g, 0.72 mmol) and TFȦH-Gly- N(Me)-Ph (0.20 g, 0.72 mmol) in DMF (10 mL) at 0 °C, NMM (0.08 mL, 0.72 mmol), HOBt (0.12 g, 0.79 mmol), and WSC (0.15 g, 0.79 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.27 g (87%); Rf(B) 0.69; HPLC K′ 7.35; mp 133–135 °C; [α]20D - 30.1; m/z 425 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.78 (s, 3H), 2.92–3.17 (m, 2H), 4.14–4.27 (m, 4H), 4.92 (m, 1H), 6.96–7.31 (m, 9H).
TFȦH-Tic-Gly-N(Me)-Ph
Boc-Tic-Gly-N(Me)-Ph (0.24 g, 0.57 mmol) was treated with TFA (1.5 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 0.24 g (95%); Rf(A) 0.39; HPLC K′ 6.22; mp 158–160 °C; [α]20D - 30.6; m/z 324 (M+H)+.
Boc-Dmt-Tic-Gly-N(Me)-Ph
To a solution of Boc-Dmt-OH (0.10 g, 0.32 mmol) and TFȦH-Tic-Gly-N(Me)-Ph (0.14 g, 0.32 mmol) in DMF (10 mL) at 0 °C, NMM (0.03 mL, 0.32 mmol), HOBt (0.05 g, 0.35 mmol), and WSC (0.07 g, 0.35 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.17 g (87%); Rf(B) 0.68; HPLC K′ 10.01; mp 141–143 °C; [α]20D −18.5; m/z 616 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.78 (s, 3H), 2.92–3.17 (m, 4H), 4.14–4.92 (m, 6H), 6.29 (s, 2H), 6.96–7.31 (m, 9H).
TFȦH-Dmt-Tic-Gly-N(Me)-Ph (5)
Boc-Dmt-Tic-Gly-N(Me)-Ph (0.14 g, 0.23 mmol) was treated with TFA (1 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 0.14 g (97%); Rf(A) 0.45; HPLC K′ 7.25; mp 150–152 °C; [α]20D −20.3; m/z 516 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.78 (s, 3H), 2.92–3.17 (m, 4H), 3.95–4.92 (m, 6H), 6.29 (s, 2H), 6.96–7.31 (m, 9H); Anal. C32H35F3N4O6: C; H; N.
Boc-Asp(OBzl)-NH-Ph
This intermediate was obtained by condensation of Boc-Asp(OBzl)- OH with aniline via mixed anhydride as reported for Boc-Gly-N(Me)-Ph: yield 0.57 g (93%); Rf (B) 0.73; HPLC K' 7.02; mp 129–132 °C; [α]20D +12.5; m/z 399 (M+H)+, 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.72–2.97 (d, 2H), 5.17–5.34 (m, 3H), 7.19–7.60 (m, 10H).
TFȦH-Asp(OBzl)-NH-Ph
Boc-Asp(OBzl)-NH-Ph was treated with TFA as reported for TFȦH-Gly-N(Me)-Ph: yield 0.54 g (96%); Rf(A) 0.75; HPLC K′ 5.02; mp 138–140 °C; [α]20D +15.2; m/z 299 (M+H)+.
Boc-Tic-Asp(OBzl)-NH-Ph
This intermediate was obtained by condensation of Boc-Tic-OH with TFȦH-Asp(OBzl)-NH-Ph via WSC/HOBt as reported for Boc-Tic-Gly-N(Me)-Ph: yield 0.21 g (88%); Rf(B) 0.82; HPLC K′ 6.43; mp 147–149 °C; [α]20D +5.2; m/z 559 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.72–3.17 (m, 4H), 4.17–4.27 (m, 2H), 4.92–5.34 (m, 4H), 6.96–7.64 (m, 14H).
TFȦH-Tic-Asp(OBzl)-NH-Ph
Boc-Tic-Asp(OBzl)-NH-Ph was treated with TFA as reported for TFȦH-Tic-Gly-N(Me)-Ph: yield 0.18 g (96%); Rf(A) 0.73; HPLC K ′4.21; mp 145–147 °C; [α]20D +5.3; m/z 459 (M+H)+.
Boc-Dmt-Tic-Asp(OBzl)-NH-Ph
This intermediate was obtained by condensation of Boc-Dmt-OH with TFȦH-Tic-Asp(OBzl)-NH-Ph via WSC/HOBt as reported for Boc-Dmt-Tic-Gly- N(Me)-Ph: yield 0.14 g (87%); Rf(B) 0.78; HPLC K′ 9.31; mp 165–167 °C; [α]20D −2.5; m/z 750 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.72–3.17 (m, 6H), 4.41–4.51 (m, 2H), 4.92–5.34 (m, 5H), 6.29 (s, 2H), 6.96–7.64 (m, 14H).
Boc-Dmt-Tic-Asp-NH-Ph
To a solution of Boc-Dmt-Tic-Asp(OBzl)-NH-Ph (0.11 g, 0.15 mmol) in methanol (30 mL) was added Pd/C (10%, 0.07 g), and H2 was bubbled for 1 h at room temperature. After filtration, the solution was evaporated to dryness. The residue was crystallized from Et2O/Pe (1:9, v/v): yield 0.09 g (95%); Rf(B) 0.67; HPLC K′ 7.19; mp 169–171 °C; [α]20D −6.4; m/z 660 (M+H)+.
TFȦH-Dmt-Tic-Asp-NH-Ph (6)
Boc-Dmt-Tic-Asp-NH-Ph was treated with TFA as reported for TFȦH-Dmt-Tic-Gly-N(Me)-Ph: yield 0.09 g (96%); Rf(A) 0.67; HPLC K′ 4.21; mp 158–160 °C; [α]20D −7.3; m/z 560 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.72–3.17 (m, 6H), 3.95–4.51 (m, 3H), 4.86–4.92 (m, 2H), 6.29 (s, 2H), 6.96–7.64 (m, 9H); Anal. C33H35F3N4O8: C; H; N.
Boc-D-Asp(OBzl)-NH-Ph
This intermediate was obtained by condensation of Boc-D-Asp(OBzl)-OH with aniline via mixed anhydride as reported for Boc-Gly-N(Me)-Ph: yield 0.38 g (92%); Rf (B) 0.73; HPLC K' 7.02; mp 129–132 °C; [α]20D −12.5; m/z 399 (M+H)+, 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.72–2.97 (d, 2H), 5.17–5.34 (m, 3H), 7.19–7.60 (m, 10H).
TFȦH-D-Asp(OBzl)-NH-Ph
Boc-D-Asp(OBzl)-NH-Ph was treated with TFA as reported for TFȦH-Gly-N(Me)-Ph: yield 0.25 g (96%); Rf(A) 0.75; HPLC K′ 5.02; mp 138–140 °C; [α]20D −15.2; m/z 299 (M+H)+.
Boc-Tic-D-Asp(OBzl)-NH-Ph
This intermediate was obtained by condensation of Boc-Tic-OH with TFȦH-D-Asp(OBzl)-NH-Ph via WSC/HOBt as reported for Boc-Tic-Gly-N(Me)-Ph: yield 0.17 g (87%); Rf(B) 0.80; HPLC K′ 6.38; mp 147–149 °C; [α]20D +8.4; m/z 559 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.73–3.19 (m, 4H), 4.21–4.29 (m, 2H), 4.90–5.31 (m, 4H), 6.96–7.64 (m, 14H).
TFȦH-Tic-D-Asp(OBzl)-NH-Ph
Boc-Tic-D-Asp(OBzl)-NH-Ph was treated with TFA as reported for TFȦH-Tic-Gly-N(Me)-Ph: yield 0.15 g (97%); Rf(A) 0.71; HPLC K′ 4.17; mp 146–148 °C; [α]20D +10.1; m/z 459 (M+H)+.
Boc-Dmt-Tic-D-Asp(OBzl)-NH-Ph
This intermediate was obtained by condensation of Boc-Dmt-OH with TFȦH-Tic-D-Asp(OBzl)-NH-Ph via WSC/HOBt as reported for Boc-Dmt-Tic- Gly-N(Me)-Ph: yield 0.11 g (89%); Rf(B) 0.75; HPLC K′ 8.98; mp 160–162 °C; [α]20D +8.6; m/z 750 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.70–3.20 (m, 6H), 4.40–4.53 (m, 2H), 4.93–5.32 (m, 5H), 6.29 (s, 2H), 6.96–7.64 (m, 14H).
Boc-Dmt-Tic-D-Asp-NH-Ph
Boc-Dmt-Tic-D-Asp(OBzl)-NH-Ph was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.08 g (95%); Rf(B) 0.64; HPLC K′ 6.95; mp 164–176 °C; [α]20D +12.8; m/z 660 (M+H)+.
TFA.H-Dmt-Tic-D-Asp-NH-Ph (7)
Boc-Dmt-Tic-D-Asp-NH-Ph was treated with TFA as reported for TFA.H-Dmt-Tic-Gly-N(Me)-Ph: yield 0.04 g (93%); Rf(A) 0.64; HPLC K′ 4.15; mp 150–152 °C; [α]20D +12.5; m/z 560 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.70–3.19 (m, 6H), 3.96–4.53 (m, 3H), 4.85–4.90 (m, 2H), 6.29 (s, 2H), 6.96–7.66 (m, 9H); Anal. C33H35F3N4O8: C; H; N.
Boc-Asp(OBzl)-N(Me)-Ph
This intermediate was obtained by condensation of Boc-Asp(OBzl)-OH with N-methylaniline via mixed anhydride as reported for Boc-Gly-N(Me)-Ph: yield 0.43 g (90%); Rf (B) 0.75; HPLC K' 7.11; mp 126–128 °C; [α]20D +10.3; m/z 413 (M+H)+, 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.72–2.97 (m, 5H), 5.17–5.34 (m, 3H), 7.10–7.31 (m, 10H).
TFA.H-Asp(OBzl)-N(Me)-Ph
Boc-Asp(OBzl)-N(Me)-Ph was treated with TFA as reported for TFA.H-Gly-N(Me)-Ph: yield 0.40 g (97%); Rf(A) 0.77; HPLC K′ 5.11; mp 135–139 °C; [α]20D +14.7; m/z 313 (M+H)+.
Boc-Tic-Asp(OBzl)-N(Me)-Ph
This intermediate was obtained by condensation of Boc-Tic-OH with TFA.H-Asp(OBzl)-N(Me)-Ph via WSC/HOBt as reported for Boc-Tic-Gly-N(Me)-Ph: yield 0.21 g (85%); Rf(B) 0.84; HPLC K′ 6.45; mp 144–146 °C; [α]20D +4.8; m/z 573 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.73–3.19 (m, 7H), 4.17–4.27 (m, 2H), 4.92–5.34 (m, 4H), 6.96–7.31 (m, 14H).
TFA.H-Tic-Asp(OBzl)-N(Me)-Ph
Boc-Tic-Asp(OBzl)-N(Me)-Ph was treated with TFA as reported for TFA.H-Tic-Gly-N(Me)-Ph: yield 0.18 g (97%); Rf(A) 0.75; HPLC K′ 4.25; mp 141–143 °C; [α]20D +4.6; m/z 473 (M+H)+.
Boc-Dmt-Tic-Asp(OBzl)-N(Me)-Ph
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA.H-Tic-Asp(OBzl)-N(Me)-Ph via WSC/HOBt as reported for Boc-Dmt-Tic-Gly-N(Me)-Ph: yield 0.13 g (88%); Rf(B) 0.81; HPLC K′ 9.36; mp 160–162 °C; [α]20D −3.5; m/z 764 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.70–3.17 (m, 9H), 4.43–4.51 (m, 2H), 4.92–5.34 (m, 5H), 6.29 (s, 2H), 6.96–7.31 (m, 14H).
Boc-Dmt-Tic-Asp-N(Me)-Ph
Boc-Dmt-Tic-Asp(OBzl)-N(Me)-Ph was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.08 g (94%); Rf(B) 0.69; HPLC K′ 7.23; mp 165–167 °C; [α]20D −7.3; m/z 674 (M+H)+.
TFA.H-Dmt-Tic-Asp-N(Me)-Ph (8)
Boc-Dmt-Tic-Asp-N(Me)-Ph was treated with TFA as reported for TFA.H-Dmt-Tic-Gly-N(Me)-Ph: yield 0.04 g (94%); Rf(A) 0.69; HPLC K′ 4.26; mp 152–154 °C; [α]20D −8.2; m/z 574 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.70–3.17 (m, 9H), 3.95–4.51 (m, 3H), 4.86–4.92 (m, 2H), 6.29 (s, 2H), 6.96–7.31 (m, 9H); Anal. C34H37F3N4O8: C; H; N.
Boc-D-Asp(OBzl)-N(Me)-Ph
This intermediate was obtained by condensation of Boc-D-Asp(OBzl)-OH with N-methylaniline via mixed anhydride as reported for Boc-Gly-N(Me)-Ph: yield 0.42 g (88%); Rf (B) 0.75; HPLC K' 7.11; mp 126–128 °C; [α]20D −10.3; m/z 413 (M+H)+, 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.72–2.97 (m, 5H), 5.17–5.34 (m, 3H), 7.10–7.31 (m, 10H).
TFA.H-D-Asp(OBzl)-N(Me)-Ph
Boc-D-Asp(OBzl)-N(Me)-Ph was treated with TFA as reported for TFA.H-Gly-N(Me)-Ph: yield 0.39 g (97%); Rf(A) 0.77; HPLC K′ 5.11; mp 135–139 °C; [α]20D −14.7; m/z 313 (M+H)+.
Boc-Tic-D-Asp(OBzl)-N(Me)-Ph
This intermediate was obtained by condensation of Boc-Tic-OH with TFA.H-D-Asp(OBzl)-N(Me)-Ph via WSC/HOBt as reported for Boc-Tic-Gly-N(Me)-Ph: yield 0.19 g (87%); Rf(B) 0.83; HPLC K′ 6.44; mp 145–147 °C; [α]20D +7.8; m/z 573 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.73–3.19 (m, 7H), 4.21–4.29 (m, 2H), 4.90–5.31 (m, 4H), 6.96–7.35 (m, 14H).
TFA.H-Tic-D-Asp(OBzl)-N(Me)-Ph
Boc-Tic-D-Asp(OBzl)-N(Me)-Ph was treated with TFA as reported for TFA.H-Tic-Gly-N(Me)-Ph: yield 0.16 g (96%); Rf(A) 0.73; HPLC K′ 4.24; mp 142–144 °C; [α]20D +9.2; m/z 473 (M+H)+.
Boc-Dmt-Tic-D-Asp(OBzl)-N(Me)-Ph
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA.H-Tic-D-Asp(OBzl)-N(Me)-Ph via WSC/HOBt as reported for Boc-Dmt-Tic-Gly-N(Me)-Ph: yield 0.12 g (88%); Rf(B) 0.79; HPLC K′ 9.08; mp 156–158 °C; [α]20D +7.8; m/z 764 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.70–3.20 (m, 9H), 4.40–4.53 (m, 2H), 4.93–5.32 (m, 5H), 6.29 (s, 2H), 6.96–7.35 (m, 14H).
Boc-Dmt-Tic-D-Asp-N(Me)-Ph
Boc-Dmt-Tic-D-Asp(OBzl)-N(Me)-Ph was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.08 g (93%); Rf(B) 0.68; HPLC K′ 7.03; mp 155–157 °C; [α]20D +11.3; m/z 674 (M+H)+
TFA.H-Dmt-Tic-D-Asp-N(Me)-Ph (9)
Boc-Dmt-Tic-D-Asp-N(Me)-Ph was treated with TFA as reported for TFA.H-Dmt-Tic-Gly-N(Me)-Ph: yield 0.04 g (96%); Rf(A) 0.68; HPLC K′ 4.23; mp 145–147 °C; [α]20D +11.7; m/z 574 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.70–3.19 (m, 9H), 3.96–4.53 (m, 3H), 4.85–4.90 (m, 2H), 6.29 (s, 2H), 6.96–7.35 (m, 9H); Anal. C34H37F3N4O8: C; H; N.
(S)-tert-butyl3-((S)-2-((benzyloxy)carbonyl)-1-(1-methyl-1H-benzo[d]imidazol-2-yl)ethylcarbamoyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate [Boc-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me)]
To a solution of Boc-Tic-NH-(S)CH(CH2-COOBzl)-Bid8 (0.27 g; 0.40 mmol) in DMF (10 mL) at room temperature, K2CO3 (0.25 g; 1.8 mmol) and, after 1 h, iodomethane (0.03 mL; 0.42 mmol) were added. The reaction mixture was stirred for 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with NaHCO3 (5% in H2O) and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.20 g (90%); Rf(B) 0.81; HPLC K′ 6.15; mp 151–153 °C; [α]20D +9.7; m/z 570 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.80–3.17 (m, 4H), 3.63 (s, 3H), 4.17–4.27 (m, 2H), 4.92–5.51 (m, 4H), 6.96–7.70 (m, 13H).
2TFA.H-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me)
Boc-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me) was treated with TFA as reported for TFA.H-Tic-Gly-N(Me)-Ph: yield 0.21 g (93%); Rf(A) 0.71; HPLC K′ 5.52; mp 144–146 °C; [α]20D +10.1; m/z 470 (M+H)+
Boc-Dmt-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me)
To a solution of Boc-Dmt-OH (0.08 g, 0.26 mmol) and 2TFA.H-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me) (0.18 g, 0.26 mmol) in DMF (10 mL) at 0 °C, NMM (0.06 mL, 0.52 mmol), HOBt (0.04 g, 0.29 mmol), and WSC (0.05 g, 0.29 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.17 g (86%); Rf(B) 0.68; HPLC K′ 9.23; mp 165–167 °C; [α]20D +3.8; m/z 760 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.92–3.20 (m, 6H), 3.63 (s, 3H), 4.41–4.51 (m, 2H), 4.92–5.51 (m, 5H), 6.29 (s, 2H), 6.96–7.70 (m, 13H).
Boc-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid(N1-Me)
Boc-Dmt-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me) was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.11 g (94%); Rf(B) 0.55; HPLC K′ 7.26; mp 166–168 °C; [α]20D +4.6; m/z 671 (M+H)+
2TFA.H-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid(N1-Me) (10)
Boc-Dmt-Tic-NH-(S)CH(CH2-COOH)-Bid(N1-Me) was treated with TFA as reported for TFA.H-Dmt-Tic-Gly-N(Me)-Ph: yield 0.05 g (96%); Rf(A) 0.69 HPLC K′ 4.56; mp 152–154 °C; [α]20D +7.3; m/z 571 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92–3.20 (m, 6H), 3.63–3.95 (m, 4H), 4.41–4.51 (m, 2H), 4.92–5.20 (m, 2H), 6.29 (s, 2H), 6.96–7.70 (m, 8H); Anal. C36H37F6N5O9: C; H; N.
tert-butyl (R)-2-((benzyloxy)carbonyl)-1-(1H-benzo[d]imidazol-2-yl)ethylcarbamate [Boc-NH-(R)CH(CH2-COOBzl)-Bid]
A solution of Boc-D-Asp(OBzl)-OH (1 g, 3.1 mmol) and NMM (0.34 mL, 3.1 mmol) in DMF (10 mL) was treated at −20 °C with IBCF (0.4 mL, 3.1 mmol). After 10 min. at −20 °C, o-phenylendiamine (0.33 g, 3.1 mmol) was added. The reaction mixture was allowed to stir while slowly warming to room temperature (1h) and was then stirred for 3 h. The solvent was evaporated, and the residue was partitioned between EtOAc and H2O. The AcOEt layer was washed with NaHCO3 (5% in H2O) and brine and dried over Na2SO4. The solution was filtered, the solvent was evaporated, and the residual solid was dissolved in glacial acetic acid (10 mL). The solution was heated at 60 °C for 1h.. After the solvent was evaporated and the residue was precipitated from Et2O/Pe (1:9, v/v): yield 1 g (82%); Rf(B) 0.52; HPLC K′ 6.50; mp 136–138 °C; [α]20D −15.8; m/z 396 (M+H)+; 1H-NMR (DMSO-d6) δ 1.40 (s, 9H), 2.67–2.92 (m, 2H), 5.34–5.51 (m, 3H), 7.19–7.70 (m, 9H).
2TFA.H2N-(R)CH(CH2-COOBzl)-Bid
Boc-NH-(R)CH(CH2-COOBzl)-Bid was treated with TFA as reported for TFA.H-Gly-N(Me)-Ph: yield 0.51 g (96%); Rf(A) 0.78; HPLC K′ 4.20; mp 142–144 °C; [α]20D −17.3; m/z 296 (M+H)+.
Boc-Tic-NH-(R)CH(CH2-COOBzl)-Bid
To a solution of Boc-Tic-OH (0.28 g, 1 mmol) and 2TFA.H2N-(R)CH(CH2-COOBzl)-Bid (0.52 g, 1 mmol) in DMF (10 mL) at 0 °C, NMM (0.2 mL, 2 mmol), HOBt (0.17 g, 1.1 mmol), and WSC (0.21 g, 1.1 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.48 g (87%); Rf(B) 0.72; HPLC K′ 5.92; mp 155–157 °C; [α]20D −1.6; m/z 556 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.80–3.17 (m, 4H), 4.17–4.27 (m, 2H), 4.92–5.51 (m, 4H), 6.96–7.70 (m, 13H).
(S)-tert-b u t y l 3-((R)-2-((benzyloxy)carbonyl)-1-(1-methyl-1H-benzo[d]imidazol-2-yl)ethylcarbamoyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate [Boc-Tic-NH-(R)CH(CH2-COOBzl)-Bid(N1-Me)]
This intermediate was obtained by alkylation of Boc-Tic-NH-(R)CH(CH2-COOBzl)-Bid with K2CO3 and iodomethane as reported for Boc-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me): yield 0.22 g (88%); Rf(B) 0.77; HPLC K′ 6.07; mp 154–156 °C; [α]20D +4.3; m/z 570 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.82–3.20 (m, 4H), 3.63 (s, 3H), 4.15–4.29 (m, 2H), 4.90–5.49 (m, 4H), 6.96–7.70 (m, 13H).
2TFȦH-Tic-NH-(R)CH(CH2-COOBzl)-Bid(N1-Me)
Boc-Tic-NH-(R)CH(CH2-COOBzl)-Bid(N1-Me) was treated with TFA as reported for TFȦH-Tic-Gly-N(Me)-Ph: yield 0.21 g (92%); Rf(A) 0.68; HPLC K′ 5.39; mp 148–150 °C; [α]20D +5.4; m/z 470 (M+H)+.
Boc-Dmt-Tic-NH-(R)CH(CH2-COOBzl)-Bid(N1-Me)
This intermediate was obtained by condensation of Boc-Dmt-OH with 2TFȦH-Tic-NH-(R)CH(CH2-COOBzl)-Bid(N1-Me) via WSC/HOBt as reported for Boc-Dmt-Tic-NH-(S)CH(CH2-COOBzl)-Bid(N1-Me): yield 0.17 g (86%); Rf(B) 0.64; HPLC K′ 8.28; mp 169–171 °C; [α]20D −2.7; m/z 760 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.94–3.22 (m, 6H), 3.63 (s, 3H), 4.40–4.52 (m, 2H), 4.92–5.48 (m, 5H), 6.29 (s, 2H), 6.96–7.70 (m, 13H).
Boc-Dmt-Tic-NH-(R)CH(CH-COOH)-Bid(N1-Me)
Boc-Dmt-Tic-NH-(R)CH(CH2-COOBzl)-Bid(N1-Me) was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.10 g (92%); Rf(B) 0.50; HPLC K′ 6.98; mp 172–174 °C; [α]20 −1.9; m/z 671 (M+H)+D −1.9; m/z 671 (M+H)+.
2TFȦH-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid(N1-Me) (11)
Boc-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid(N1-Me) was treated with TFA as reported for TFȦH-Dmt-Tic-Gly- N(Me)-Ph: yield 0.07 g (96%); Rf(A) 0.58 HPLC K′ 4.02; mp 161–163 °C; [α]20D +2.2; m/z 571 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.90–3.22 (m, 6H), 3.61–3.93 (m, 4H), 4.42–4.53 (m, 2H), 4.90–5.18 (m, 2H), 6.29 (s, 2H), 6.96–7.70 (m, 8H); Anal. C36H37F6N5O9: C; H; N.
2TFȦH-Tic-NH-(R)CH(CH2-COOBzl)-Bid
Boc-Tic-NH-(R)CH(CH2-COOBzl)-Bid was treated with TFA as reported for TFȦH-Tic-Gly-N(Me)-Ph: yield 0.18 g (93%); Rf(A) 0.66; HPLC K′ 5.35; mp 149–151 °C; [α]20D +6.3; m/z 456 (M+H)+.
Boc-Dmt-Tic-NH-(R)CH(CH2-COOBzl)-Bid
This intermediate was obtained by condensation of Boc-Dmt-OH with 2TFȦH-Tic-H2N-(R)CH(CH2-COOBzl)-Bid via WSC/HOBt as reported for Boc-Dmt-Tic-NH-(S)CH(CH -COOBzl)-Bid(N1-Me): yield 0.15 g (87%); Rf(B) 0.61; HPLC K′ 8.01; mp 174–176 °C; [α]20D −3.5; m/z 747 (M+H)+; 1H-NMR (DMSO-d6) δ 1.36–1.40 (d, 9H), 2.35 (s, 6H), 2.63–3.17 (m, 6H), 4.42–4.53 (m, 2H), 4.92–5.51 (m, 5H), 6.29 (s, 2H), 6.96–7.70 (m, 13H).
Boc-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid
Boc-Dmt-Tic-NH-(R)CH(CH2-COOBzl)-Bid was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.09 g (93%); Rf(B) 0.46; HPLC K′ 6.84; mp 176–178 °C; [α]20D −2.8; m/z 657 (M+H)+.
2TFẠH-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid (12)
Boc-Dmt-Tic-NH-(R)CH(CH2-COOH)-Bid was treated with TFA as reported for TFȦH-Dmt-Tic-Gly-N(Me)-Ph: yield 0.06 g (96%); Rf(A) 0.52; HPLC K′ 3.92; mp 166–168 °C; [α]20D +3.5; m/z 557 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.90–3.17 (m, 6H), 3.95–4.52 (m, 3H), 4.90–5.21 (m, 2H), 6.29 (s, 2H), 6.96–7.70 (m, 8H); Anal. C35H35F6N5O9: C; H; N.
Boc-Asp(OBzl)-NH-Bzl
This intermediate was obtained by condensation of Boc- Asp(OBzl)-OH with benzylamine via mixed anhydride as reported for Boc-Gly-N(Me)-Ph: yield 0.45 g (90%); R (B) 0.77; HPLC K' 7.18; mp 126–128 °C; [α]20D −11.2; m/z 413 (M+H)+, 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.63–2.88 (d, 2H), 4.46 (s, 2H), 5.17–5.34 (m, 3H), 7.06–7.19 (m, 10H).
TFȦH-Asp(OBzl)-NH-Bzl
Boc-Asp(OBzl)-NH-Bzl was treated with TFA as reported for TFȦH-Gly-N(Me)-Ph: yield 0.42 g (96%); Rf(A) 0.78; HPLC K′ 5.18; mp 135–147 °C; [α]20D +14.3; m/z 313 (M+H)+.
Boc-Tic-Asp(OBzl)-NH-Bzl
This intermediate was obtained by condensation of Boc-Tic-OH with TFȦH-Asp(OBzl)-NH-Bzl via WSC/HOBt as reported for Boc-Tic-Gly-N(Me)-Ph: yield 0.22 g (87%); Rf(B) 0.86; HPLC K′ 6.51; mp 144–146 °C; [α]20D +4.7; m/z 573 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.63–3.17 (m, 4H), 4.17–4.46 (m, 4H), 4.92–5.34 (m, 4H), 6.96–7.19 (m, 14H).
TFȦH-Tic-Asp(OBzl)-NH-Bzl
Boc-Tic-Asp(OBzl)-NH-Bzl was treated with TFA as reported for TFȦH-Tic-Gly-N(Me)-Ph: yield 0.18 g (97%); Rf(A) 0.77; HPLC K′ 4.29; mp 140–142 °C; [α]20D +4.7; m/z 473 (M+H)+.
Boc-Dmt-Tic-Asp(OBzl)-NH-Bzl
This intermediate was obtained by condensation of Boc-Dmt-OH with TFȦH-Tic-Asp(OBzl)-NH-Bzl via WSC/HOBt as reported for Boc-Dmt-Tic-Gly- N(Me)-Ph: yield 0.13 g (88%); Rf(B) 0.82; HPLC K′ 9.38; mp 160–162 °C; [α]20D −1.9; m/z 764 (M+H)+; 1H-NMR (DMSO-d6) δ 1.38–1.42 (d, 9H), 2.35 (s, 6H), 2.63–3.17 (m, 6H), 4.41–4.51 (m, 4H), 4.92–5.34 (m, 5H), 6.29 (s, 2H), 6.96–7.19 (m, 14H).
Boc-Dmt-Tic-Asp-NH-Bzl
Boc-Dmt-Tic-Asp(OBzl)-NH-Bzl was treated with Pd/C and H2 as reported for Boc-Dmt-Tic-Asp-NH-Ph: yield 0.09 g (96%); Rf(B) 0.71; HPLC K′ 7.24; mp 164–166 °C; [α]20D −5.2; m/z 674 (M+H)+.
TFȦH-Dmt-Tic-Asp-NH-Bzl (13)
Boc-Dmt-Tic-Asp-NH-Bzl was treated with TFA as reported for TFȦH-Dmt-Tic-Gly-N(Me)-Ph: yield 0.05 g (96%); Rf(A) 0.69; HPLC K′ 4.29; mp 154–166 °C; [α]20D −6.7; m/z 574 (M+H)+; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.63–3.17 (m, 6H), 3.95–4.51 (m, 5H), 4.86–4.92 (m, 2H), 6.29 (s, 2H), 6.96–7.14 (m, 9H); Anal. C34H37F3N4O8:C; H; N.
Pharmacology
Competitive Binding Assays
Opioid receptor affinities were determined under equilibrium conditions [2.5 h room temperature (23 °C)] in competition assays using brain P2 synaptosomal membranes prepared from Sprague-Dawley rats.40, 41 Synaptosomes were preincubated to remove endogenous opioid peptides and stored at −80 °C in buffered 20% glycerol.40, 42 Each analogue was analyzed in duplicate assays using five to eight dosages and three to five independent repetitions with different synaptosomal preparations (n values are listed in Table 1 in parenthesis and results are mean ± SE). Unlabeled peptide (2 μM) was used to determine non-specific binding in the presence of 1.9 nM [3H]deltorphin II (45.0 Ci/mmol, Perkin Elmer, Boston, MA; KD = 1.4 nM) for δ-opioid receptors and 3.5 nM [3H]DAMGO (50.0 Ci/mmol), Amersham Bioscience, Buckinghamshire, U. K.; KD = 1.5 nM) for μ-opioid receptors. Glass fibre filters (Whatman GFC) were soaked in 0.1% polyethylenimine in order to enhance the signal-to-noise ratio of the bound radiolabeled-synaptosome complex, and the filters were washed thrice in icecold buffered BSA,40 and the affinity constants (Ki) were calculated according to Cheng and Prusoff..43
Biological Activity in Isolate Tissue Preparations
The myenteric plexus longitudinal muscle preparations (2–3 cm segments) from the small intestine of male Hartley strain of guinea pigs (GPI) measured μ-opioid receptor agonism, and a single mouse vas deferens (MVD) was used to determine δ-opioid receptor agonism as described previously.44 The isolated tissues were suspended in organ baths containing balanced salt solutions in a physiological buffer, pH 7.5. Agonists were tested for the inhibition of electrically evoked contraction and expressed as IC50 (nM) obtained from the dose-response curves. The IC50 values represent the mean ± SE of five or six separate assays, and the δ-antagonist potencies in the MVD assay were determined against the δ agonist deltorphin-II, while μ antagonism (GPI assay) used the μ agonist endomorphin-2. Antagonism is expressed as pA2 determined using the Schild Plot.45
Animals for in vitro and in vivo studies
Laboratory animals were used under protocols approved and governed by the Animal Care and Use Committees of Tohoku Pharmaceutical University, University of Ferrara and the National Institute of Environmental Health Sciences.
In vivo Assessment of Opioid Bioactivity
Male Swiss-Webster mice (20–25 g, Taconic, Germantown, NY) were housed on a 12-h light/dark cycle with free access to food and water and experimental protocol approved by the NIEHS Animal Care and Use Committee. Intracerebroventricular injection (icv) used a Hamilton microsyringe with disposable 26-gauge needle that was inserted 2.3–3 mm into the ventricular sinus at the bregma as described;46 the total volume administered was 5 μL (peptide in saline). Upon completion of the study, mice were sacrificed according ACUC protocols and those animals having needle tract 2 mm lateral from the midline were counted as being valid. Hot-plate test consisted of animals placed on an electrically heated plate (55 ± 0.1 °C, IITC MODEL 39D Hot Plate analgesia meter, IITC Inc, Woodland Hills, CA) 10 min after icv administration. Hot-plate latency (HPL) is the interval between placement of mice onto the hot plate and observing movement consisting of either jumping, licking or shaking their hind paws; a baseline latency of 5–10 sec (pre-response time) and maximal cut off time of 30 sec. Measurements were repeated every 10 min and testing was terminated when the HPL was close to the pre-response time.
Spinal effects used of a tail-flick instrument (Columbus Instruments, Columbus, OH). Radiant heat was applied on the dorsal surface of the tail and the latency before removal of the tail from the onset of the radiant heat is defined as the tail-flick latency. The baseline was to 2–3 sec (pre-response time) and a cut off time was set at 8 sec to avoid external heat-related damage. The time sequence was the same as described for the HPL test47, 48
Statistical Analysis
Statistical significance of the data was estimated by one-way analysis of variance (ANOVA) followed by Dunnett's test using the computer software program JMP (SAS Institute Inc, Cary, NC) and considered significant at p < 0.05. Minimum Effective Dose (MED) is the minimum dose of compound showing statistically significant antinociceptive effect expressed as the area under the time-response curve (AUC) value compared to a saline-treated group. The AUC was obtained by plotting the response time (sec) on the ordinate and time (min) on the abscissa after administration of the compounds. The percent maximum possible effect (% MPE) was calculated as follows: ([post-drug response latency — pre-drug response latency]/[cutoff time (30 s) — pre-drug response latency]) x100.
Acknowledgments
This study was supported in part by the University of Cagliari (GB), the University of Ferrara (SS) and in part by the Intramural Research Program of the NIH and NIEHS (LHL and EDM).
Abbreviations
In addition to the IUPAC-IUB Commission on Biochemical Nomenclature (J. Biol. Chem. 1985, 260, 1 4–42), this paper uses the following additional symbols and abbreviations:
- AcOEt
ethyl acetate
- AcOH
acetic acid
- Bid
1H-benzimidazole-2-yl
- Boc
tert-butyloxycarbonyl
- DAMGO
[D-Ala2,N-Me-Phe4,Gly-ol5]enkephalin
- DEL C
deltorphin II (H-Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2)
- DMF
N,N-dimethylformamide
- DMSO-d6
hexadeuteriodimethyl sulfoxide
- Dmt
2′6′-dimethyl-L-tyrosine
- DPDPE
(D-Pen2,D-Pen5)-enkephalin
- endomorphin-2
H-Tyr-Pro-Phe-Phe-NH2
- Et2O
diethyl ether
- GPI
guinea-pig ileum
- HOBt
1-hydroxybenzotriazole
- HPLC
high performance liquid chromatography
- MALDI-TOF
matrix assisted laser desorption ionization time-of-flight
- MVD
mouse vas deferens
- NMM
4-methylmorpholine
- NLX
naloxone
- NTI
naltrindole
- pA2
negative log of the molar concentration required to double the agonist concentration to achieve the original response
- Pe
petroleum ether
- PL017
H-Tyr-Pro-(N-Me)Phe-D-Pro-NH2
- TEA
triethylamine
- TFA
trifluoroacetic acid
- Tic
1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
- TLC
thin-layer chromatography
- WSC
1-ethyl-3-[3′-dimethyl)aminopropyl]-carbodiimide hydrochloride
- Z
benzyloxycarbonyl
Footnotes
Supporting Information Available: Chemistry general methods, enzymatic degradation general methods, elemental analysis, MS and HPLC data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Salvadori S, Attila M, Balboni G, Bianchi C, Bryant SD, Crescenzi O, Guerrini R, Picone D, Tancredi T, Temussi PA, Lazarus LH. δ opioidmimetic antagonists: prototypes for designing a new generation of ultraselective opioid peptides. Mol. Med. 1995;1:678–689. [PMC free article] [PubMed] [Google Scholar]
- (2).Temussi PA, Salvadori S, Amodeo P, Bianchi C, Guerrini R, Tomatis R, Lazarus LH, Tancredi T. Selective opioid dipeptides. Biochem. Biophys. Res. Commun. 1994;198:933–939. doi: 10.1006/bbrc.1994.1133. [DOI] [PubMed] [Google Scholar]
- (3).Schiller PW, Nguyen TM-D, Weltrowska G, Wilkes BC, Marsden BJ, Lemieux C, Chung NN. Differential stereochemical requirements of μ vs δ opioid receptors for ligand binding and signal transduction: development of a class of potent and highly δ-selective peptide antagonists. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11871–11875. doi: 10.1073/pnas.89.24.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Salvadori S, Balboni G, Guerrini R, Tomatis R, Bianchi C, Bryant SD, Cooper PS, Lazarus LH. Evolution of the Dmt-Tic pharmacophore: N-terminal methylated derivatives with extraordinary δ opioid antagonist activity. J. Med. Chem. 1997;40:3100–3108. doi: 10.1021/jm9607663. [DOI] [PubMed] [Google Scholar]
- (5).Balboni G, Guerrini R, Salvadori S, Bianchi C, Rizzi D, Bryant SD, Lazarus LH. Evaluation of the Dmt-Tic pharmacophore: conversion of a potent δ-opioid receptor antagonist into a potent δ agonist and ligands with mixed properties. J. Med. Chem. 2002;45:713–720. doi: 10.1021/jm010449i. [DOI] [PubMed] [Google Scholar]
- (6).Balboni G, Onnis V, Congiu C, Zotti M, Sasaki Y, Ambo A, Bryant SD, Jinsmaa Y, Lazarus LH, Trapella C, Salvadori S. Effect of lysine at C-terminus of the Dmt-Tic opioid pharmacophore. J. Med. Chem. 2006;49:5610–5617. doi: 10.1021/jm060741w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tryoen-Tóth P, Décaillot FM, Filliol D, Befort K, Lazarus LH, Schiller PW, Schmidhammer H, Kieffer BL. Inverse agonism and neutral antagonism at wild-type and constitutively active mutant delta opioid receptors. J. Pharmacol. Exp. Ther. 2005;313:410–421. doi: 10.1124/jpet.104.077321. [DOI] [PubMed] [Google Scholar]
- (8).Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J. Med. Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- (9).Aguila B, Coulbault M, Léveillé F, Davis A, Borsodi A, Balboni G, Salvadori S, Jauzac P, Allouche S. In vitro and in vivo pharmacological profile of UFP-512, a novel selective delta-opioid receptor agonist; correlations between desensitization and tolerance. Br. J. Pharmacol. 2007;152:1312–1324. doi: 10.1038/sj.bjp.0707497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus l. H., Regoli D, Guerrini R, Salvadori S, Caló G. Anxiolytic and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides. 2008;29:93–103. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- (11).Ryu EK, Wu Z, Chen K, Lazarus LH, Marczak ED, Sasaki Y, Ambo A, Salvadori S, Ren C, Zhao H, Balboni G, Chen X. Synthesis of a potent and selective 18F-labeled δ-opioid receptor antagonist derived from the Dmt-Tic pharmacophore for positron emission tomograpgy imaging. J. Med. Chem. 2008;51:1817–1823. doi: 10.1021/jm7014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Snook LA, Milligan G, Kieffer BL, Massotte D. μ-δ opioid receptor functional interaction: insight using receptor-G protein fusions. J. Pharmacol. Exp. Ther. 2006;318:683–690. doi: 10.1124/jpet.106.101220. [DOI] [PubMed] [Google Scholar]
- (13).Snook LA, Milligan G, Kieffer BL, Massotte D. Co-expression of mu and delta opioid receptors as receptor-G protein fusion enhances both mu and delta signalling via distinct mechanisms. J. Neurochem. 2008;105:865–873. doi: 10.1111/j.1471-4159.2008.05215.x. [DOI] [PubMed] [Google Scholar]
- (14).Salvadori S, Trapella C, Fiorini S, Negri L, Lattanzi R, Bryant SD, Jinsmaa Y, Lazarus LH, Balboni G. A new opioid designed multiple ligand derived from the μ opioid agonist endomorphin-2 and the δ opioid antagonist pharmacophore Dmt-Tic. Bioorg. Med. Chem. 2007;15:6876–6881. doi: 10.1016/j.bmc.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lee YS, Petrov R, Park CK, Ma SW, Davis P, Lai J, Porreca F, Vardanyan R, Hruby VJ. Development of novel enkephalin analogues that have enhanced opioid activities at both μ and δ opioid receptors. J. Med. Chem. 2007;50:5528–5532. doi: 10.1021/jm061465o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dai X, Cui SG, Li SR, Chen Q, Wang R. Melatonin attenuates the development of antinociceptive tolerance to delta-, but not to mu-opioid receptor agonist in mice. Behav. Brain Res. 2007;182:21–27. doi: 10.1016/j.bbr.2007.04.018. [DOI] [PubMed] [Google Scholar]
- (17).Jutkiewicz EM. The antidepressant-like effects of delta-opioid receptor agonists. Mol. Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- (18).Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki TR. Role of δ-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem. 2006;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- (19).Hill MP, Hille CJ, Brotchie JM. Delta-opioid receptor agonists as a therapeutic approach in Parkinson's disease. Drug News Perspect. 2000;13:261–268. [PubMed] [Google Scholar]
- (20).Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival-unravelling mechanism and revealing new indications. Pharmacol. Rev. 2004;23:351–366. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- (21).Watson MJ, Holt JD, O'Neill SJ, Wei K, Pendergast W, Gross GJ, Gengo PJ, Chang KJ. ARD-353 [4-((2R,5S)-4-(R)-(4-diethylcarbamoxylphenyl)(3-hydroxyphenyl)methyl-2,5dimethylpiperazin-1-ylmethyl)benzoic acid], a novel nonpeptide δ receptor agonist, reduces myocardial infarct size without central effects. J. Pharmacol. Exp. Ther. 2006;316:423–430. doi: 10.1124/jpet.105.092742. [DOI] [PubMed] [Google Scholar]
- (22).Pol O, Palacio JR, Puig MM. The expression of δ-and κ-opioid receptors is enhanced during intestinal inflammation in mice. J. Pharmacol. Exp. Ther. 2003;306:455–462. doi: 10.1124/jpet.103.049346. [DOI] [PubMed] [Google Scholar]
- (23).Stein C, Schäfer M, Machelska H. Attaching pain at its source: new perspectives on opioids. Nat. Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- (24).Balboni G, Guerrini R, Salvadori S, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Conversion of the potent δ-opioid agonist H-Dmt-Tic-NH-CH2-Bid into δ-opioid antagonists by N1-benzimidazole alkylation. J. Med. Chem. 2005;48:8112–8114. doi: 10.1021/jm058259l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Salvadori S, Fiorini S, Trapella C, Porreca F, Davis P, Sasaki Y, Ambo A, Marczak ED, Lazarus LH, Balboni G. Role of benzimidazole (Bid) in the δ-opioid agonist pseudopeptide H-Dmt-Tic-NH-CH2-Bid (UFP-502) Bioorg. Med. Chem. 2008;16:3032–3038. doi: 10.1016/j.bmc.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–181. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Vergura R, Valenti E, Hebbes CP, Gavioli EC, Spagnolo B, McDonald J, Lambert DG, Balboni G, Salvadori S, Regoli D, Calò G. Dmt-Tic-NH-CH2-Bid (UFP-502), a potent DOP receptor agonist: in vitro and in vivo studies. Peptides. 2006;27:3322–3330. doi: 10.1016/j.peptides.2006.07.015. [DOI] [PubMed] [Google Scholar]
- (28).Nestor JJ, Jr., Horner BL, Ho TL, Jones GH, McRae G, Vickery BH. Synthesis of a novel class of heteroaromatic amino acids and their use in the preparation of analogues of Luteinizing Hormone-Releasing Hormone. J. Med. Chem. 1984;27:320–325. doi: 10.1021/jm00369a016. [DOI] [PubMed] [Google Scholar]
- (29).Lee YS, Nyberg J, Moye S, Agnes RS, Davis P, Ma SW, Lai J, Porreca F, Vardanyan R, Hruby VJ. Understanding the structural requirements of 4-anilinopiperidine analogues for biological activities at μ and δ opioid receptors. Bioorg. Med. Chem. Lett. 2007;17:2161–2165. doi: 10.1016/j.bmcl.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Codd EE, Carson JR, Colburn RW, Dax SL, Desai-Krieger D, Martinez RP, McKown LA, Neilson LA, Pitis PM, Stahle PL, Stone DJ, Streeter AJ, Wu WN, Zhang SP. The novel, orally active, delta opioid RWJ-394674 is biotransformed to the potent mu opioid RWJ-413216. J. Pharmacol. Exp. Ther. 2006;318:1273–1279. doi: 10.1124/jpet.106.104208. [DOI] [PubMed] [Google Scholar]
- (31).Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic delta opioid agonists in rats: comparison with pentylenetetrazol. J. Pharmacol. Exp. Ther. 2006;317:1337–1348. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- (32).Danielsson I, Gasior M, Stevenson GW, Folk JE, Rice KC, Negus SS. Electroencephalographic and convulsant effects of the delta opioid agonist SNC80 in rhesus monkeys. Pharmacol. Biochem. Behav. 2006;85:428–434. doi: 10.1016/j.pbb.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wentland MP, Sun X, Cohen DJ, Bidlack JM. Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. Part 6: opioid receptor binding properties of cyclic variants of 8-carboxamidocyclazocine. Bioorg. Med. Chem. 2008;16:5653–5664. doi: 10.1016/j.bmc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kosson P, Bonney I, Carr DB, Lipkowski AW. Endomorphins interact with tachykinin receptors. Peptides. 2005;26:1667–1669. doi: 10.1016/j.peptides.2005.02.006. [DOI] [PubMed] [Google Scholar]
- (35).Pello OM, Martínez-MuŇoz L, Parrillas V, Serrano A, Rodrígues-Frude JM, Toro MJ, Lucas P, Monterrubio M, Martínez-A C, Mellado M. Ligand stabilization of CXCR4/δ-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur. J. Immunol. 2008;38:537–549. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- (36).Koda Y, Del Borgo M, Wessling ST, Lazarus LH, Okada Y, Toth I, Blanchfield JT. Synthesis and in vitro evaluation of a library of modified endomorphin 1 peptides. Bioorg. Med. Chem. 2008;16:6286–6296. doi: 10.1016/j.bmc.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lesscher HM, Bailey A, Burbach JP, Van Ree JM, Kitchen I, Gerrits MA. Receptor-selective changes in mu-, delta- and kappa-opioid receptors after chronic naltrexone treatment in mice. Eur. J. Neurosci. 2003;17:1006–1012. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- (38).Cahill CM, Holdridge SV, Morinville A. Trafficking of δ-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol. Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- (39).Archer—Lahlou E, Audet N, Amraei MG, Huard K, Paquin-Gobeil M, Pineyro G. Src promotes delta opioid receptor (DOR) desensitization by interfering with receptor recycling. J. Cell. Mol. Med. 2008 doi: 10.1111/j.1582-4934.2008.00308.x. in press. doi:10.1111/j.1582-4934.2008.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Lazarus LH, Salvadori S, Santagada V, Tomatis R, Wilson WE. Function of negative charge in the “address domain” of deltorphins. J. Med. Chem. 1991;34:1350–1355. doi: 10.1021/jm00108a017. [DOI] [PubMed] [Google Scholar]
- (41).Lazarus LH, Salvadori S, Attila M, Grieco P, Bundy DM, Wilson WE, Tomatis R. Interaction of deltorphin with opioid receptors: Molecular determinants for affinity and selectivity. Peptides. 1993;14:21–28. doi: 10.1016/0196-9781(93)90006-3. [DOI] [PubMed] [Google Scholar]
- (42).Lazarus LH, Wilson WE, de Castglione R, Guglietta A. Dermorphin gene sequence peptide with high affinity and selectivity for δ-opioid receptors. J. Biol. Chem. 1989;264:3047–3050. [PubMed] [Google Scholar]
- (43).Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which cause 50 per cent (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- (44).Sasaki Y, Sasaki A, Niizuma H, Goto H, Ambo A. Endomorphin 2 analogues containing Dmp residue as an aromatic amino acid surrogate with high μ-opioid receptor affinity and selectivity. Bioorg. Med. Chem. 2003;11:675–678. doi: 10.1016/s0968-0896(02)00601-6. [DOI] [PubMed] [Google Scholar]
- (45).Arunlakshana Q, Schild HO. Some quantitative uses of drug antagonists. Br. J. Pharmcol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J. Pharmacol. Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- (47).Jinsmaa Y, Marczak ED, Fujita Y, Shiotani K, Miyazaki A, Li T, Tsuda Y, Ambo A, Sasaki Y, Bryant SD, Okada Y, Lazarus LH. Potent in vivo antinociception and opioid receptor preference of the novel analogue [Dmt1]endomorphin-1. Pharmacol. Biochem. Behav. 2006;84:252–258. doi: 10.1016/j.pbb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- (48).Marczak ED, Jinsmaa Y, Li T, Bryant SD, Tsuda Y, Okada Y, Lazarus LH. [N-Allyl-Dmt1]-Endomorphins are μ-opioid receptor antagonists lacking inverse agonist properties. J Pharmacol Exp Ther. 2007;323:374–380. doi: 10.1124/jpet.107.125807. [DOI] [PubMed] [Google Scholar]
- (49).Kosterlitz HW, Watt AJ. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone) Br. J. Pharmacol. 1968;33:266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]