Abstract
Background & Aims
Chronic psychological stress is associated with visceral hyperalgesia and increased expression of spinal NK1 receptors (NK1R). We aimed to identify the role of spinal microglia in this process.
Methods
Male Wistar rats were exposed to water avoidance (WA) or sham stress 1 hour each day for 10 days and given daily injections of minocycline, the p38 inhibitor SB203580, or saline. Phosphorylation levels of the kinase p38 (P-p38), the microglia marker OX42, NK1R, and IκBα were assessed by immunoblotting and/or immunostaining of spinal samples collected at Day 11. The visceromotor response (VMR) to colorectal distension at baseline and following WA were also assayed in rats given injections of minocycline, SB203580 or vehicle. The effects of fractalkine were assessed on the VMR in rats exposed to minocycline or vehicle.
Results
P-p38 protein levels and immunoreactivity were increased in stressed rats and co-localized with OX42-positive cells and neurons in the dorsal horn. This increase was reversed by minocycline or SB203580 exposure. Stress-induced increased NK1R expression was blocked by minocycline but not SB203580. WA-induced decreased IκBα expression was blocked by minocycline and SB203580. WA-induced hyperalgesia was blocked by minocycline and SB203580 IT. Fractalkine-induced hyperalgesia was blocked by minocycline.
Conclusions
This is the first demonstration that stress-induced activation of spinal microglia has a key role in visceral hyperalgesia and associated spinal NK1R upregulation.
Introduction
Glia in the central nervous system (CNS) refer to several distinct non-neuronal cell types including microglia, oligodendrocytes and astrocytes that carry out homeostatic surveillance, acting as exquisite sensors of changes in their microenvironment.1 Specifically, microglia play a key role in the initiation or maintenance of hyperalgesia and allodynia as demonstrated in animal models of chronic pain associated with peripheral inflammation or nerve injury.2 Upon stimulation, microglia activity shifts from a “resting” to a “reactive” state, acquiring functional phenotypes constituted by transcriptional profiles and non-transcriptional changes, leading to the release of cytokines and chemokines.3 Though, the mechanisms triggering microglia activation and the downstream pathways leading to amplification of the nociceptive signaling are only partially understood. Microglia express a multitude of receptors for neurotransmitters and may be activated by mediators including excitatory amino acids or prostaglandins, some of which may be released from sensory nerve terminals or post-synaptic neurons in the dorsal horn of the spinal cord. Substances produced and released from activated microglia include a range of proinflammatory mediators such as cytokines (IL-1β, IL-6, TNF-α), chemokines, NO, prostaglandins, all of which can act on afferent nerve terminals and spinal sensory neurons and increase neuronal excitability.4
Recent reports have demonstrated microglia responsiveness to environmental stressors in the absence of tissue injury. Microglia activation in different regions of the brain, including the hippocampus, has been described in response to restraint stress in rats or chronic restraint stress in mice.5, 6 Stress was also found to induce sensitization of microglia immune reactivity. Microglia can be “primed” by stress and exhibit phenotypic changes that may lead to potentiation of the CNS immune response to further peripheral stimulation or immune/proinflammatory challenge.7 To our knowledge, the stress responsiveness of microglia in the spinal cord has not been studied yet. This response may have important implication for pain disorders with enhanced stress sensitivity, such as fibromyalgia, interstitial cystitis, or irritable bowel syndrome.8-10 Only one study reported a potential role of spinal microglia activation in visceral pain associated with neonatal colonic irritation in rats.11
In the present study, we were particularly interested in the effect of chronic stress on spinal microglia activation and whether it contributes to the development of visceral hyperalgesia in a rat model. To date, the role of microglia in models of functional chronic pain (pain not associated with injury) and visceral pain amplification has not been investigated. We have previously shown that repeated WA stress in male Wistar rats results in sustained visceral hyperalgesia in the absence of colonic inflammatory changes.12 However, an increased expression of spinal NK1R was observed in this model, and the visceral hyperalgesia was abolished by a NK1R antagonist.13 Based on these considerations, we examined whether chronic WA stress in rats leads to spinal microglia activation and whether it contributes to stress-induced visceral hyperalgesia and the modulation of spinal NK1Rs. In addition, we verified the concept that spinal microglia activation has a modulatory influence on visceral nociception by testing the effect of an exogenous microglia activator on the visceral nociceptive response.
Materials & Methods
Animals
The study used adult male Wistar rats (250-275 g) (Harlan, IN) maintained on a normal light-dark cycle, housed in pairs or singly when equipped with a chronic intrathecal catheter, and provided with food and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee at the VA Greater Los Angeles Healthcare System.
Water avoidance (WA) stress protocol
Rats are placed on a block (8 × 8 × 10 cm) affixed to the center of a Plexiglas® cage (25 × 25 × 45 cm) filled with fresh room temperature water (25 °C) to within 1 cm of the top of the block (WA), or kept empty (sham WA) for 1 hour daily, for 10 consecutive days, as described in previous reports.12
Surgical implantation of chronic intrathecal catheter and osmotic mini-pump
Rats were anesthetized with pentobarbital sodium (45 mg/kg, IP), and a 8.5 cm PE5 tube (polyethylene tubing-5, OD 0.14′, ID 0.006′, Spectranetics, Colorado Springs, CO, USA), connected to a 4 cm PE10 was inserted through a small puncture in the atlanto-occipital membrane of the cisterna magna such that the caudal tip reached the lumbar enlargement of the spinal cord. The tip of the PE10 tubing was connected to an osmotic mini-pump (volume 200 μl, 0.5 μL/hr, model 2002, Alzet, Cupertino, CA, USA) positioned subcutaneously in a small pocket between the scapulas. Catheters were primed with vehicle/drugs before implantation. When possible, the catheter position was verified in each animal by postmortem examination of the spinal cord.
Implantation of electromyographic (EMG) electrodes and assessment of visceromotor response (VMR) to colorectal distension (CRD)
Rats were anesthetized with pentobarbital sodium (45 mg/kg, IP). Electrodes (Teflon-coated stainless steel wire, AstraZeneca, Mölndal, Sweden) were stitched into the external oblique musculature, for EMG recordings as previously described.14 The visceral stimulus employed was distension of the descending colon and rectum using a well established and validated method.14
Western Blotting
Animals were anesthetized with 4% isoflurane and decapitated. The spinal cords were hydroextruded with 5 ml of iced saline and the lumbar L6S1 spinal cord segment was dissected, snap frozen and stored in −70 °C until processed for Western blotting as previously described.15 Antibodies used were: OX42, P-p38, total p38, NK1R, IκBα. The intensity of immunoreactive bands was quantified using Image Quant software (Molecular Dynamics, Sunnyvale, CA) and normalized relative to β-actin.
Immunohistochemistry (IHC)
Animals were anesthetized with sodium pentobarbital (50 mg/kg, IP) and perfused transcardially with 0.9% heparinized saline followed by 4% paraformaldehyde. Spinal cords were removed, fixed and immunostained for phosphorylated p38 (P-p38), OX42, or NeuN, as described previously.15 Images were captured using a fluorescent microscope (Nikon, Melville, NY, USA) and a confocal microscopy system (Biorad, Hemel Hemstead, UK) operated by Biorad Lasersharp 2000 software.
Drugs
Endotoxin-free rat recombinant CX3CL1/fractalkine, chemokine Domain, aa25-100 (RD system, Minneapolis, MN, USA) or vehicle (1×PBS, 0.1 mM, pH 7.2) was injected IT (25 ng or 40 ng, in 5 μL followed by 10 μL vehicle). The MAPK p38 inhibitor SB203580 hydrochloride (Calbiochem, Gibbstown, NJ, USA) or vehicle (0.9% saline) was delivered IT via an osmotic pump connected to the chronically implanted IT catheter. It was delivered at the rate of 4 μg/day with a flow rate of 0.5 μl/hour. Minocycline hydrochloride, a potent inhibitor of microglia activation16 (Sigma, St. Louis, MO, USA) or vehicle (0.9% saline) was injected either acutely via the IT catheter (100 μg/rat in 10 μL followed by 10 μL vehicle) or delivered chronically via a subcutaneously implanted osmotic pump connected to the IT catheter at the rate of 120 μg/day, 0.5 μl/hour.
More details are available in supplemental materials referring to the recording of VMR to CRD, Western Blotting, immunohistochemistry and drugs, including concentration of antibodies and justification of drugs doses.
Experimental design
Experiment 1: Evidence of spinal microglia activation after chronic WA stress: immunohistochemistry and Western Blotting
1- Groups of rats (n=4 in each group) were exposed to chronic WA stress or sham WA. On day 11, rats were sacrificed and samples of lumbar spinal cord (L6S1) were collected and processed for immhunohistochemistry (IHC) for P-p38, OX42, or the neuronal marker NeuN. Some sections were double-labeled for P-p38/OX42 or P-p38 and NeuN.
2 - Between day 1 and day 10, 4 groups of 4 animals each were exposed to WA stress or sham WA and received continuous spinal infusion of minocycline or vehicle via osmotic pump connected to an IT catheter (120 μg/day, 0.5 μL/hour from day 1 to day 10). Twenty-four hours after the end of the last WA or sham WA session (day 11), rats were sacrificed and samples of lumbar spinal cords (L6S1) were collected and processed for Western Blotting for P-p38 and OX42.
Experiment 2: Effect of minocycline and SB203580 on stress-induced up-regulation of spinal NK1R and NFκB activation
Groups of 4 rats were implanted with chronic IT catheters connected to osmotic pumps and were exposed to daily WA or sham WA. Four groups received treatments with either SB203580 or vehicle (4 μg/day, 0.5 μl/hour) from day 1 to day 10. Samples of lumbar spinal cord (L6S1) were collected at day 11 and processed for Western Blotting for NK1R and IκBα. We measured IκBα as an index of NFκB activation as described in prior reports.17 Similarly, we assessed NK1R and IκBα expression in samples from rats treated with minocycline IT or vehicle (120 μg/day, 0.5 μL/hour from day 1 to day 10).
Experiment 3: Effect of minocycline treatment on chronic WA stress-induced visceral hyperalgesia
Eight groups of 8 rats implanted with EMG electrodes were exposed to chronic WA or sham WA. Four groups received a daily IP injection of minocycline (20 mg/kg, 1 ml/kg) or vehicle, one-hour prior to WA or sham WA from day 1 to day 10. Four other groups of rats were equipped with chronic intrathecal catheters connected to subcutaneous osmotic pumps continuously delivering either minocycline (120 μg/day, 0.5 μL/hour) or vehicle from day 1 to day 10. In both series of experiments, we performed a baseline recording of the VMR to CRD before beginning the treatment (day 0) and then again, 24 hours after the last WA or sham WA sessions (day 11).
Experiment 4: Effect of the MAPK p38 inhibitor SB203580 on chronic WA stress-induced visceral hyperalgesia
Groups of 8 rats were surgically implanted with EMG electrodes and IT catheters connected to subcutaneous osmotic pumps. Animals were exposed to daily 1-hour sessions of WA or sham WA and received treatments with either SB203580 (4 μg/day, 0.5 μl/hour) or vehicle, from day 1 to day 10. Before the beginning of treatments, animals were recorded for baseline response to CRD and then again 24 hours after the end of the stress protocol at day 11.
Experiment 5: Effect of the microglia activating factor fractalkine on visceral sensitivity in control non-stressed rats
Four groups of 8 rats were surgically implanted with EMG electrodes and IT catheters externalized for single injection. We first measured a baseline response to CRD. Then rats were injected with either minocycline or vehicle (100 μg/rat in 10 μL IT single injection). Injections were performed in animals placed in the restraining device used for the CRD protocol. One hour later, rats were injected IT with either fractalkine or vehicle (4 μg/rat in 5 μL) and the VMR to CRD was recorded 30 minutes later.
Data presentation and statistical analysis
The effect of stress and treatments on proteins expression and EMG responses were analyzed using one- and two-way ANOVA for repeated measures followed by Bonferroni post-test comparisons, respectively. This method of analysis has been previously validated in a similar model of EMG measurement in response to CRD.12 The numbers of rats from which data were effectively collected are indicated in the figure legends.
RESULTS
Experiment 1: Evidence of spinal microglia activation after chronic WA stress: qualitative immunohistochemistry and Western Blotting
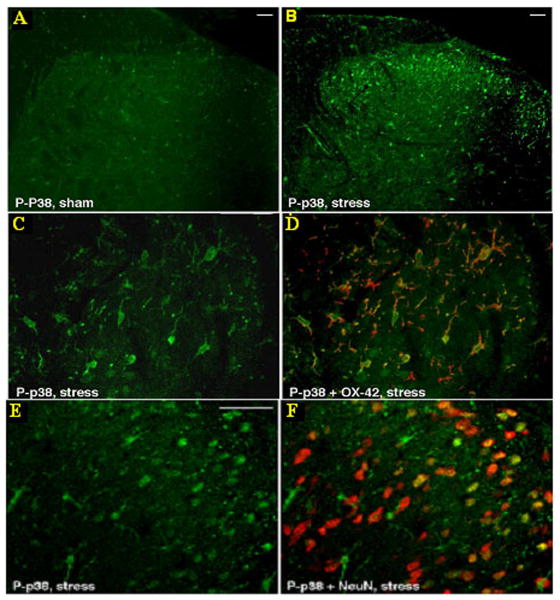
Based on prior studies showing that functional microglia activation can be assessed by measuring p38 phosphorylation,18 we performed IHC for P-p38 in sections of spinal cord (L6S1) from WA and sham WA rats. As shown in figure 1, although basal activation of p38 was evident in sham spinal cords (figure 1A), IHC revealed greater P-p38 staining in the spinal dorsal horn from stressed rats compared with controls (figure 1B). P-p38 positive cells were mainly localized in the superficial layers of the spinal cord dorsal horn with fewer P-p38 positive cells located in the deep dorsal horn. Immunoreactivity for P-p38 co-localized with OX42 positive cells (microglia) (figure 1C-D) as well as with NeuN positive cells (neurons) in laminae I and II in spinal sections from stressed rats (figure 1E-F).
Figure 1. Representative micrograph of immunostaining for P-p38 alone or co-stained with OX42 and NeuN on L6S1 dorsal spinal cord sections.
Spinal P-p38 staining is increased in animals exposed to WA stress (B) compared with control (A) (bar=20 μm). In samples from stressed rats, P-p38 staining is found to co-localize with OX42 positive cells (D) as well as NeuN positive cells (F) (bar=50 μm in C, D, E, F).
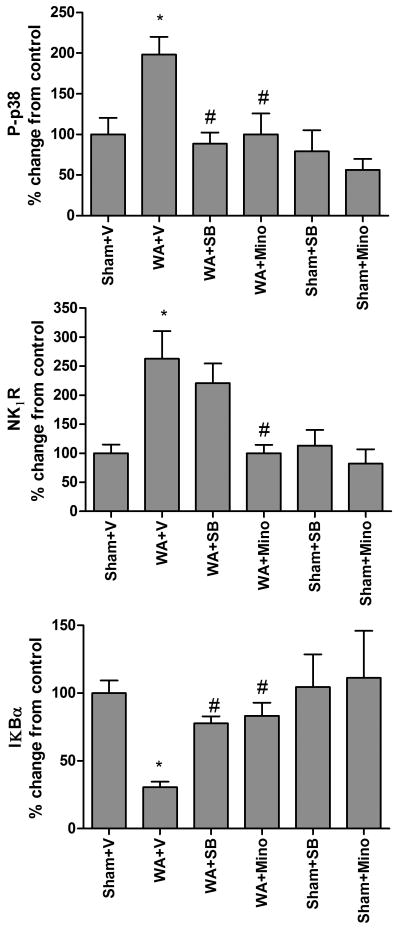
Western Blotting revealed increased levels of P-p38 in WA rats compared with control. This increase was inhibited by treatment with minocycline IT or with SB203580 IT (figure 2). There was no effect of minocycline or SB203580 on the level of P-p38 in sham WA rats compared with vehicle treatment. The expression of OX42, quantified by Western Blotting, was not different in WA rats compared with controls (data not shown).
Figure 2. Effect of chronic treatment with minocycline (120μg/day, IT) and the p38 inhibitor SB 203580 (4 μg/day, IT) on stress-induced regulation of spinal P-p38, NK1Rs, and IKBα: proteins levels assessed by Western Blot analysis.
P-p38: Greater level of P-p38 is observed in chronic WA compared with sham WA. This effect is blocked by both minocycline and SB 203580. NK1R: Chronic WA-induced increased levels of spinal NK1R is blocked by minocycline, but is not affected by SB203580. IKBα: Chronic WA induces increased level of spinal IKBα. Both minocycline and SB203580 blocked this effect. Treatments had no effect in sham WA. n=4 in each group. Data are Mean ± SEM expressed as % control, * (compared to sham WA + vehicle) or # (compared to WA + vehicle) P<0.05, One way ANOVA followed by Bonferroni post-test.
Experiment 2: Effect of minocycline and SB203580 on stress-induced up-regulation of spinal NK1R and NFκB activation
Western blot showed increased expression of NK1R in rats exposed to stress compared with controls. This increase was completely blocked by minocycline IT. In contrast, the p38 inhibitor SB203580 failed to block the NK1R increase. Reduced levels of IκBα were observed in WA+Vehicle compared with sham WA+Vehicle. This decrease was partially blocked by minocycline IT and the p38 inhibitor SB203580. Neither minocycline, nor SB203580 affected the levels of NK1R or IκBα in sham WA rats (figure 2).
Experiment 3: Functional role of stress-induced spinal microglia activation in the development of visceral hyperalgesia
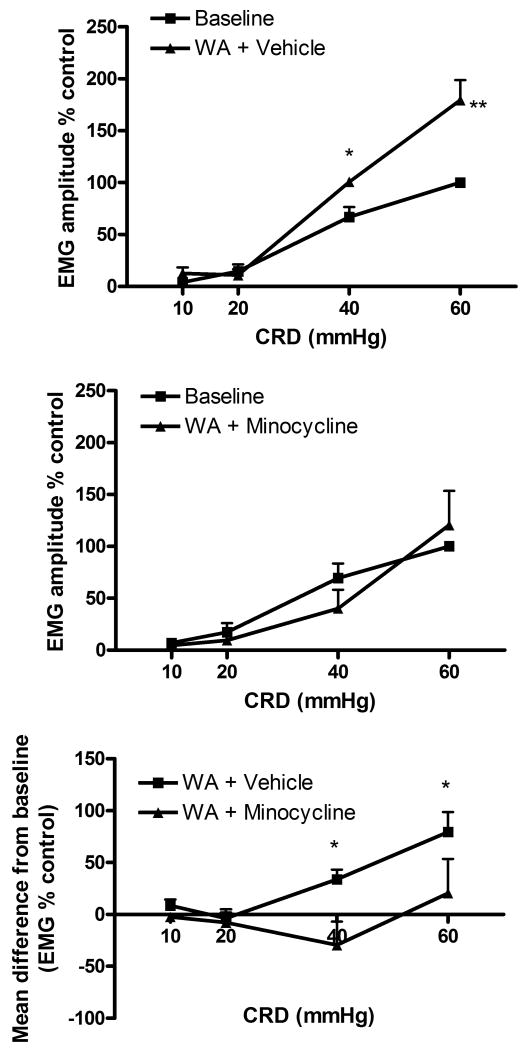
Stressed rats treated with vehicle showed significant increase of VMR to CRD at day 11 compared with baseline at 40 and 60 mmHg. These results are consistent with our previous report that chronic WA induces visceral hyperalgesia.12 In contrast, in rats exposed to WA stress and pre-treated daily with minocycline IP, the VMR to CRD observed at day 11 was not different from baseline. (Figure in supplemental material.) Rats exposed to WA and receiving spinal infusion of vehicle exhibited a significant increase of the VMR to CRD at day 11 compared with baseline. This stress-induced increase of VMR was blocked by spinal infusion of minocycline (figure 3). Neither IT or IP treatments with vehicle nor with minocycline affected the VMR to CRD in sham WA animals at day 11 compared with baseline (data not shown).
Figure 3. Effect of chronic treatment with minocycline (120 μg/day, IT) on stress-induced visceral hyperalgesia.
Stressed rats treated with vehicle showed increased EMG response to CRD compared with baseline (n=8) (top panel) whereas rats treated with minocycline failed to show increased EMG response at day 11 (n=7) (middle panel). *P<0.05 significantly different from baseline. The bottom panel illustrates the significant difference between the effect of minocycline and vehicle with *P<0.05 significantly different from vehicle. Data are Mean ± SEM. Two way ANOVA followed by Bonferroni post-test.
Experiment 4: Effect of the MAPK p38 inhibitor SB203580 on chronic WA stress-induced visceral hyperalgesia
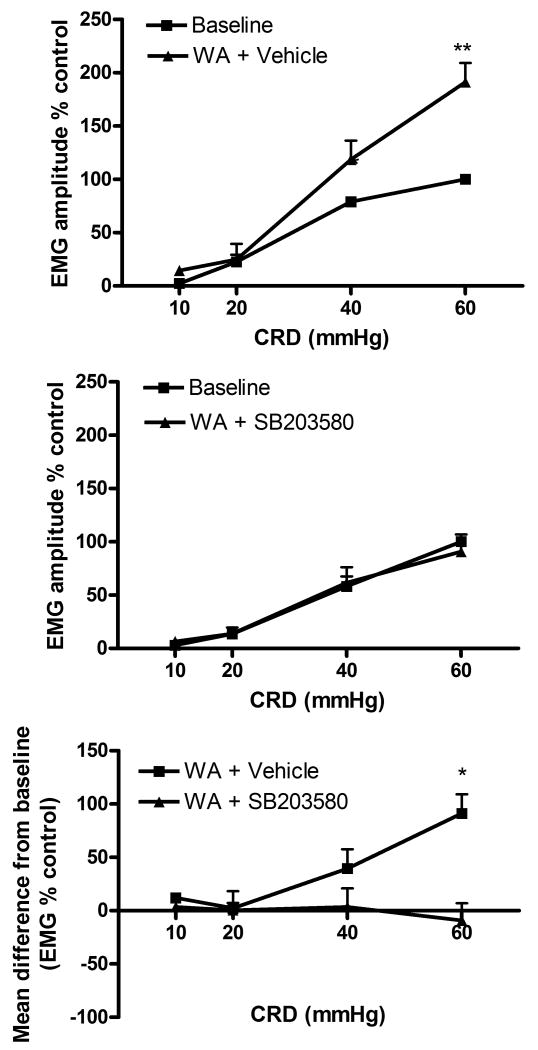
Similarly to the results seen in experiment 3, rats exposed to WA and receiving spinal infusion of vehicle exhibited a significant increase of VMR to CRD at day 11 from baseline. This effect was reduced in rats treated with spinal infusion of SB203580 (figure 4). The VMR to CRD in sham WA rats was not affected by treatments (data not shown).
Figure 4. Effect of chronic treatment with the p38 inhibitor SB203580 (4 μg/day/IT) on stress-induced visceral hyperalgesia.
Stressed rats treated with vehicle showed increased EMG response to CRD compared with baseline (n=4) (top panel) whereas rats treated with SB203580 showed no increase of the EMG response at day 11 (n=6) (middle panel). *P<0.05 significantly different from baseline. The bottom panel illustrates the significant difference between the effect of SB203580 and vehicle with *P<0.05 significantly different from vehicle. Data are Mean ± SEM. Two way ANOVA followed by Bonferroni post-test.
Experiment 5: Effect of the microglia-activating factor fractalkine on visceral sensitivity in control non-stressed rats
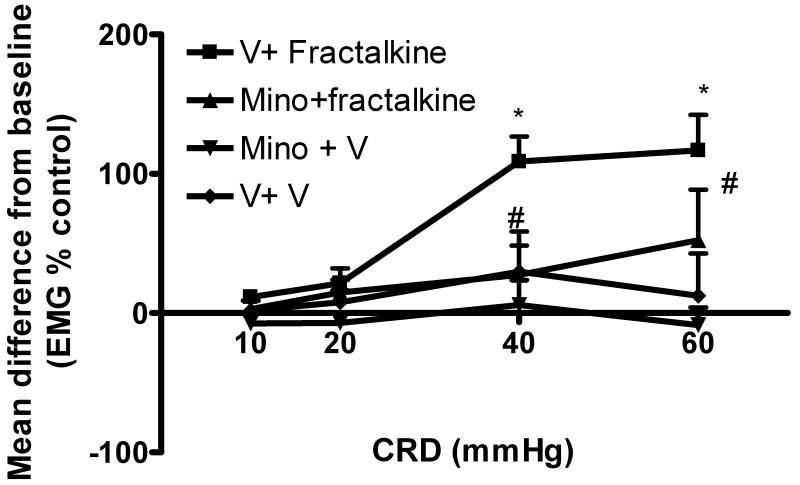
In order to further corroborate the involvement of microglia activation in the development of visceral hyperalgesia, we evaluated the effect of fractalkine on visceral response to CRD. In preliminary studies, we tested the effect of a single injection of fractalkine at the doses 25 or 40 ng/rat in 5 μL on VMR to CRD in rats. VMR to CRD was measured first at baseline and then 30 minutes after fractalkine injection. The VMR to CRD was significantly increased after injection of 40 ng/rat, but not after the lower dose (data not shown). In experiment 5, IT injection of 40 ng of fractalkine resulted in a large increase of the VMR to CRD compared to baseline. However, in rats treated with minocycline (100 μg/rat in 10 μL, IT) 1 hour prior to fractalkine (4 μg/rat in 5 μL IT), the increased response was significantly reduced. We observed no change from baseline in the VMR to CRD in rats treated with minocycline and vehicle or with vehicle only (figure 5).
Figure 5. Effect of the microglia activating factor fractalkine on visceral sensitivity in control non-stressed rats.
Fractalkine (40 ng/rat, IT) induces increased EMG response compared with baseline (n=7) while vehicle had no effect (n=4). This effect is prevented by prior treatment with minocycline IT (100 μg/rat, n=5). Minocycline alone had no effect (n=5). Data are Mean change from baseline± SEM, *P<0.05 significantly different from vehicle+vehicle, # P<0.05 significantly different from vehicle+ fractalkine, Two way ANOVA followed by Bonferroni post-test.
DISCUSSION
In the present study, we provide novel evidence that exposure to chronic WA stress leads to microglia activation in the lumbar spinal cord. We observed increased immunoreactivity for phosphorylated p38 MAPK (P-p38) in spinal cord sections from stressed rats and elevated spinal levels of P-p38 by Western Blot analysis. P-p38 immunostaining co-localized with the microglia marker OX42, but also with the neuronal marker NeuN, in line with previous reports showing that activation of spinal p38 is associated with microglia activation in models of inflammatory hyperalgesia.19 As the level of spinal OX42 protein was not increased in stressed rats, our findings suggest activation of resident microglia rather than recruitment or proliferation of microglia.
While functional microglia activation has been characterized by a variety of phenotypic transformations depending on the nature and intensity of the stimulus (ranging from morphological modifications20 to increased expression of cell-surface markers such as MHC-II or CD11b (OX42),21) activated microglia may display only a subset or variations of these phenotypes such as increased P-p38 in the absence of increased OX42. Our findings are in agreement with this notion that structural changes in microglia do not necessarly parallel activation of the p38 signaling pathway.19 The reduction of stress-induced increase of P-p38 by the microglia inhibitor minocycline22 further supports the hypothesis that microglia is a key source of increased spinal P-p38 after chronic stress.
Even though microglia activation by neonatal colon irritation has been reported,11 to our knowledge, we provide the first demonstration of microglia activation in the spinal cord in the absence of peripheral/nerve inflammation or injury. These findings are in agreement with emerging evidence suggesting that stress may affect immune responses within the CNS, and that, under certain conditions, stress can potentiate inflammatory responses to subsequent peripheral immune stimulation.7 Thus, increased brain level of IL-1β has been described after inescapable tail shock,5 social isolation,23 or immobilization stress 6 in rats. The role of microglia as a cellular effector in stress-induced CNS immune activation is further supported by recent findings showing that chronic restraint stress in mice induces brain microglia activation and proliferation24 and further studies showing that restraint stress in rats induces microglia activation in the hippocampus.7 The pattern of microglia activation in our model may be related to our experimental stressor and to the spinal site, as opposed to brain regions showing microglia proliferation in response to more severe stressors in the studies cited above.
Our finding that intrathecal or peripheral administration of minocycline prior to WA stress blocks visceral hyperalgesia confirms the important role of stress-induced microglia activation in the development of visceral hyperalgesia. These findings are consistent with a body of literature showing the preventive effect of systemic or intrathecal injection of minocycline on the development of hyperalgesia or allodynia in experimental models of exacerbated somatic pain following sciatic nerve inflammation,25 or peripheral inflammation.15 It also extends recent observations that minocycline blocks visceral hyperalgesia in a rat model of sensitization induced by neonatal colonic irritation.11 Although, the biochemical mechanism of action of minocycline is not clear, it has been reported that inhibition of p38 MAPK activation may play a key role.26 The lack of effect of minocycline on basal nociception strongly supports an important role of microglia in the facilitation of the processing of noxious input at the spinal level rather than in the transmission of normal nociceptive signals.
To date, while extensive studies have addressed the role of spinal microglia in somatic pain sensitization, the possible role of spinal microglia in the modulation of visceral nociception has been largely unexplored. The present data, together with the previous report from Saab et al 11 support the new notion that spinal microglia activation is a potential important factor contributing to the sensitization of visceral nociception in different rat models of visceral hyperalgesia. A modulatory influence exerted by spinal microglia on visceral nociception is further supported by our findings that spinal injection of the microglia activator fractalkine induces visceral hyperalgesia, also shown in Saab et al.11 These data corroborate previous work showing that spinal administration of fractalkine produces mechanical allodynia and thermal hyperalgesia,27 whereas blockage of spinal CX3CR1 attenuates neuropathic pain or prevent its development.27, 28 Together, these data further support the concept that transmission of visceral nociceptive signals may be enhanced in various conditions of spinal microglia activation.
Our finding that the p38 MAPK inhibitor SB203580 prevents stress-induced p38 phosphorylation as well as stress-induced visceral hyperalgesia further supports the concept that functional activation of spinal microglia is involved in visceral pain following chronic stress. Indeed, the large literature documenting that p38 MAPK inhibition reduces allodynia and hyperalgesia in several models of inflammatory and neuropathic pain points to an important role of this signaling pathway in pain processing.29 However, the mechanism(s) by which p38 MAPK activation occurs in our stress model and the downstream targets by which p38 may contribute to spinal sensitization are not well understood. Activation of transcription factors, such as protein kinases, post-transcriptional regulation by mRNA stabilization or activation or enhancement of the activity of other substrates, such as calcium-dependent phospholipase A, have all been described as potential pathways downstream to p38 phosphorylation and have been associated with the facilitation of pain-related signaling.30
Among the array of signaling pathways engaged by p38 activation, the production and secretion of cytokines and chemokines from activated microglia has been proposed to play a key role in spinal sensitization. Increased expression of TNFα, IL-1β, and IL-6 during peripheral inflammation or nerve injury31, 32 may activate dorsal horn neurons, an event associated with exaggerated pain states. It is known that p38 MAPK can regulate the synthesis of these molecules.33 In addition, the production of mediators such as NO or prostaglandins are, at least in part, p38-dependent.34 The release of such pro-inflammatory mediators, and their role in visceral hyperalgesia were not assessed in the present study but will be addressed in a follow up study. Cytokines may act at different levels in the modulation of nociception such as modulating transcriptional pathways leading to up-regulation of receptors or channels resulting in increased sensitivity to peripheral stimuli, or they may increase the strength of synaptic neurotransmission between primary and second-order neurons. For example, several studies have described a modulatory role of cytokines on sodium channels or transient receptor potential (TRP) channels35 as well as on the modulation of AMPA, NMDA, and NK1 receptors trafficking, and expression in the CNS and in isolated cell systems.36-39
We previously showed that chronic WA stress leads to increased expression of NK1R in dorsal horn neurons and this up-regulation plays an important role in visceral hyperalgesia.13 Similar results have been reported in models of peripheral inflammation associated with persistent pain.40 In the present study, we showed that stress-induced increased NK1R expression is blocked by minocycline. These findings, along with prior reports showing that IL-1β or IL-6 can affect NK1R gene expression,39 provide converging lines of evidence suggesting a role of cytokines released from activated microglia during chronic WA stress, in the increased expression of spinal NK1Rs on neurons. In a recent report, NFκB was found to be an important factor in IL-1β-mediated increase of the expression of NK1R in astrocytes41 and in isolated human macrophages.39 We found that the NF-κB inhibitor IκBα is decreased after chronic stress, suggesting that NFκB activation can be blocked by minocycline treatment. We hypothesize that increased NFκB availability occurs in part in spinal neurons, upon activation of cytokine neuronal receptors by cytokines released from activated microglia. In turn, the transcriptional unit of NFκB may act on the NK1R gene promoter to enhance its expression in neurons. We cannot exclude the possibility that NFκB may be activated in microglia as well, but this was not verified in our study. The effect of minocycline, blocking the effect of stress on IκBα, further supports the role of microglia mediators in the signaling pathways modulating NK1R expression. Surprisingly, we observed that treatment with the p38 inhibitor during chronic stress partially blocked the reduced level of IκBα but had no effect on NK1R expression. While we anticipated that inhibition of p38 activation, via blockade of mediators release from microglia, would block both NFκB activation and NK1Rs up-regulation, the lack of effect on NK1R over-expression may be related to parallel signaling pathways engaged in microglia activation during stress. For example, activation of ERK1/2 in microglia has been reported in rat models of chronic pain.42 In addition, ERK can activate the transcriptional activity of transcription factors such as p65 (which was found independent of p38 activity)43 or CREB.42 One may speculate that spinal NK1R expression may be under the control of microglia mediators released upon activation of other MAPKs. Although cytokines production and the role of ERK or JNK have not been explored in our model, their contribution to NK1R dependent or independent neuronal sensitization will be assessed in future studies. Finally, a partial inhibitory effect of SB203580 on neuronal p38 activation cannot be ruled out. Prior reports indicating that p38 may influence NFκB release and IκBα phosphorylation independently from its transcriptional activity (p38 does not phosphorylate p65),43 are in agreement with the effect observed in our study (a p38 inhibitor blocks the effect of stress on IκBα level but does not affect NK1R expression). Our findings that SB203580 blocks stress-induced visceral hyperalgesia, but does not prevent NK1R up-regulation challenges the generally accepted concept that in rodents, spinal NK1R play a major role in the mechanisms of visceral hyperalgesia, including stress-induced visceral hyperalgesia13 (demonstrated by us and others in various animal models as stated above). It is conceivable that multiple mechanisms contribute to the development and maintenance of stress-induced visceral hyperalgesia, including spinal microglia activation and upregulation of spinal NK1R.
In conclusion, the present study provides new evidence for a role of spinal microglia activation in the development of visceral hyperalgesia in a model of chronic psychological stress in rats associated with up-regulation of the spinal NK1R system. These findings open new perspectives in the understanding of the mechanisms underlying chronic pain conditions associated with enhanced stress responsiveness.
Acknowledgments
The authors would like to thank Ms. Jennifer Drader and Cathy Liu for their editorial assistance in preparing this manuscript.
Grant support: R21 DK071767 (SB), P50 DK64539 (EAM), R24 AT00281 (EAM), DK 48351 (EAM), RO1 NS16541 (TLY), R01 DA002110 (TLY),RO1 DK 47343 (CP), P01 DK 33506 (CP), and an Arthritis Foundation Fellowship (CIS)
Abbreviations
- CNS
central nervous system
- CRD
colorectal distension
- CSF
cerebrospinal fluid
- EMG
electromyographic
- IHC
immunohistochemistry
- IP
intraperitoneal
- IT
intrathecal
- MAPK
MAP kinase
- NK1R
Neurokinin 1 receptors
- NO
nitric oxide
- VMR
visceromotor response
- WA
water avoidance
Footnotes
Conflict of interest: Emeran A Mayer, Consultant: Prometheus, GSK, Danone, Nestle, Lilly, Takeda, Sanofi, Industry research support: Danone, GSK. Sylvie Bradesi: research support from GSK.
References
- 1.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 2.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–83. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14:166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 4.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–95. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. Journal of Neuroscience. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami M, Kuraishi Y, Yamaguchi T, Nakai S, Hirai Y, Satoh M. Immobilization stress induces interleukin-1 beta mRNA in the rat hypothalamus. Neuroscience Letters. 1991;123:254–256. doi: 10.1016/0304-3940(91)90944-o. [DOI] [PubMed] [Google Scholar]
- 7.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, Behavior. and Immunity. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Bradley LA. Pathophysiologic mechanisms of fibromyalgia and its related disorders. J Clin Psychiatry. 2008;69 2:6–13. [PubMed] [Google Scholar]
- 9.Bennett EJ, Piesse C, Palmer K, Badcock CA, Tennant CC, Kellow JE. Functional gastrointestinal disorders: Psychological, social, and somatic features. Gut. 1998;42:414–420. doi: 10.1136/gut.42.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theoharides TC. Treatment approaches for painful bladder syndrome/interstitial cystitis. Drugs. 2007;67:215–35. doi: 10.2165/00003495-200767020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Saab CY, Wang J, Gu C, Garner KN, Al-Chaer ED. Microglia: a newly discovered role in visceral hypersensitivity? Neuron Glia Biol. 2007;2:271–277. doi: 10.1017/S1740925X07000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: A new model for sustained visceral hyperalgesia. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC, Sternini C, Pothoulakis C, Mayer EA. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology. 2006;130:1729–1742. doi: 10.1053/j.gastro.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: Physiological and pharmacological characterization of pseudoaffective reflexes in the rat. Brain Research. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 15.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. European Journal of Neuroscience. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 16.Zemke D, Majid A. The potential of minocycline for neuroprotection in human neurologic disease. Clinical Neuropharmacology. 2004;27:293–298. doi: 10.1097/01.wnf.0000150867.98887.3e. [DOI] [PubMed] [Google Scholar]
- 17.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 18.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. Journal of Neurochemistry. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 19.Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38á isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. Journal of Neurochemistry. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 21.Streit WJ, Walater SA, Pennell NA. Reactive microgliosis. Progress in Neurobiology. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 22.Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167–71. doi: 10.1016/j.neulet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behavioural Brain Research. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 24.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. Journal of Neuroimmunology. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–23. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 28.Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–82. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 29.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs. 2004;5:71–5. [PubMed] [Google Scholar]
- 31.Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. Journal of Neuroscience. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 33.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003;14:1153–7. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- 35.Schafers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008 doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 36.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 37.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–28. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bursztajn S, Rutkowski MD, Deleo JA. The role of the N-methyl-D-aspartate receptor NR1 subunit in peripheral nerve injury-induced mechanical allodynia, glial activation and chemokine expression in the mouse. Neuroscience. 2004;125:269–75. doi: 10.1016/j.neuroscience.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, Pasha A, Valenick L, Sougioultzis S, Zhao D, Pothoulakis C. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci U S A. 2003;100:2957–62. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- 41.Guo CJ, Douglas SD, Gao Z, Wolf BA, Grinspan J, Lai JP, Riedel E, Ho WZ. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia. 2004;48:259–66. doi: 10.1002/glia.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Wesselborg S, Bauer MK, Vogt M, Schmitz ML, Schulze-Osthoff K. Activation of transcription factor NF-kappaB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J Biol Chem. 1997;272:12422–9. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 44.Schafers M, Sorkin LS, Geis C, Shubayev VI. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003;347:179–82. doi: 10.1016/s0304-3940(03)00695-5. [DOI] [PubMed] [Google Scholar]
- 45.Lin CS, Tsaur ML, Chen CC, Wang TY, Lin CF, Lai YL, Hsu TC, Pan YY, Yang CH, Cheng JK. Chronic intrathecal infusion of minocycline prevents the development of spinal-nerve ligation-induced pain in rats. Reg Anesth Pain Med. 2007;32:209–16. doi: 10.1016/j.rapm.2007.01.005. [DOI] [PubMed] [Google Scholar]