Abstract
Bone marrow transplantation is a curative treatment for many diseases, including leukemia, autoimmune diseases, and a number of immunodeficiencies. Recently, it was claimed that bone marrow cells transdifferentiate, a much desired property as bone marrow cells are abundant and therefore could be used in regenerative medicine to treat incurable chronic diseases. Using a Cre/loxP system, we studied cell fusion after bone marrow transplantation. Fused cells were chiefly Gr-1+, a myeloid cell marker, and found predominantly in the bone marrow; in parenchymal tissues. Surprisingly, fused cells were most abundant in the kidney, Peyer’s patches, and cardiac tissue. In contrast, after cell fusion with embryonic stem cells, bone marrow cells were reprogrammed into new tetraploid pluripotent stem cells that successfully differentiated into beating cardiomyocytes. Together, these data suggest that cell fusion is ubiquitous after cellular transplants and that the subsequent sharing of genetic material between the fusion partners affects cellular survival and function. Fusion between tumor cells and bone marrow cells could have consequences for tumor malignancy.—Bonde, S., Pedram, M., Stultz, R., Zavazava, N. Cell fusion of bone marrow cells and somatic cell reprogramming by embryonic stem cells.
Keywords: cre/loxP, transdifferentiation, lacZ, β-gal
Whether bone marrow cells are multipotent has been widely studied with controversial outcomes. One report claimed that a single bone marrow-derived cell was induced to proliferate down several different lineages, which were then successfully engrafted into a variety of target tissues (1). Many labs, however, failed to verify this claim, which might have an effect on regenerative medicine. Similarly, reports that bone marrow-derived cells could differentiate into neural cells (2, 3) were contradicted when other investigators failed to demonstrate this property (4). Thus, encouraging reports of bone marrow’s developmental plasticity have often been difficult to verify. Nevertheless, animal studies suggested that bone marrow cells can differentiate into cells that regenerate infarcted myocardium (5), a particularly interesting observation because infused therapeutic cells could be readily delivered to a live host. But this approach had mixed results in clinical trials. In one trial, bone marrow cells delivered to the heart were eliminated swiftly within 24–48 h (6); and in three other clinical trials, attempts to treat cardiac infarctions with autologous bone marrow cells also gave disappointing results, as one study failed to show any effect, and the other two claimed left ventricular function improvement by only 2–3% (7,8,9). Indeed, others demonstrated that bone marrow cells infused in myocardium maintain their bone marrow phenotype (10), contradicting the notion of transdifferentiation. It is therefore possible that improved function of damaged organs after bone marrow transplantation might be due to cytokine production by the hematopoietic cells and mesenchymal stem cells rather than due to transdifferentiation.
Despite varying results in clinical trials, laboratory studies continue to show that bone marrow cells hold promise for treating damage from myocardial infarction. In a recent manuscript, Fazel et al. (11) demonstrated that c-kit+ bone marrow-derived cells could contribute to the recovery of heart function after myocardial infarction. In these studies, bone marrow-derived c-kit+ cells proliferated in the damaged heart in which they created a proangiogenic milieu by increasing VEGF expression and reversing the cardiac ratio of angiopoietin-1 to angiopoetin-2. Recent work supports these studies by showing that postnatal cardiac progenitor cells contain isl1+ cells that can enter cardiogenesis (12). Moreover, Bailey et al. (13) reported that myeloid lineage progenitors can give rise to vascular endothelium.
It is unclear what mechanisms might allow bone marrow-derived cells to restore cardiac function after an ischemic insult. Some studies suggest that spontaneous cell fusion allows cells to transdifferentiate (14). Cell fusion is distinct from intrinsic differentiation of pluripotent stem cells and remains poorly studied. Hypothetically, bone marrow cells delivered to the heart can fuse with cardiomyocytes weakened by infarction, and the resulting hybrid cells might restore cardiac function. In support of this theory, one report showed that after fusion, bone marrow cells could adopt the phenotype of recipient cells (15). However, while such results suggest that bone marrow-derived hybrid cells could regenerate damaged tissues, others claim that they contribute to pathology. For example, Terashima et al. (16) raised concerns when they reported that diabetic neuropathy is caused by hybrid cells created when marrow-derived proinsulin-expressing cells fused with nerve cells. Despite this, the data so far raise the possibility that stem cells produce their benefits by fusing with target cells, making it critical to determine the extent of cell fusion and elucidate the cellular characteristics of fused cells. The benefits of such studies could extend beyond treating the damaged cardiac myocardium. For example, cells formed after fusing bone marrow cells with weakened parenchymal cells (such as hepatocytes) might revitalize a damaged liver. Moreover, in allogenic combinations, fused cells expressing major histocompatibility complex (MHC) types of both fusion partners might induce transplantation tolerance if proven that after transplantation they evade the immune system of the recipient.
Our interest has been to determine whether, in the uninjured host, bone marrow cells fuse with hematopoietic and nonhematopoietic cells in vivo. Our experiments here were sparked by previous observations that bone marrow cells fuse with Purkinje cells, cardiomyocytes, and hepatocytes (17, 18); however, in those studies, the extent to which bone marrow cells fused with both hematopoietic and nonhematopoietic cells was not fully addressed. To more closely examine cell fusion, our experiments here use the Cre-loxP system (19) that allows a reporter gene to be expressed only in fused cells. In our studies, bone marrow cells from Cre-transgenic mice were transplanted into syngeneic mice that are transgenic for floxed loxP sites. These studies indicated that after bone marrow transplantation, fused cells were detectable in the blood circulation and parenchymal organs.
MATERIALS AND METHODS
Cell lines and animals
The C57BL/6 (H-2b) cell line was maintained in culture as we recently described (20). Culture medium was changed daily, and the cells were passaged every 2 or 3 d. MRL (H-2Kk), 129SvJ (H-2b), and 129GT9 (R26R) mice at the age of 6–8 wk were purchased from Jackson Laboratories (Bar Harbor, ME, USA); the CAG-CATZ mice were a generous gift by Dr. Robin Davisson (University of Iowa) and were bred at our VA Animal Care Center. The Cre transgenic mice were the 129-Tg(Prm-cre)58Og/J purchased from the Jackson Laboratories. These mice are of the 129S4/SvJae strain origin. All animal procedures were approved by the University of Iowa Board of Animal Studies.
Construction of shuttle vectors
The FIV-shuttle-vector backbone (pVETL Cmcs) was obtained from the University of Iowa Vector Core and used for developing the final shuttle vectors: pVETL-Puro, which contains a puromycin acetyltransferase gene, under the control of a mouse polyglycerate kinase gene promoter (PGK); and pVETL-puro-Cre, which, in addition to puro, contains an enhanced Cre-recombinase gene. To construct pVETL-Puro, a plasmid called pPGK-Puro (a kind gift of Dr. John Murnane, University of California, San Francisco, CA, USA) was used as the template to amplify by PCR the puro coding sequence plus its immediate upstream promoter fragment from mouse PGK. PCR primers were designed to incorporate terminal MfeI restriction sites. The 1.2-kb PGK-Puro fragment was amplified using a proofreading DNA polymerase (pfuUltra HF Polymerase; Stratagene, La Jolla, CA, USA) and cloned into the unique MfeI site pVETL Cmc. The resultant vector, pVETL-Puro, was selected following colony PCR analysis (to determine the direction of the insert) and sequencing, to ensure that there were no mutations that occurred during the cloning procedure. This construct was the shuttle vector used to generate FIV-Puro. To make the FIV-Cre shuttle vector, we isolated DNA from AdCre M2 (Microbix Biosystems, Mississauga, ON, Canada), an adenovirus containing a modified Cre-recombinase gene under the control of a modified CMV promoter (MCMV), which includes an intron and nuclear localization signals for enhanced expression and appropriate localization. The 1.8-kb MCre fragment was amplified using a proofreading DNA polymerase plus forward and reverse DNA primers that incorporated restriction sites for NotI and SalI, respectively. The NotI-SalI fragment was consequently cloned into the pVETL-Puro, which had been prepared by double digestion with NotI and SalI. The integrity of the cloned fragment was confirmed by DNA sequencing.
Packaging the shuttle vectors into viral particles
The packaging and generation of high-titer viral particles for FIV-Puro and FIV-puro-Cre were carried out by the University of Iowa’s Gene Transfer Vector Core. The protocols for second-generation FIV packaging and pseudotyping, which enable the FIV to infect a wide range of mammalian cells, have been established by the University of Iowa Gene Transfer Vector Core and described previously (21). Briefly, pseudotyped FIV-puro-Cre particles were obtained by simultaneously transfecting the human kidney 293T cells with the FIV shuttle vector (pVETL-puro-MCre), FIV packaging vector (pCFIVΔofr2 Δvif), and an envelope construct (pCMV-G) in a 3:2:1 ratio, using standard calcium phosphate methods. Harvesting the viral particles and viral titer evaluation were carried out as described previously (21).
Stable transduction of embryonic stem cells (ESCs) via infection with FIV-Cre
High-titer viral preps (5×107 CFU/ml) were used to infect ESCs of syngeneic (C57BL/6, H-2b) cells, respectively, prior to infusion into the GtR26 reporter mice (H-2b). Stem cells (1×105 in 6-well plates) were incubated with ∼50 viral particles/cell for 4 h in ESC medium lacking nucleosides and containing only 2% FBS. Following infection, cells were washed with regular ESC medium and left to recover for 48 to72 h. Puromycin was then added (2 μg/ml), and after selection, several puromycin-resistant colonies from each set were transferred to 24-well plates and expanded for further cloning and characterization.
Bone marrow preparation
Bone marrow from B6;129GT9(ROSA) (Jackson Laboratories) mice was collected by flushing both tibiae and femurs with RPMI medium 1640 (Gibco Life Technologies, Grand Island, NY, USA). Red blood cells were depleted using ammonium chloride. Bone marrow cells were plated at a density of 2–4 × 107 cells/9.5 cm2 in Iscove’s modified Dulbecco’s medium (Gibco Life Technologies) supplemented with 10% fetal calf serum, 100 U/ml pen/strep (Gibco Life Technologies), and 10 mg/ml glutamine (complete IMDM medium). After 48 h, nonadherent cells were removed, fresh medium was added, and cells were cultivated until further use.
Bone marrow transplantation and flow cytometry
To determine engraftment of bone marrow cells in allogenic recipients, C57BL/6 bone marrow cells (H2b) were retrieved as described above, and 107 cells were transplanted in sublethally irradiated MRL mice (H2k). To protect against rejection, animals were treated with an anti-CD40L antibody (BD Biosciences, San Jose, CA, USA), as described previously (22). At 60 d posttransplantation, peripheral blood was retrieved, and donor and recipient cells were detected by costaining with a PE-conjugated H2b− and a GFP-conjugated H2k− antibody (both BD Biosciences). After erythrocyte lysis using ammonium chloride, cell fluorescence was measured using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). As controls, equal volumes of 20 μl of peripheral blood from donor and nontransplanted recipient mice were mixed and costained with both antibodies to rule out false-positive antibody stains.
Cocultures of ESCs and bone marrow cells
Bone marrow cells and Cre-expressing ESCs were cocultured on a 1% gelatin-coated plate at a ratio of 1:1, and a density of 2 × 105 cells/ml in ESC culture medium, DMEM with 4.5 mg/ml d-glucose, 15% ECS-quality FCS, 10 mM MEM nonessential amino acids, 2 mM l-glutamine, 100 U/100 μg pen/strep, 1000 U/ml leukemia inhibitory factor (LIF; Chemicon, Temecula, CA, USA), 6 ml nucleosides, and 0.2 mM β-mercaptoethanol. After 4 d, cocultures were washed and fixed in 2% paraformaldehyde for 10 min and then analyzed by 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) staining. As a negative control, monocultures of nontransduced C57 ESCs were similarly stained. X-gal staining was performed as described previously (23). lacZ expression was determined by flow cytometry using the ImageneGreen C12 FDG lacZ Gene Expression Kit (Molecular Probes, Eugene, OR, USA). To determine the phenotype of fused cells, the cells were costained with PE-conjugated antibodies against Gr-1, B220, CD3, and CD45. Cell fluorescence was measured on a FACScan (BD Biosciences).
Determining gene expression by RT-PCR
The mRNAs analyzed by RT-PCR were Cre recombinase, Nanog-1, Oct-4, CD45, and GAPDH. The primers for the PCR have been published (23). In addition, the CAT gene was analyzed using previously described oligonucleotides (24).
Differentiation of fused cells into beating cardiomyocytes and karyotyping
To differentiate fused cells into beating cardiomyocytes, we used a previously reported procedure (25). To induce cardiac differentiation, ESCs were plated onto specialty plates (Costar ultra-low-attachment clusters; Costar, Corning, NY, USA) containing the culture medium lacking supplemental LIF. After 5 d of culture, the embryoid bodies were plated onto tissue culture dishes. Spontaneous contractile activity was noticed 8 d after removing LIF. The expression of cardiac tissue genes was performed by PCR of the genes NKx2.5, ANF, MEF2C, β-MHC, and α-MHC, as described previously (26).
To count chromosomes after cell fusion, cells were plated and treated with vinblastine sulfate, then removed by trypsin and introduced to a hypotonic solution. Cells were fixed with Carnoy’s fixative, washed, and placed dropwise on preheated glass slides. Chromosomes were visualized after slides were stained with 10% Giemsa stain in Gurr buffer (VWR, Lutterworth, UK) and allowed to air dry.
LacZ detection in parenchymal tissues
To detect fused cells in parenchymal tissues, frozen sections of the heart or liver from Cre-expressing C57BL/6 mice were obtained 21 d posttransplantation with R26R bone marrow cells. They were costained for lacZ and with antibodies against either myosin heavy chain or albumin, respectively. All antibodies were purchased from BD Biosciences. Staining was documented using confocal microscopy. Alternatively, frozen sections of the transplanted animals were β-gal stained using bromo-4-chloro-3-indolyl β-d-galactoside (Sigma, St. Louis, MO, USA).
RESULTS
Bone marrow cells fuse posttransplantation
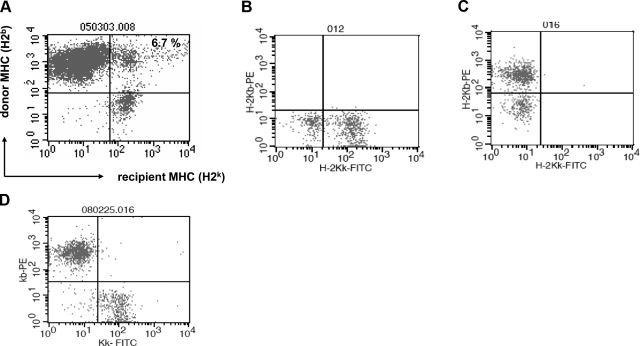
To study cell fusion of transplanted bone marrow cells, bone marrow cells (H2b) derived from 129SvJ mice were transplanted into MRL mice (H2k, as described previously (27, 28). Recipient mice were treated with an anti-CD154 antibody, to prevent rejection. Permanent engraftment of bone marrow cells was achieved in 75% of the animals using this protocol. At 60 d after transplantation, we analyzed the chimeric animals by whole-blood staining with antibodies against donor and recipient MHC antigens and observed that cells expressing donor MHC engrafted robustly (Fig. 1A). Further, we detected a distinct population of 6.7% that expressed both donor and recipient MHC class I antigens. Altogether, the percentage of the cells coexpressing donor and recipient MHC in peripheral blood was 4.5 ± 2.2%. We interpreted these data as evidence that donor bone marrow cells fused with circulating recipient hematopoietic cells. As controls for the antibody specificities, peripheral blood lymphocytes from nonchimeric MRL (Fig. 1B) and nonchimeric C57BL/6 mice (Fig. 1C) were similarly stained and showed no double-positive cells. In addition, 20-μl samples of blood from a control C57BL/6 mouse and from a control MRL mouse, respectively, were mixed, and the cells were costained with antibodies against H2b and H2k, respectively, to further rule out any false-positive stains (Fig. 1D). Our results indicate that bone marrow cells fuse with recipient cells posttransplantation, a previously unrecognized phenomenon, which might have consequences in the treatment of hematopoietic malignancies.
Figure 1.
Evidence of cell fusion by flow cytometry after bone marrow transplantation. A) C57BL/6 bone marrow cells (H2b) were transplanted into MRL allogenic recipient mice (H2k), and the animals were treated with an anti-CD40L antibody to prevent rejection. Recipient mice became chimeric, as depicted by whole-blood dual stain of donor and recipient MHC class I antigens 60 d posttransplantation. A distinct cell population coexpressing donor and recipient MHC was detected, suggesting cell fusion. B, C) As controls for the antibody specificities, whole-blood lymphocytes from nonchimeric MRL mice (B) and nonchimeric C57BL/6 mice (C) were similarly stained and showed no double-positive cells. D) In addition, a mixture of equal volumes of donor and recipient whole blood was similarly costained and analyzed by flow cytometry. There was no evidence of any cells expressing both donor and recipient MHC antigens.
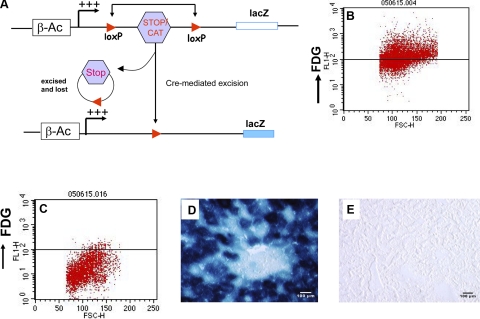
To more definitively verify that these cells were truly a result of cell fusion, we employed the Cre/loxP system—a technique used extensively in conditional gene expression (19). In this system, one cell type is transgenic for Cre recombinase, whereas the other is transgenic for a reporter gene blocked for read-through expression by flanked lox P sites. Thus, if the two cell types fuse, then Cre recombines the tandem loxP sites, positioning the reporter gene adjacent to the promoter, so it can be expressed. As depicted in Fig. 2A, we used either the R26R mouse (17) or the Cag-CATZ mouse, which harbors a CAT gene driven by the chicken β-actin promoter and flanked by loxP sites (24).
Figure 2.
Detection of cell fusion by the Cre/loxP sytem. A) Schematic diagram of the floxed loxP transgenic mouse and how Cre recombines at loxP sites. Note that between the loxP sites was either a STOP codon (ROSA26R mice) or the CAT gene (CAT CAGZ). B–E) C57BL/6 mice were infected with the adenoviral vector expressing lacZ. Mice were sacrificed 48 h later, and splenocytes were stained with a fluorescent lacZ substrate, whereas the liver was stained for lacZ by immunohistochemistry. Splenocytes from a representative infected animal strongly stained for lacZ (B), whereas the control cells remained negative (C). Similarly, liver histological sections from both animals were stained for lacZ, showing strong lacZ expression in the infected animal (D), but not in the control (E).
Initially, we confirmed lacZ staining by infusing an adenovirus expressing the lacZ gene into C57BL/6 mice and harvesting livers and spleens after 48 h. LacZ-expressing splenocytes were then visualized using a FITC-conjugated lacZ substrate (Fig. 2B). Control splenocytes from an animal infected with an empty adenovirus were negative for LacZ (Fig. 2C). In addition, histological sections of the livers of these animals were stained using X-gal staining. The results showed that the liver infected with the lacZ-containing adenovirus was strongly positive (Fig. 2D), but not that of control animals (Fig. 2E). Thus, these initial experiments confirmed lacZ expression by two different methods.
In vitro cell fusion of bone marrow cells
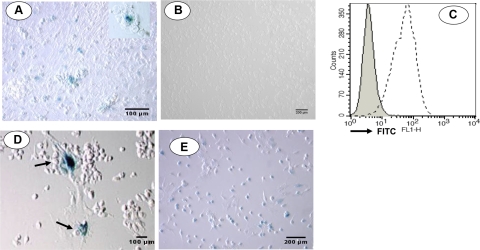
To exploit the Cre-loxP system for detecting cell fusion of bone marrow cells in vitro, bone marrow cells retrieved from C57BL/6 mice (transgenic for Cre recombinase) were cultured at 37°C for 3 d. Nonadherent cells were discarded, and bone marrow cells of the R26R mouse (transgenic for floxed loxP sites) were added. After coculturing the cells for another 5 d, nonadherent cells were again discarded, and the remaining cells were stained for lacZ. A significant number of cells stained positive for lacZ, confirming the presence of fused cells (Fig. 3A). Furthermore, lacZ-positive cells were larger than the negative cells, consistent with cell-cell fusion. Controls of cocultures of nontransgenic cells remained negative (Fig. 3B). Further, to detect fusion between bone marrow cells and ESCs, we constructed a Cre-recombinase FIV vector for the transduction of C57BL/6 ESCs. Following puromycin selection, Cre expression in the isolated clones was confirmed by staining with an anti-Cre-recombinase antibody (Fig. 3C).
Figure 3.
In vitro cell fusion. A) To detect cell fusion in vitro, bone marrow cells from loxP transgenic mice (R26R) were cocultured with bone marrow cells from Cre-recombinase transgenic mice, and lacZ expression was visualized after 4 d. A large number of adherent cells stained blue, indicating the presence of fused cells. Inset: fused cells at higher magnification. Cell viability was >98% in these cultures, ruling out contamination from dead cells. B) Control cells coincubated with non-Cre+ bone marrow cells remained negative. C) To detect fusion between bone marrow cells and ESCs, Cre was cloned and transfected into C57BL/6 ESC. Cre expression was confirmed by flow cytometry using an anti-Cre antibody. Control cells remained negative (solid line). D) To determine whether Cre was functional in these cells, they were transfected with adenovirus containing a floxed lacZ gene and stained for lacZ. Cells postivie for lacZ were observed (arrows), indicating that the cloned Cre was functional. E) To detect fusion between ESCs and bone marrow cells, Cre-ESCs were cocultured with loxP transgenic bone marrow cells and stained for lacZ. Many positive cells that stained for lacZ were indeed identified.
The enzymatic activity of Cre in these clones was further confirmed by their injection with an adenovirus containing a floxed lacZ gene (AdfloxlacZ1; Microbix Biosystems). The infected cells were stained with X-gal 24 h later, as evidence of lacZ expression (Fig. 3D).
To determine whether bone marrow cells fuse with ESCs, bone marrow cells transgenic for loxP were cocultured with ESCs stably expressing Cre recombinase. Fused cells were again detected using β-gal staining (Fig. 3E). Thus, these in vitro studies demonstrated that hematopoietic bone marrow cells fuse with both bone marrow cells and ESCs, respectively, in in vitro cocultures. Moreover, cell fusion was spontaneous, as it occurred without the need for medium supplements, such as PEG, which has been previously applied to induce cell fusion (29).
Cell fusion in vivo and phenotype characterization of fused cells
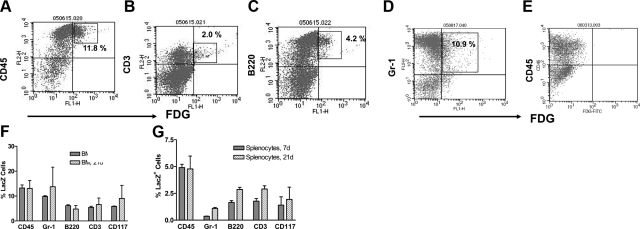
After these in vitro studies, we wondered to what extent cell fusion spontaneously occurs in vivo. Although a number of manuscripts have addressed cell fusion of bone marrow cells in vivo, the phenotype of the resulting hybrid cells has not been well addressed. To approach this question, we transplanted floxed loxP transgenic mouse (R26R)-derived bone marrow cells into Cre-recombinase transgenic C57BL/6 mice. Animals were sacrificed after either 7 or 21 d, and bone marrow cells or splenocytes were costained with a fluorescent lacZ substrate plus antibodies against CD45, CD3, B220, Gr-1, and CD117 to determine their phenotype. Cell fluorescence was measured on a FACScan. Our data on bone marrow stains are represented in Fig. 4A–D. As controls, we transplanted bone marrow cells from the R26R loxP transgenic mice in C57BL/6 wild-type mice with no Cre. Bone marrow cells were derived after 21 d and double stained for CD45 and lacZ (Fig. 4E). No lacZ-staining cells were detected in this control, as expected.
Figure 4.
Detection of fused cells after transplanting bone marrow. A–D) To detect cell fusion in vivo, bone marrow cells were transplanted into loxP/lacZ transgenic mice derived from a Cre-secreting C57BL/6 mouse. Mice were sacrificed on d 21, and both splenocytes and bone marrow cells were analyzed for cell fusion by flow cytometry, using the fluorescent FDG lacZ substrate. To determine which leukocyte subsets fused, cells were costained for CD45 (A), CD3 (B), B220 (C), and Gr-1 (D). E) Control bone marrow cells obtained from a R26R mouse transplanted with wild-type C57BL/6 bone marrow cells. F, G) Summary of the data on fused cells in bone marrow (BM; F) and spleen (G) at d 7 and 21 posttransplantation, respectively; n = 3 animals/group.
In the bone marrow of transplanted animals, generally <10% of cells were double positive for CD45 and lacZ (Fig. 4F). Interestingly, these percentages corresponded to the number of fused cells detected in peripheral blood, while only about half that number was detected in splenocytes (Fig. 4G). Fused cells were either CD3+, B220+, CD117+, or Gr-1+. In the bone marrow, most of the fused cells were Gr-1+, while in the spleen, mostly B220+ cells costained for lacZ, but not T cells. So far, however, it is not clear whether this distribution pattern of hybrid cells indicates the sites of cell fusion or whether hybrid cells and their progeny migrated to these sites after fusion. In this model, the degree of mixed chimerism after bone marrow transplantation was overall 20–35%.
Bone marrow cells fuse with parenchymal cells
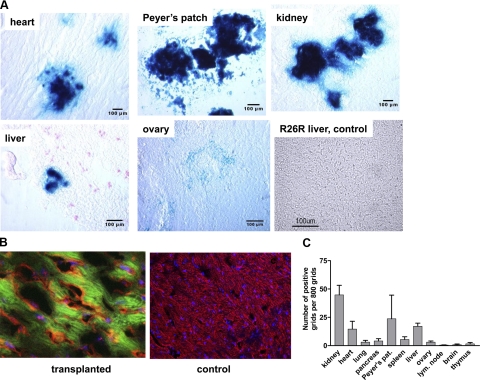
To determine fused cells in parenchymal tissues, we analyzed histological sections by immunohistochemical staining for lacZ posttransplantation. Frozen sections of the heart, kidney, liver, ovaries, Peyer’s patches, spleen, lymph nodes, brain, and thymus were prepared and stained for lacZ. Representative stains are shown in Fig. 5A. The highest numbers were detected in the kidney, followed by the Peyer’s patches and the heart. In contrast, no fused cells were detected in the brain and few in the thymus. We included a control histological section from a nontransplanted R26R mouse stained for LacZ that remained negative on staining.
Figure 5.
Evidence of cell fusion in parenchymal tissues. A) Histological sections of bone marrow-transplanted animals were stained for lacZ at 21 d posttransplantation. Substantial numbers of fused cells were detected in the heart, Peyer’s patches, and kidney, with fewer cells in the liver and ovaries. R26R liver section from a nontransplanted animal was used as control for the lacZ stain. No lacZ-staining cells were detected, as expected. B) To further characterize fused cells, cardiac sections were stained with PE for myosin heavy chain and with FITC for lacZ. Cardiac tissue was counterstained with Torpo3. Left panel: section showing costaining confirms cell fusion between the transplanted bone marrow cells and cardiomyocytes. Right panel: control section remained negative for lacZ. C) Bar graph summarizes the relative cell numbers counted. Ten histological sections were studied for each organ; ≥800 grids were counted to obtain the mean of the positive grids.
To confirm that the fused cells in the cardiac tissue were with cardiac cells and not with other hematological cells, histological sections were costained for myosin heavy chain and lacZ by immunofluorescence. Indeed, costained cardiac tissue detected (Fig. 5B) but not in the control sections that remained positive for myosin heavy chain only. To quantitate the number of fused cells, 10 histological sections were stained, and ≥800 grids on each slide were regulated. The results are summarized in Fig. 5C. Contrary to previously published findings (30), we did not detect many fused cells in the liver. This may be because cell fusion occurs more often with dividing cells (17), such as is the case after tissue injury. Our recipient animals were uninjured pretransplantation.
ESCs reprogram bone marrow cells to pluripotent cells
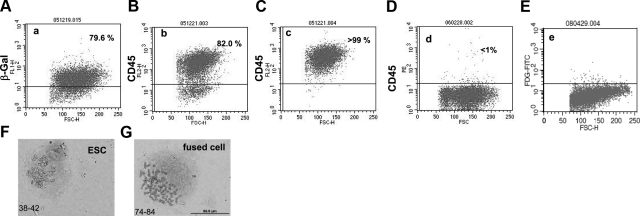
Because our experiments so far suggested that cocultured ESCs and bone marrow cells fuse (Fig. 3), we were interested in the nature of the hybrid cells after fusion. To examine hybrid cells, the C57BL/6 Cre-expressing ESCs were cocultured for 4 d with CAG CATZ-derived bone marrow cells, which are transgenic for the floxed loxP sites. The cell mixture was treated with puromycin to remove nonfused bone marrow cells, as they do not express the puromycin resistance gene. After another 4 d, our cultures contained fused cells and nonfused Cre-expressing ESCs. We reasoned that after fusion, the hybrid cells down-regulate CD45, a hematopoietic cell marker not detectable on nondifferentiated ESCs. After selecting with puromycin, the remaining cells were stained with a green fluorescent lacZ substrate, and ∼79% were positive for β-gal (Fig. 6A). We further confirmed this result by staining for the hematopoietic cell marker CD45, because we postulated that recently fused cells should still express CD45, even if they may eventually change their phenotype. We found that ∼82% of the cells stained for CD45 (see Fig. 6B). The hybrid cells were then purified to >99% by magnetic bead separation (Fig. 6C). Surprisingly, after another 6 d in culture, almost all of the cells had lost CD45 expression (Fig. 6D). This finding may suggest that after cell fusion, ESCs reprogram somatic hematopoietic cells to adopt an ESC phenotype. As a control, R26R bone marrow cells were cocultured with wild-type C56BL/6 ESCs not transduced with Cre, and the procedure was repeated. The cells remained negative for lacZ (Fig. 6E).
Figure 6.
ESCs reprogram adult hematopoietic cells after cell fusion. At 4 d after coculturing ESCs with bone marrow cells, the cell mixture was treated with puromycin to remove unfused bone marrow cells. At that stage, 79.6% of the cells were lacZ positive (A), which correlated well with the 82% that we measured for CD45+ cells (B). To purify the fused cells based on CD45 expression, immunomagnetic beads were used, yielding a near pure cell population (C). Surprisingly, after another 6 d of culture, >99% of the cells had lost CD45 expression (D). As controls, C57BL/6 ESC cocultured with R26R bone marrow cells showed no FDG-positive stains (E). Chromosomes in the fused cells were subsequently counted (F), showing that fused cells had double the number of chromosomes to those detected in ESCs (G).
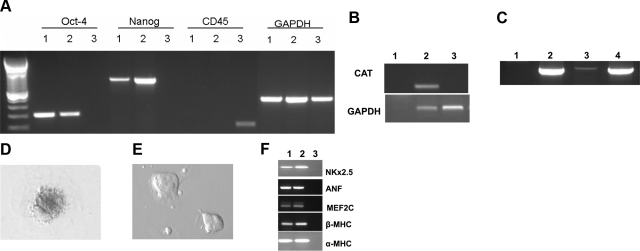
Fused cells should contain twice the nuclear material of nonfused partners. As predicted, our hybrid cells contained twice as many chromosomes (72 to 84) as ESCs alone or bone marrow cells (38 to 42; Fig. 6F). To further determine whether these hybrid cells adopted an embryonic phenotype, we used RT-PCR to assay for expression of both the ESC-specific transcription factors Oct4 and Nanog-1 and the hematopoietic cell marker CD45. Consistent with the hybrid cells adopting an ESC phenotype, they were positive for Oct-4 and Nanog-1, but negative for CD45. Conversely, bone marrow cells were negative for ESC transcription factors, but positive for CD45 (Fig. 7A).
Figure 7.
Molecular evidence for cell fusion and reprogramming of somatic cells to ESCs. A) RT-PCR for OCT4, Nanog, CD45 and GAPDH was performed on ESCs (lane 1), fused cells (lane 2), or on bone marrow (lane 3). B) To verify cell fusion, fused cells were tested for presence of the CAT gene, which should have been lost gene after Cre recombination. Genomic DNA of the CAT CAGZ bone marrow cells was positive (lane 2), while the fused cells (lane 3) and the water control (lane 1) were negative. C) PCR amplification was performed on the lacZ gene, which was found in the genomic DNA of bone marrow cells from the CAT-CAGZ mouse (lane 2), cDNA of fused cells (lane 3), and in the positive control DNA extracted from the liver of a mouse after infection with adenovirus containing lacZ (lane 4). Water control remained negative (lane 1). D–F) Beating cardiomyocytes differentiated from fused ESCs (D) and those from wild-type ESCs (E) were stained for lacZ. Results further confirmed that fused cells transcribed the lacZ gene. Further, expression of cardiac genes NKx2.5, ANF, MEF2C, β-MHC, and α-MHC in the beating cells was confirmed by PCR (F). Lane 1, positive control of cardiac tissue from a murine heart; lane 2, samples from beating cardiomyocytes generated from beating cardiomyocytes; lane 3, water control.
We next sought to confirm that the ESC-like cells were derived from fused cells by testing whether they contained the CAT gene, because in fused cells, Cre recombinase should excise the CAT gene at the loxP sites. PCR using CAT gene-specific primers showed that while bone marrow cells of the CAG CATZ mouse were CAT positive, the fused cells were negative (Fig. 7B). To further confirm excision of the floxed loxP sites, we tested for lacZ expression by RT-PCR and found that cDNA from the CAG CATZ mice was negative for lacZ, while the genomic DNA was positive (as expected, since without Cre, the CAT gene prevents read-through expression of lacZ; see Fig. 7C). Conversely, in fused cells, Cre-mediated recombination would excise the CAT gene, positioning lacZ adjacent to the β-actin promoter, thereby allowing lacZ expression. As positive control, we observed that cDNA from livers of animals infected with the adeno lacZ virus stained positive. We interpreted these results as confirmation that the ESC-like cells were generated by cell fusion.
Although we demonstrated that the fused cells expressed ESC genes, it was unclear whether, like pluripotent ESCs, they could differentiate into other tissues; so we differentiated them into beating cardiomyocytes. This process takes ∼13 d in culture and indeed led the fused cells to fully differentiate into beating cardiomyocytes. We further stained the beating cardiomyocytes for lacZ. As expected, the cells were positive for lacZ, (Fig. 7D). Lastly, we performed PCR for cardiac genes NKx2.5, ANF, MEF2C, β-MHC, and α-myosin using cDNA from cardiac tissue and from our beating cardiomyocytes derived from fused cells. Bands representing these genes were detected (Fig. 7F). Altogether, our data suggest that after cellular transplants, cell fusion occurs. The data further affirm that ESCs can reprogram the cellular contents of somatic cells to adopt an ESC phenotype after cell fusion.
DISCUSSION
Some investigators have suggested that transplanted bone marrow cells can give rise to cells of different lineages (1) through a process generally termed as transdifferentiation. Others hypothesized that such a property might allow bone marrow cells to repair infarcted myocardium in vivo (5). The latter claim led to clinical trials that had mixed results (7,8,9). These studies led us to examine cell fusion in a mouse model. We suspected that in vivo, transdifferentiation might be a result of cell fusion of donor bone marrow cells with host cells. Thus, we chose to determine which cell types might fuse with transplanted bone marrow cells.
In vitro, we showed that cell fusion was common when cells were cocultured. Particularly, we showed that when bone marrow cells from different mouse strains were coincubated, a subpopulation emerged, which expressed donor and recipient MHC antigens. To examine this further, we transplanted bone marrow cells in both allogenic and syngeneic combinations. Interestingly, cell fusion was observed in both cases. When tested, most of the fused cells were of the myeloid lineage; furthermore, most of the fused cells appeared localized in the bone marrow and spleen. The scope of our studies, however, did not allow establishment of whether these were the sites of cell fusion or whether fused cells migrate to these compartments. Flow cytometry showed that fused cells expressed MHC antigens of both fusion partners; thus, there is no dominant expression of the MHC genes from either. To our knowledge, this study shows for the first time that cell fusion preferentially occurs with Gr-1+ cells in the bone marrow. Of particular interest was the observation that CD117-expressing cells fused with bone marrow cells, allowing speculation on whether such fused cells have the capability to reconstitute bone marrow.
Previous studies of cell fusion in parenchymal tissues showed abundant evidence of cell fusion in the liver after hepatic injury. Here, our animals had no specific organ damage except the sublethal irradiation in the allogenic recipients of bone marrow cells. Interestingly, we observed relatively moderate cell numbers of fused cells in the liver, but higher cell numbers in the kidney, heart, and Peyer’s patches. Presumably, cell fusion occurs in tissues with rapidly dividing cells, such as in the damaged liver (30), yet that assumption is challenged by our data, as the kidney and heart traditionally do not show high rates of cell division. Since these fused cells were viable, it is of interest to probe whether they can divide in vivo and, for example, reconstitute bone marrow cells. However, in vitro, although cells generated by fusion of bone marrow cells can be cultivated up to 6 wk, they eventually die off.
Cell fusion between somatic cells is less likely to yield highly dividing cells, as somatic cells are fully differentiated. Therefore, we studied cell fusion between bone marrow cells and ESCs. Cell fusion was further confirmed by a double karyotype in fused cells. More interestingly, fused cells were reprogrammed into new tetraploid ESCs. We observed down-regulation of the CD45 pan-leukocyte marker and expression of the ESC transcription factors Nanog-1 and Oct-4, suggesting that ESCs indeed reprogram somatic cells into embryonic cells. To prove this point, we successfully formed embryoid bodies and beating cardiomyocytes. These findings show the remarkable capability of ESCs to override the adult somatic cell program, as seen after nuclear transfer (31). Our results are similar to prior findings by Ying et al. (32), who also detected tetraploid cells after cell fusion with ESCs. Morphogens such as Nodal (33, 34), TGF-β, and cripto-1 (35) might play a role in ESC fusion, as these molecules are highly involved in cell signaling and cell cycling in pluripotent cells. These growth factors are currently under investigation in our lab.
The data on somatic cell fusion presented here cast doubt on earlier conclusions that suggested transdifferentiation of bone marrow cells. Our data are in agreement with reports from Weismann’s laboratory (10, 14), which observed little evidence of transdifferentiation after bone marrow transplantation. Using the Cre/loxP system that is generally used for conditional expression of genes (19), we showed that, indeed, cell fusion occurs and that the fused hematopoietic cells are detected in the bone marrow, Peyer’s patches, and spleen. Terada et al. (15) concluded in their own experiments that spontaneously fused bone marrow cells adopt the phenotype of the recipient cells, which might be interpreted as dedifferentiation. Thus, cell fusion appears to be a physiological process that occurs spontaneously, not requiring tissue damage to have preceded. The fused cells do not appear to be cancerous, as we were able to detect fused cells in healthy mice 5 mo after bone marrow transplantation, albeit at lower cell numbers than were detected early after transplantation. The current data not only provide evidence that fused cells are stable long term in vivo, but that some theses on transdifferentiation require a revisit and more cautious interpretation.
Acknowledgments
This work was made possible by a VA Merit Review award and by National Institutes of Health/National Heart Lung and Blood Institute grant R01 HLO73015. We thank the University of Iowa Gene Transfer Vector Core for providing the FIV vectors and Chantal Allamargot (University of Iowa Central Microscopy Research Facility) for technical assistance. We thank John Engelhardt (Department of Anatomy and Cell Biology, University of Iowa) for useful discussions.
References
- Krause D S. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Brazelton T R, Rossi F M V, Keshet G I, Blau H M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross K J, Harta G, Maki R A, McKercher S R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Castro R F, Jackson K A, Goodell M A, Robertson C S, Liu H, Shine H D. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine D M, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Wollert K C, Meyer G P, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp W H, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld J G, Smith H J, Taraldsrud E, Grogaard H K, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann J E, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Assmus B, Honold J, Schachinger V, Britten M B, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali N D, Tonn T, Dimmeler S, Zeiher A M. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey D G, Hamm C W, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher A M, for REPAIR-AMI investigators Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Balsam L B, Wagers A J, Christensen J L, Kofidis T, Weissman I L, Robbins R C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel R D, Keating A, Li R K. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz K L, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin L Z, Cai C L, Lu M M, Reth M, Platoshyn O, Yuan J X J, Evans S, Chien K R. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A S, Willenbring H, Jiang S, Anderson D A, Schroeder D A, Wong M H, Grompe M, Fleming W H. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers A J, Sherwood R I, Christensen J L, Weissman I L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz D M, Nakano Y, Meyer E M, Morel L, Petersen B E, Scott E W. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Terashima T, Kojima H, Fujimiya M, Matsumura K, Oi J, Hara M, Kashiwagi A, Kimura H, Yasuda H, Chan L. The fusion of bone-marrow-derived proinsulin-expressing cells with nerve cells underlies diabetic neuropathy. Proc Natl Acad Sci U S A. 2005;102:12525–12530. doi: 10.1073/pnas.0505717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo J, Fike J, Lee H, Pfeffer K, Lois C, Morrison S, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Rizvi A Z, Swain J R, Davies P S, Bailey A S, Decker A D, Willenbring H, Grompe M, Fleming W H, Wong M H. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci U S A. 2006;103:6321–6325. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24:2192–2201. doi: 10.1634/stemcells.2006-0022. [DOI] [PubMed] [Google Scholar]
- Stein C S, Kang Y, Sauter S L, Townsend K, Staber P, Derksen T A, Martins I, Qian J, Davidson B L, McCray P B., Jr In vivo treatment of hemophilia A and mucopolysaccharidosis type VII using nonprimate lentiviral vectors. Mol Ther. 2001;3:850–856. doi: 10.1006/mthe.2001.0325. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell F R, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak M Z. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Agah R, Frenkel P A, French B A, Michael L H, Overbeek P A, Schneider M D. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C, Maioli M, Asara Y, Santoni D, Scarlata I, Cantoni S, Perbellini A. Butyric and retinoic mixed ester of hyaluronan: a novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells. J Biol Chem. 2004;279:23574–23579. doi: 10.1074/jbc.M401869200. [DOI] [PubMed] [Google Scholar]
- Arai A, Yamamoto K, Toyama J. Murine cardiac progenitor cells require visceral embryonic endoderm and primitive streak for terminal differentiation. Dev Dyn. 1997;210:344–353. doi: 10.1002/(SICI)1097-0177(199711)210:3<344::AID-AJA13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- Wekerle T, Kurtz J, Ito H, Ronquillo J, Dong V, Zhao G, Schaffer J. Allogeneic bone marrow transplantation with co-stimulatory blockade in macrochimerism and tolerance without cytoreductive host teratment. Nat Med. 1999;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- Takei S, Yamamoto M, Cui L, Yue F, Johkura K, Ogiwara N, Iinuma H, Okinaga K, Sasaki K. Phenotype-specific cells with proliferative potential are produced by polyethylene glycol-induced fusion of mouse embryonic stem cells with fetal cardiomyocytes. Cell Transplant. 2005;14:701–708. doi: 10.3727/000000005783982693. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhallmy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Campbell K H, McWhir J, Ritchie W A, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Ying Q L, Nichols J, Evans E P, Smith A G. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Vallier L, Touboul T, Brown S, Cho C, Bilican B, Alexander M, Cedervall J, Chandran S, Ahrlund-Richter L, Weber A, Pedersen R A. Signalling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells [E-pub ahead of print] Stem Cells. 2009 doi: 10.1002/stem.199. doi: 10.1002/stem.199. [DOI] [PubMed] [Google Scholar]
- Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers L E, Trotter M W, Cho C H, Martinez A, Rugg-Gunn P, Brons G, Pedersen R A. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizzi L, Postovit L M, Margaryan N V, Seftor E A, Abbott D E, Seftor R E, Salomon D S, Hendrix M J. Emerging roles of nodal and Cripto-1: from embryogenesis to breast cancer progression. Breast Dis. 2008;29:91–103. doi: 10.3233/bd-2008-29110. [DOI] [PMC free article] [PubMed] [Google Scholar]