Abstract
FK506 binding protein 12.6 kDa (FKBP12.6), a protein that regulates ryanodine Ca2+ release channels, may act as an important regulator of insulin secretion. In this study, the role of FKBP12.6 in the control of insulin secretion and blood glucose is clarified using FKBP12.6−/− mice. FKBP12.6−/− mice showed significant fed hyperinsulinemia but exhibited normoglycemia, fasting normoinsulinemia, and normal body weight compared with wild-type (WT) littermate control mice. Deletion of FKBP12.6 resulted in enhanced glucose-stimulated insulin secretion (GSIS) both in vivo and in vitro, a result that is due to enhanced glucose-induced islet Ca2+ elevation. After a high-fat dietary challenge (HF diet) for 3 mo, FKBP12.6−/− mice displayed higher body weight, hyperinsulinemia, and lower fed blood glucose concentrations compared with WT mice. FKBP12.6−/− mice displayed hyperinsulinemia, and resistance to HF diet-induced hyperglycemia, suggesting that FKBP12.6 plays an important role in insulin secretion and blood glucose control, and raising the possibility that it may be a potential therapeutic target for the treatment of type 2 diabetes.—Chen, Z., Li, Z., Wei, B., Yin, W., Xu, T., Kotlikoff, M. I., Ji, G. FKBP12.6-knockout mice display hyperinsulinemia and resistance to high-fat diet-induced hyperglycemia.
Keywords: pancreatic islets, diabetes, Ca2+, insulin secretion
Energy homeostasis is controlled by insulin secretion from pancreatic β cells and sensitivity to the insulin signal in target tissues, such as the liver, fat, and skeletal muscle. In pancreatic β cells, elevated blood glucose concentrations increase the ATP/ADP ratio, depolarize the cell membrane, and lead to increases in Ca2+ concentration via L-type voltage-gated channels and Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER). Elevated intracellular Ca2+ concentrations then trigger insulin secretion (1). It has been thought that ryanodine receptors (RyRs) play an important role in insulin secretion and diabetes development (2,3,4,5,6,7). However, the underlying mechanisms of RyR involvement in the regulation of insulin secretion and diabetes development are not well understood.
FKBP12.6, a member of the peptidyl-prolyl isomerase family, is widely expressed in many cell types. FKBP12.6 is generally considered to be a functional regulator of ryanodine receptor 2 (RyR2). It has been shown that FKBP12.6 plays an important role in cardiac and smooth-muscle excitation-contraction coupling (8,9,10,11). The possibility that FKBP12.6 plays a role in insulin secretion is supported by in vitro studies, suggesting that cADPR causes the dissociation of FKBP12.6 from RyR2, which is then followed by increases in cytosolic Ca2+ concentration in pancreatic β cells (12, 13). However, the potential function of FKBP12.6 in insulin secretion and glucose metabolism remains unclear.
The FKBP12.6−/− mouse provides a unique model for investigating the role of FKBP12.6/RyR2 in insulin secretion and glucose metabolism. Deletion of FKBP12.6 results in one of three different disease phenotypes, depending on the genetic background of the line used (8); cardiac hypertrophy is observed in FKBP12.6−/− male mice with a 129 or C57 genetic background line (9); stress-induced cardiac arrhythmias (8) are observed in mice with a DBA genetic background; and impaired glucose-induced insulin secretion is observed in FKBP12.6−/− mice with an ICR genetic background (14). In this study, we used FKBP12.6−/− mice with a 129 genetic background to study the role of FKBP12.6 in regulating metabolism. Our results show that FKBP12.6−/− mice display hyperinsulinemia and resistance to high-fat (HF) diet-induced hyperglycemia.
MATERIALS AND METHODS
Animals
All animal procedures described in this study were performed in adherence with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996), and with the approval from the Institute of Biophysics Committee for Animal Care.
FKBP12.6−/− mice with a 129/Sv/Ev genetic background were used throughout this study. Development of FKBP12.6−/− mice has previously been described in detail (9). FKBP12.6−/− (129/Sv/Ev) mice were mated with wild-type (WT; 129/Svpaslco-Crl) mice, and the resulting heterozygous mice were subsequently mated to produce the littermates used in the present experiments. One-month-old male mice were placed on either a control diet or an HF diet for 8 mo. The fat source was lard and comprised 45% of the total calories in the diet. Mice were housed in sterile barrier facilities with equal day/night periods. Unless otherwise stated, paired littermates were used for the study to randomize genetic variation.
Measurement of blood glucose and glucose tolerance tests
Blood glucose levels were measured from tail venous blood using an automatic glucometer (Accu-Check; Roche Diagnostics, Mannheim, Germany). Age-matched mice were deprived of food overnight for 15 h and then injected intraperitoneally (i.p.) with d-glucose (2 g/kg body weight) to test for glucose tolerance. Blood samples were obtained from the tail tip at the times indicated.
Insulin tolerance tests
Insulin tolerance tests were performed by measuring blood glucose levels at the indicated time points following a single i.p. injection of 0.75 U/kg body weight regular human insulin (humulin; Eli Lilly & Co., Fegersheim, France). Food was withdrawn for 4 h before the start of the experiment.
Serum insulin levels
Blood was collected from the retroorbital sinus. Serum insulin levels were measured using a rat/mouse insulin ELISA kit (Linco Research, St. Charles, MO, USA).
Islet morphology
Pancreata were removed, fixed overnight in 10% buffered formalin solution, and embedded in paraffin. Sections were obtained and stained with hematoxylin and eosin using standard methods. Image J software (U.S. National Institutes of Health, Bethesda, MD, USA) was used to analyze islet morphology.
Pancreatic insulin content
Pancreatic insulin content was measured with a rat/mouse insulin ELISA kit (Linco Research) after insulin extraction with acidic ethanol (0.1 M HCl in 95% ethanol) and was normalized to protein content.
Islet isolation
Islets from 2-mo-old adult male mice that had been killed by cervical dislocation were isolated according to a published procedure (15). Briefly, islets were obtained by incubating small sections of pancreatic tissue for 25 min in Hank’s buffered solution, containing 137 mM NaCl, 5.4 mM KCl, 1.3 mM CaCl2·2H2O, 0.8 mM MgSO4·7H2O, 0.44 mM KH2PO4, 0.34 mM Na2HPO4·12H2O, 5 mM d-glucose, 4.2 mM NaHCO3, 1–2 mg/ml collagenase P (Roche Diagnostics), and 1 mg/ml BSA (pH 7.4). Isolated islets were cultured in RPMI 1640 medium supplemented with 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10 mM glucose at 37°C (5% CO2) for 1–2 d.
Insulin release from islets
Insulin release from pancreatic islets was measured as described previously (16). Briefly, 15 islets, which had been cultured overnight, were incubated in 200 μl Krebs-Ringer bicarbonate buffer (KRBB) composed of (in mM) 129 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 5 NaHCO3, 0.1% BSA, and 3 glucose (pH 7.4) at 37°C for 1 h to determine basal insulin release. Islets were then challenged with 16.7 mM glucose at 37°C for 30 min. Insulin concentration was determined using a rat/mouse insulin ELISA kit (Linco Research).
Measurement of islet Ca2+ concentration
Islets attached to coverslips were cultured for 1–2 d and then loaded with the Ca2+-sensitive dye fluo-4 AM (10μM) (Molecular Probes, Eugene, OR, USA) and 0.1% pluronic acid F-127 in a standard solution containing (in mM) 140 NaCl, 5.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 3 glucose (pH 7.4 adjusted with NaOH) for 30 min at room temperature. The cover slips (1 mm) with attached islets were placed to a perfusion chamber with a perfusion rate of 1.5 ml/min. Ca2+ was measured by using a laser scanning confocal microscope, attached to an Olympus IX-70 inverted microscope, using a Plan Apo ×60 oil objective (1.4 numerical aperture; Olympus, Tokyo, Japan). The Ca2+-dependent fluorescence intensity ratio (F/F0) was plotted as a function of time. All chemicals were from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Statistical analysis
Statistical analysis was performed using SigmaPlot (Systat Software Inc., San Jose, CA, USA). Values given are means ± se. Data were tested for significance using the Student’s t test. Only results with values of P < 0.05 were considered statistically significant.
RESULTS
Deletion of FKBP12.6 enhanced insulin secretion both in vivo and in vitro
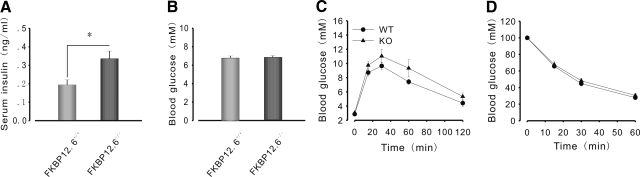
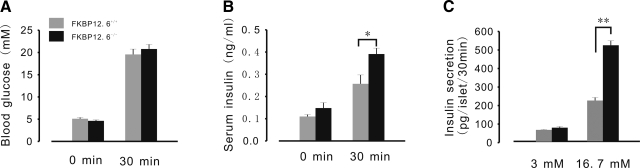
To examine whether FKBP12.6 is involved in insulin secretion and blood glucose control, we measured insulin and blood glucose concentration continuously in 2-mo-old FKBP12.6-knockout (KO) and WT littermates. FKBP12.6−/− mice showed higher serum insulin concentrations than WT mice (0.34±0.042 and 0.19±0.028 ng/ml, respectively; P<0.05, n=8–10) and normoglycemia when they were allowed to feed freely (Fig. 1A, B). Under fasting conditions, FKBP12.6−/− mice showed normal levels of blood glucose and serum insulin (Fig. 2A, B). Glucose (3 g/kg body weight) was administered to mice that had been subjected to 15 h of food deprivation overnight to measure glucose-stimulated insulin secretion (GSIS) in vivo (Fig. 2B). After a 30-min challenge with glucose, serum insulin levels increased by 163.8% in FKBP12.6−/− mice, but only by 134.7% in WT mice (0.391±0.026 and 0.257±0.04 ng/ml, respectively; P<0.05, n=6). Simultaneously, blood glucose levels were measured, and results indicated that there were no significant differences between them (Fig. 2A). Furthermore, we performed GSIS with isolated islets from FKBP12.6−/− and control littermates (Fig. 2C). As expected, islets from FKBP12.6−/− mice secreted significantly more insulin than those from control mice at a high glucose concentration (16.7 mM) (524.27±24.75 and 222.84±16.46 pg/islet/30 min, respectively; P<0.01, n=6). However, there were no significant differences in insulin secretion at low glucose concentrations (3 mM). Taken together, these results indicate that deletion of FKBP12.6 enhances glucose-induced insulin secretion and gives rise to hyperinsulinemia.
Figure 1.
Glucose homeostasis in 2-mo-old FKBP12.6−/− mice. A, B) Serum insulin (A) and blood glucose (B) in FKBP12.6−/− mice (n=8–10) and control littermates (n=8–10) allowed to feed freely. FKBP12.6−/− mice showed hyperinsulinemia and normoglycemia. C) Glucose tolerance test in FKBP12.6−/− mice (n=8) and control littermates (n=8). No significant differences were found in blood glucose levels after injection with glucose (2 g/kg body weight i.p.). D) Insulin tolerance test in FKBP12.6−/− mice (n=6) deprived of food for 4 h and control littermates (n=6). FKBP12.6−/− mice exhibited normal insulin sensitivity. *P < 0.05.
Figure 2.
Deletion of FKBP12.6 results in enhanced insulin secretion. A, B) Blood glucose and serum insulin levels in FKBP12.6−/− mice (n=6) and control littermates (n=6) injected with glucose (3g/kg body weight i.p.). Blood was collected before (0 min) and 30 min after glucose injection. At 30 min after glucose application, a significant increase in serum insulin levels (B) was observed in FKBP12.6−/− mice, while blood glucose levels (A) were similar between KO and control littermates. C) Glucose stimulated insulin secretion from isolated islets. Following overnight culture, 15 islets were exposed to 3 mM glucose for 1 h as basal insulin secretion, and then exposed to 16.7 mM glucose for 30 min. Insulin concentrations were determined by ELISA; n = 6 mice/group. *P < 0.05; **P < 0.01.
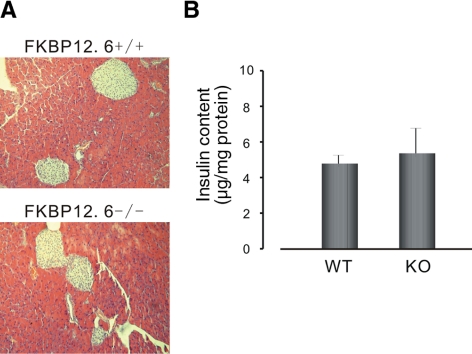
The enhanced insulin secretion in FKBP12.6−/− mice described above could have resulted from an increase in the number of islets and/or the insulin content of the islets. We, therefore, performed a morphological analysis of pancreatic sections from FKBP12.6−/− and WT mice to investigate this question. Our experiments demonstrated that there was no significant difference in the number of islets between FKBP12.6−/− and WT mice (1.89±0.39 and 1.9±0.34 islets/section of pancreas, respectively, P>0.05 n=3). The results of a comparative study of islet size indicated that islets in FKBP12.6−/− and WT mice were of a similar size. Moreover, there was no significant difference in pancreatic insulin content between the two types of mice (Fig. 3B). These studies indicate that deletion of FKBP12.6 does not result in changes in islet number, size, or insulin content.
Figure 3.
FKBP12.6−/− mice show normal islet histology and pancreatic insulin content. A) Pancreatic sections from FKBP12.6−/− mice and control littermates were stained by hematoxylin and eosin (HE). B) Insulin content in pancreata from FKBP12.6−/− mice (n=5) and control littermates (n=5).
Deletion of FKBP12.6 enhances glucose- and KCl-induced Ca2+ release
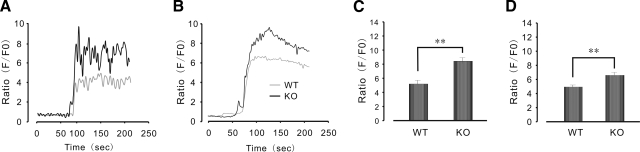
It has previously been reported that deletion of FKBP12.6 enhances stimulation-induced Ca2+ elevation in smooth-muscle (10, 11) and cardiac myocytes (9). In the following experiments, we examined whether enhanced insulin secretion was due to a large Ca2+ release in islets from FKBP12.6−/− mice. As shown in Fig. 4A, C, Ca2+ release was significantly higher in FKBP12.6-null islets than in WT islets after stimulation with a 16.7 mM glucose load (8.44±0.45 and 5.23±0.4 F/F0, respectively; P<0.01, n=15–20). Similarly, as shown in Fig. 4B, D, the amplitude of Ca2+ transients in FKBP12.6−/− islets was also greater than that in WT islets after stimulation with a 25 mM KCl load (6.61±0.33 and 4.91±0.23 F/F0, respectively; P<0.01, n=15–20). These results indicate that deletion of FKBP12.6, which is mainly associated with RyR2, results in higher levels of Ca2+, which then lead to higher levels of insulin secretion in pancreatic islets.
Figure 4.
Enhanced Ca2+ response to glucose or KCl application in isolated FKBP12.6−/− islets. A, B) Response of Ca2+ to glucose application (16.7 mM) (A) and to KCl application (25 mM) (B) in FKBP12.6−/− and control islets. C, D) Summary data of glucose stimulation (C) and KCI stimulation (D) of Ca2+ response. n = 15–20 islets/group. **P < 0.01.
FKBP12.6−/− mice are resistant to HF-diet-induced hyperglycemia
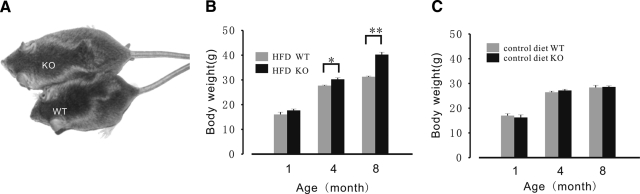
In the above experiments, we demonstrated that while FKBP12.6−/− mice display hyperinsulinemia, they still exhibit normoglycemia. In our next series of experiments, we examined the effects of a long-term HF diet on β-cell function in FKBP12.6−/− mice. Body weights of WT and FKBP12.6−/− male mice fed an HF diet for 1, 4, and 8 mo are shown in Fig. 5B. Body weight of these mice was similar before the onset of the HF diet when 1 mo old. After following an HF diet for 3 mo, body weight of FKBP12.6−/− mice increased markedly compared to WT mice. After following the HF diet for 7 mo, the body weight of FKBP12.6−/− mice was 28% heavier (40.24±0.95 vs. 31.28±0.26 g; P<0.01, n=8–10) (Fig. 5B). In contrast, the body weights of FKBP12.6−/− and WT mice given the control diet were similar. As expected, administration of an HF diet to WT mice significantly increased their body weight (P<0.05) and plasma cholesterol concentration (7.93±0.11 and 3.42±0.11 mM, respectively; P<0.01, n=8–10).
Figure 5.
FKBP12.6−/− mice develop obesity after an HF diet. A) Representative male FKBP12.6−/− mice and control littermates fed an HF diet for 5 mo. B, C) Body weight of FKBP12.6−/− mice (B) and WT control mice (C) fed control or HF diets. WT and FKBP12.6−/− mice fed control or HF diets were weighed 1×/mo during the course of the experiment. Diets were started when mice were 1 mo old. Data are means ± se from mo 1, 4, and 8 for WT [control, n=10; HF diet (HFD), n=10] and KO (control, n=10; HFD, n=8). *P < 0.05; **P < 0.01.
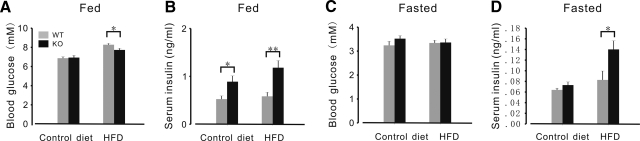
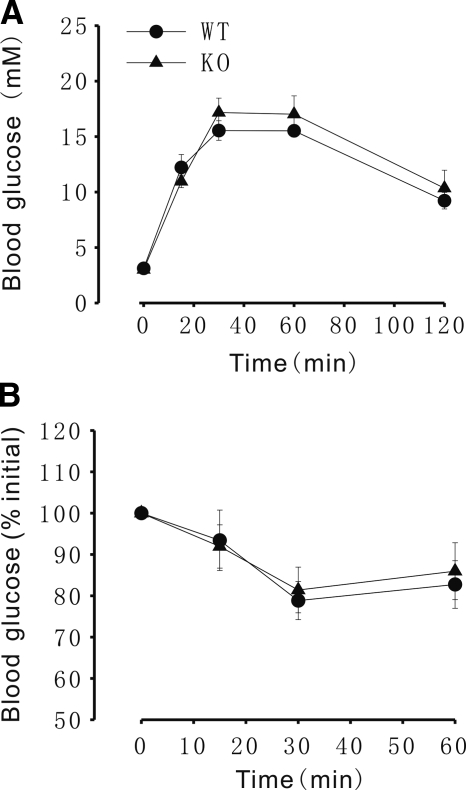
We also measured insulin and blood glucose concentration in FKBP12.6−/− and WT littermates after mice had been fed an HF diet for 4–5 months. There were no significant differences in feeding or fasting blood glucose concentrations between WT and FKBP12.6−/− mice fed a control diet (Fig. 6C). However, FKBP12.6−/− mice fed an HF diet exhibited significantly lower feeding blood glucose concentrations than WT mice (7.73±0.17 and 8.28±0.13 mM, respectively; P<0.05, n=8–10; Fig. 6A), though glucose tolerance (Fig. 7A), insulin sensitivity (Fig. 7B), and fasting blood glucose concentrations were similar between these two types of mice (Fig. 6C). Although the fasting blood concentrations were similar between the control and HF diet groups, the feeding blood glucose concentrations were significantly higher in both FKBP12.6−/− and WT mice fed an HF diet than in those fed a control diet (7.73±0.168 and 6.93±0.22, 8.28±0.134 and 6.867±0.153 mM, respectively; P<0.01, n=8–10). The serum insulin levels of feeding FKBP12.6−/− mice were higher than those of WT mice fed either the control diet or the HF diet (0.889±0.121 and 0.525±0.067, 1.181±0.148 and 0.585±0.085 ng/ml, respectively; P<0.05, n=8–10; Fig. 6B), and the serum insulin levels of food-deprived KO mice were also higher than those of WT mice fed an HF diet. However, fasting insulin concentrations were similar between FKBP12.6−/− and WT mice fed control diets (Fig. 6D). Although the number and size of the islets in FKBP12.6−/− and WT mice fed an HF diet were greater than those in mice fed a control diet, the number and size of islets in FKBP12.6−/− mice fed an HF diet were similar to those in WT mice fed an HF diet (3.38±0.31 and 3.6±0.41 islets/section of pancreas, respectively; P>0.05, n=3).
Figure 6.
Glucose homeostasis in 4- to 5-mo-old FKBP12.6−/− mice and WT control mice on control and HF diets. A, B) Blood glucose (A) and serum insulin levels (B) from feeding mice. C, D) Blood glucose (C) and serum insulin levels (D) from food-deprived mice. n = 7–8/group. *P < 0.05; **P < 0.01.
Figure 7.
Glucose tolerance and insulin sensitivity in 4- to 5-mo-old FKBP12.6−/− mice and WT control mice fed an HF diet. A) Glucose tolerance test. B) Insulin tolerance test. n = 5–6/group.
DISCUSSION
The results of the present study show that deletion of FKBP12.6 enhances glucose-stimulated insulin secretion mainly via increasing glucose-stimulated Ca2+ oscillation in islets. FKBP12.6−/− mice display feeding hyperinsulinemia and resistance to HF-diet-induced hyperglycemia. Our results have firmly established a direct role of FKBP12.6/RyR2 in regulating glucose-stimulated Ca2+ oscillation and GSIS in pancreatic islets.
The possible role of FKBP12.6/RyR2 in glucose signaling in pancreatic β cells has been reported in the literature (2, 3, 5,6,7). In the present study, we show that FKBP12.6−/− mice exhibited significant fed hyperinsulinemia (Figs. 1A and 6B) and higher insulin secretion after application of a high glucose load both in vivo and in vitro (Fig. 2). These results may be associated with enhanced high-glucose-induced islet Ca2+ release (Fig. 4), suggesting that deletion of FKBP12.6 leads to instability and sensitivity of RyR2 to stimulation by high concentrations of glucose. In contrast, FKBP12.6−/− mice showed normal fasting insulin levels (Fig. 2B), and similar basal insulin secretion from FKBP12.6−/− and WT isolated islets under low-glucose concentrations (3 mM) (Fig. 3B), suggesting that the RyR2 open probability is similar in FKBP12.6−/− and WT pancreatic β cells under these conditions. This is consistent with previous reports that have demonstrated that deletion of FKBP12.6 enhances stimulation-induced Ca2+ elevation in smooth-muscle (10, 11) and cardiac myocytes (9).
It has previously been reported that increases in the number, size, and/or insulin content of islets may lead to hyperinsulinemia (17). However, changes in islet morphology were not observed here in FKBP12.6−/− mice (Fig. 3A), and insulin content of the pancreas was similar for FKBP12.6−/− and WT mice (Fig. 3B), suggesting that deletion of FKBP12.6 does not affect islet morphology or insulin synthesis, but rather results in increased Ca2+ release.
Although FKBP12.6−/− mice showed feeding hyperinsulinemia, blood glucose levels were similar to those of the WT mice. Glucose tolerance and insulin sensitivity in 2-mo-old FKBP12.6−/− mice were not changed (Fig. 1C, D). Why does dose-enhanced insulin secretion not improve glucose tolerance? One possible explanation is that deletion of FKBP12.6 only enhances the second phase of insulin secretion (Supplemental Fig. 2), which is not sufficient to improve glucose tolerance.
Noguchi et al. (14) recently reported that deletion of FKBP12.6 impairs GSIS in another strain of FKBP12.6−/− mice, a result that is inconsistent with our finding that FKBP12.6−/− deletion enhances glucose-induced insulin secretion. There are several other important differences between our FKBP12.6−/− mice and the ICR strain, which they used. First, their mice showed normal serum insulin concentrations in both the fed and fasting states. In contrast, our mice displayed hyperinsulinemia in the fed state. Second, glucose intolerance was observed in their FKBP12.6−/− mice, but there was no significant difference in glucose tolerance between our FKBP12.6−/− and control mice. Third, RyR2 expression was significantly reduced in islets from their FKBP12.6−/− mice, but not in the islets from our animals (Supplemental Fig. 1). Although we have no appropriate explanation for these discrepancies, two important differences should be noted: different FKBP12.6 exons were replaced to generate the KO mice used in these two studies (exon3 in our FKBP12.6−/− mice and exon1 in the Noguchi et al. study; ref. 14); and different background strains of mice were used (129/Sv/Ev mice and ICR mice). This may contribute at least in part to the different results obtained in the two studies.
We also challenged FKBP12.6−/− mice with an HF diet. WT and FKBP12.6−/− mice fed an HF diet gained a significant amount of body weight during the treatment (Fig. 5). The body weight of KO mice was higher than that of WT mice after 3 mo on an HF diet (Fig. 5), and FKBP12.6−/− mice showed feeding hyperinsulinemia and lower blood glucose concentrations compared with WT mice (Fig. 6). These results indicate that the FKBP12.6−/− mice tolerated an HF diet better than WT mice. However, we did not observe improved glucose tolerance in KO mice (Fig. 7) than in WT mice fed an HF diet. This might be due to normal blood glucose concentrations in food-deprived HF-diet WT and KO mice (Fig. 6C, D). We noted that either WT or KO mice fed an HF diet did not show hyperglycemia under fasting conditions, although their nonfasting blood glucose concentrations were much higher than those given a control diet (Fig. 6). This may result from a different response of the 129 mouse strain to HF-diet-induced fasting hyperglycemia (18, 19).
CONCLUSIONS
FKBP12.6−/− mice display fed hyperinsulinemia and enhanced GSIS. These phenotypes may result from increases in islet Ca2+ responsiveness to glucose. FKBP12.6−/− mice also showed resistance to HF diet-induced hyperglycemia. Our results indicated that FKBP12.6 plays an important role in glucose-stimulated Ca2+ oscillation and GSIS. Furthermore, these mice might be useful models for understanding the role of intracellular Ca2+ stores in insulin secretion.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (007CB512100 to G.J.), the U.S. National Institutes of Health (HL45239 and DK65992 to M.K.), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-50 to G.J.), the National Foundation of Science and Technology (30770520 and 2009CB918701); and the 863 research program (2006AA02A106).
References
- Henquin J-C, Ishiyama N, Nenquin M, Ravier M A, Jonas J-C. Signals and pools underlying biphasic insulin secretion. Diabetes. 2002;51:S60–S67. doi: 10.2337/diabetes.51.2007.s60. [DOI] [PubMed] [Google Scholar]
- Islam M S. The ryanodine receptor calcium channel of β-cells: molecular regulation and physiological significance. Diabetes. 2002;51:1299–1309. doi: 10.2337/diabetes.51.5.1299. [DOI] [PubMed] [Google Scholar]
- Bruton J D, Lemmens R, Shi C-L, Persson-Sjogren S, Westerblad H, Ahmed M, Pyne N J, Frame M, Furman B L, Islam M S. Ryanodine receptors of pancreatic β-cells mediate a distinct context-dependent signal for insulin secretion. FASEB J. 2002;17:301–302. doi: 10.1096/fj.02-0481fje. [DOI] [PubMed] [Google Scholar]
- Empson R M, Galione A. Cyclic ADP-ribose enhances coupling between voltage-gated Ca2+ entry and intracellular Ca2+ release. J Biol Chem. 1997;272:20967–20970. doi: 10.1074/jbc.272.34.20967. [DOI] [PubMed] [Google Scholar]
- Gamberucci A, Fulceri R, Pralong W, Banhegyi G, Marcolongo P, Watkins S L, Benedetti A. Caffeine releases a glucose-primed endoplasmic reticulum Ca2+ pool in the insulin secreting cell line INS-1. FEBS Lett. 1999;446:309–312. doi: 10.1016/s0014-5793(99)00220-3. [DOI] [PubMed] [Google Scholar]
- Holz G G, Leech C A, Heller R S, Castonguay M, Habener J F. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic β-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7–37) J Biol Chem. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J D, Kuang S, Misler S, Polonsky K S. Ryanodine receptors in human pancreatic beta cells: localization and effects on insulin secretion. FASEB J. 2004;18:878–880. doi: 10.1096/fj.03-1280fje. [DOI] [PubMed] [Google Scholar]
- Wehrens X H, Lehnart S E, Huang F, Vest J A, Reiken S R, Mohler P J, Sun J, Guatimosim S, Song L S, Rosemblit N, D'Armiento J M, Napolitano C, Memmi M, Priori S G, Lederer W J, Marks A R. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Xin H B, Senbonmatsu T, Cheng D S, Wang Y X, Copello J A, Ji G J, Collier M L, Deng K Y, Jeyakumar L H, Magnuson M A, Inagami T, Kotlikoff M I, Fleischer S. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- Ji G, Feldman M E, Greene K S, Sorrentino V, Xin H-B, Kotlikoff M I. RYR2 proteins contribute to the formation of Ca2+ sparks in smooth muscle. J Gen Physiol. 2004;123:377–386. doi: 10.1085/jgp.200308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Feldman M, Doran R, Zipfel W, Kotlikoff M I. Ca2+-induced Ca2+ release through localized Ca2+ uncaging in smooth muscle. J Gen Physiol. 2006;127:225–235. doi: 10.1085/jgp.200509422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem. 1997;272:3133–3136. doi: 10.1074/jbc.272.6.3133. [DOI] [PubMed] [Google Scholar]
- Okamoto H. The CD38-cyclic ADP-ribose signaling system in insulin secretion. Mol Cell Biochem. 1999;193:115–118. [PubMed] [Google Scholar]
- Noguchi N, Yoshikawa T, Ikeda T, Takahashi I, Shervani N J, Uruno A, Yamauchi A, Nata K, Takasawa S, Okamoto H, Sugawara A. FKBP12.6 disruption impairs glucose-induced insulin secretion. Biochem Biophys Res Commun. 2008;371:735–740. doi: 10.1016/j.bbrc.2008.04.142. [DOI] [PubMed] [Google Scholar]
- Chen L, Koh D S, Hille B. Dynamics of calcium clearance in mouse pancreatic beta-cells. Diabetes. 2003;52:1723–1731. doi: 10.2337/diabetes.52.7.1723. [DOI] [PubMed] [Google Scholar]
- Kang L, He Z, Xu P, Fan J, Betz A, Brose N, Xu T. Munc-13–1 is required for the sustained release of insulin from pancreatic β-cells. Cell Metab. 2006;3:463–468. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Heit J J, Karnik S K, Kim S K. Intrinsic regulators of pancreatic β-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- Biddinger S B, Almind K, Miyazaki M, Kokkotou E, Ntambi J M, Kahn C R. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Almind K, Kahn C R. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.