Abstract
The physiology of two metabolites of vitamin A is understood in substantial detail: retinaldehyde functions as the universal chromophore in the vertebrate and invertebrate eye; retinoic acid regulates a set of vertebrate transcription factors, the retinoic acid receptor superfamily. The third member of this retinoid triumvirate is retinol. While functioning as the precursor of retinaldehyde and retinoic acid, a growing body of evidence suggests a far more fundamental role for retinol in signal transduction. Here we show that retinol is essential for the metabolic fitness of mitochondria. When cells were deprived of retinol, respiration and ATP synthesis defaulted to basal levels. They recovered to significantly higher energy output as soon as retinol was restored to physiological concentration, without the need for metabolic conversion to other retinoids. Retinol emerged as an essential cofactor of protein kinase Cδ (PKCδ), without which this enzyme failed to be activated in mitochondria. Furthermore, retinol needed to physically bind PKCδ, because mutation of the retinol binding site rendered PKCδ unresponsive to Rol, while retaining responsiveness to phorbol ester. The PKCδ/retinol complex signaled the pyruvate dehydrogenase complex for enhanced flux of pyruvate into the Krebs cycle. The baseline response was reduced in vitamin A-deficient lecithin:retinol acyl transferase-knockout mice, but this was corrected within 3 h by intraperitoneal injection of vitamin A; this suggests that vitamin A is physiologically important. These results illuminate a hitherto unsuspected role of vitamin A in mitochondrial bioenergetics of mammals, acting as a nutritional sensor. As such, retinol is of fundamental importance for energy homeostasis. The data provide a mechanistic explanation to the nearly 100-yr-old question of why vitamin A deficiency causes so many pathologies that are independent of retinoic acid action.—Acin-Perez, T., Hoyos, B., Zhao, F., Vinogradov, V., Fischman, D. A., Harris, R. A., Leitges, M., Wongsiriroj, N., Blaner, W. S., Manfredi, G., Hammerling, U. Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis.
Keywords: intermediary metabolism, pyruvate dehydrogenase complex, respiration, retinoid metabolism, signal transduction
As we approach the centennial of vitamin A’s discovery (1, 2) it is curious that despite much effort, the physiological significance of vitamin A itself, as opposed to that of the vitamin A metabolites, retinoic acid (3, 4) and retinaldehyde (5), has not yet been fully deciphered. One reason might be that the multiplicity of cellular and molecular targets of vitamin A (retinol) creates such complexity that a common reductionist approach has failed to yield the clearcut molecular cause-and-effect relationships required for a mechanistic understanding of its actions. Our laboratory discovered that the cRaf and protein kinase C (PKC) families of serine/threonine kinases harbor high-affinity retinol-binding sites in their regulatory domains, pointing to their involvement in signal transduction (6, 7). However, with 11 PKC and 3 Raf isoforms as candidate vitamin A target molecules, all potentially responding simultaneously to manipulations of the vitamin A status, the complexity of the problem has proved overwhelming, even when defined cell types were studied. Diverse responses were observed in vitamin A-deficient cells, e.g., depolymerization of actin filaments in fibroblasts (8) and depolarization of mitochondrial membranes in lymphocytes (9), but a common denominator underlying such changes has proved elusive. The most dramatic consequence of vitamin A deprivation in lymphocytes has been rapid, programmed cell death (10,11,12) due to an acute energy crisis precipitated by the malfunction of oxidative phosphorylation (13). These results highlight the mitochondria as novel targets of vitamin A action. Because signaling and biochemical events seemed conveniently sequestered to mitochondria, the opportunity arose to investigate retinol’s influence on oxidative phosphorylation in a more simplified setting. As we report here, this reductionist approach has yielded persuasive evidence for a positive role of vitamin A in the signal cascade modulating pyruvate dehydrogenase (PDH) activity and thereby mitochondrial function. Using both pharmacological and genetic approaches, we found that vitamin A acts as a cofactor enabling the activation of protein kinase Cδ (PKCδ) in mitochondria. The activated kinase in turn stimulated the PDH complex (14, 15) via still unidentified intermediates that increased the output of acetyl-coenzyme A (acetyl-CoA), resulting in higher oxygen consumption and ATP synthesis. These findings define a biologically relevant function of vitamin A not requiring prior activation of retinol by metabolic conversion. Vitamin A serves as a major signal activating the PKCδ isoform, up-regulating the PDH complex and thereby driving oxidative phosphorylation within the mitochondrion. Because mitochondria are ubiquitous to all nucleated cells, this connection to bioenergetics may explain the pleiotropic and largely unexplained pathologies (including immune deficiency (16) and male sterility (17) resulting from vitamin A deficiency that are not reversible by retinoic acid.
MATERIALS AND METHODS
Biological reagents
Rottlerin (18) and GO6976 were obtained from Calbiochem (San Diego, CA, USA). Phorbol myristoyl acetate (PMA) and phosphatase inhibitor cocktail were from Sigma-Aldrich (St. Louis, MO, USA).
The following Western blot antibodies were used: anti-PDHE1 and anti-VDAC (Invitrogen, Carlsbad, CA, USA); anti-PKCδ and anti-Tim23 (BD Biosciences, San Jose, CA, USA); anti-phospho-PKCδ (Thr505; Cell Signaling, Boston, MA, USA); anti-PDK1 (Assay Designs, Ann Arbor, MI, USA); anti-phospho-PDHE1 (Ser-293) and PDK3 (Novus Biologicals, Littleton, CO, USA). Antibodies to PDK2, PDK4, PDP1, and PDP2 were provided by R.A.H.
Mouse strains
C57BL/6 mice were from Jackson Laboratories (Bar Harbor, ME, USA). The LRAT−/− strain has been described (19, 20). Mouse embryo fibroblasts (MEFs) were derived from 13.5-d-old mouse embryos of C57Bl/6 mice or LRAT−/− mice. The PKCδ−/− MEF cell line was provided by one of us (M.L). Two-month-old male wild-type and LRAT−/− mice from a mixed C57Bl6/129sv genetic background were placed for 2 wk on vitamin A-deficient but otherwise nutritionally complete purified diet formulated to the AIN-93M recommendations. At this point the livers of the LRAT−/− mice contained no detectable retinol or retinyl ester, whereas the livers of wild-type mice contained retinol (54.7±12.8 nmol/g liver) and relatively high levels of retinyl ester. Livers of LRAT−/− mice maintained on the vitamin A-deficient diet for 2 wk were used for isolation of vitamin A-deficient mitochondria. A group of these mice were restored to vitamin A sufficiency by IP injection of 1 mg retinol dissolved in corn oil 3 h before harvesting their livers.
Cell culture and transfection
MEFs and Jurkat cells were grown in Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), l-glutamine, 1 mM pyruvate, and 4.5 g/L glucose. For vitamin A depletion, cells were incubated for 18 h in serum-free TLB medium (DMEM supplemented with 4.5 g/L glucose, 0.05% bovine serum albumin, 5 mg/L transferrin, 1 μM linoleic acid, and 2 mM glutamine).
The pBABE-puro and MigR1 retroviral, mammalian expression vectors were purchased from Addgene (Cambridge, MA, USA). The full-length wild-type PKCδ gene was inserted into pBABE-puro and MigR1 vectors by blunt-end ligation using DNA polymerase I (Klenow) from New England Biolabs (Ipswich, MA, USA). For viral assembly, the plasmids were transfected into 293T cells together with pCL packaging vector using FuGene reagent (Roche Diagnostic, Indianapolis, IN, USA) and grown in DMEM containing 10% FBS. Viral supernatants were harvested after 48 h and used to transfect PKC δ−/− MEFs using 8 mg/ml of polybrene. Stable PKC δ-expressing cell lines were derived by selection in puromycin (pBABE vector) or by FACS sorting for the marker gene, GFP. PKCδ expression was verified by Western blot. The retinol nonbinding mutant PKCδ was created by exchanging both the δC1a and δC1b domains with the αC1b domain. The mutant gene was cloned into the pBABE retroviral vector and expressed in PKCδ−/− MEFs as described above.
Measurement of oxidative phosphorylation in cells and isolated mitochondria
Intact cells (1×106) were used for O2 consumption measurements in an oxygraph equipped with a Clark electrode. Mouse liver mitochondria were isolated as described previously (21), and state III O2 consumption driven by specific respiratory chain complexes was measured on 75–100 μg of mitochondrial protein as described previously (22). All reagents were purchased from Sigma-Aldrich.
Pyruvate dehydrogenase activity was determined spectrophotometrically in isolated mitochondria (100–300 μg of protein) by measuring the increase in absorbance at 340 nm of a reaction medium containing 20 mM HEPES, 0.2 mM MgCl2, 0.05 mM CaCl2, 0.3 mM cocarboxylase, 0.5 mM NAD+, 1 mM DTT, 5 mM pyruvate, and 0.24 mM coenzyme A.
COX enzymatic activity was measured spectrophotometrically in isolated mitochondria (2–5 μg of protein) as described previously (23).
ATP synthesis in isolated mitochondria (15–25 μg of protein) or in cells permeabilized with digitonin (1×106 cells) was measured using the kinetic luminescence assay described by Vives-Bauza et al. (24).
Titration of retinoids
Dose-response relations of retinoids in intact cells were determined by incubating MEFs with graded concentrations of retinoid for 2 h, followed by measurement of ATP synthase activity. The optimum effect was observed at nominal concentrations of 2–4 μM.
MEFs were cultured in 5 μM retinol for 15 min or 18 h. Retinoids were extracted with butanol/acetonitrile and analyzed by reversed-phase high-pressure liquid chromatography on a C18 column as described previously (10, 25). Eluted retinol metabolites were identified by their UV spectra and mass spectrometry.
Demonstration that intermediary phosphatases are required
Isolated mitochondria were incubated in the presence of increasing concentrations of retinol in MAITE medium (10 mM Tris-HCl, pH 7.4; 25 mM sucrose; 75 mM sorbitol; 100 mM KCl; 10 mM K2HPO4; 0.05 mM EDTA; 5 mM MgCl2; and 1 mg/ml BSA) in the absence or presence of a cocktail of phosphatase inhibitors (Sigma-Aldrich) for 10 min.
Immunoblot analyses
To determine the phosphorylation levels of PKCδ and PDHE1 in MEFs, homogenates of cells or crude mitochondria (10 μg of protein) were separated by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE), electroblotted onto PVDF filters (Bio-Rad, Hercules, CA, USA), and immunoblotted with PKCδ and PDHE1 antibodies.
Analysis of the phosphoproteome of mitochondria
For isoelectric focusing of mitochondrial samples, 100 μg of protein was processed with a Ready Prep 2D Cleanup kit (Bio-Rad) and resuspended in 125 μl of rehydratation buffer (Bio-Rad). Samples were applied to 3–10 or 7–10 IPG strips (Bio-Rad) and incubated overnight at room temperature. Isoelectric focusing and 2-D SDS-PAGE were run under standard conditions, and proteins were transferred onto a PVDF filters. Control samples were treated with calf intestinal phosphatase for 1 h at 37°C to dephosphorylate mitochondrial proteins.
PKC δ phosphotransferase activity
Isolated mitochondria (500 μg) were lysed in 200 μl lysis buffer (50 mM Tris HCl, pH 7.5; 150 mM NaCl; 2 mM EDTA; 1 mM EGTA; 1% Triton X-100; 25 μg/ml leupeptin; 25 μg/ml aprotinin; 1 mM PMSF; 1 mM sodium orthovanadate; 30 mM sodium fluoride; and 30 mM b-glycerophosphate). PKCδ was immunoprecipitated overnight and collected on protein G-sepharose beads (GE Healthcare, Piscataway, NJ, USA). The immunoprecipitates were washed 3 times in lysis buffer containing 0.5 M NaCl and 3 times with kinase buffer (20 mM Tris HCl, pH 7.5, and 10 mM MgCl2). The kinase reaction was performed in 20 μl kinase buffer containing 50 μM ATP, 500 μg/ml histone Type III-S (Sigma-Aldrich), and 5 mCi [γ-32P]ATP (6000 Ci/mmol; Perkin-Elmer, Wellesley, MA, USA) for 20 min at 37°C. After separation by SDS-PAGE on 7.5% gels, the top half containing PKC bands was transferred to the PVDF membrane and subjected to autoradiography, followed by Western blot analysis with PKC δ antibody; the bottom half containing histone was gel dried and exposed to autoradiography. Western blots and autoradiographs were quantified by densitometry using Quantity One software (Bio-Rad). Kinase activity values were normalized for the amount of PKCδ immunoprecipitated.
Statistical analyses
Comparisons between groups were made using 1-way ANOVA. Pairwise comparisons were made by post hoc Fisher PLSD test. Differences were considered statistically significant at P < 0.05. Data analyses were performed using the statistical program StatView (Adept Scientific, Bethesda, MD, USA). In all experiments, error bars indicate sd.
RESULTS
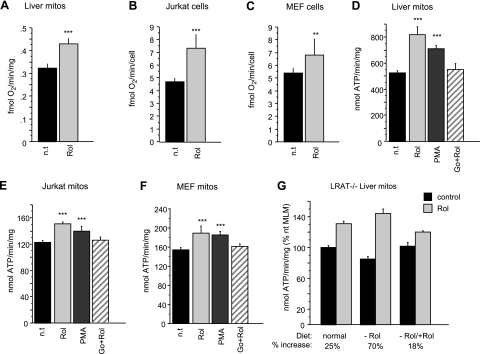
We previously demonstrated that cultured cells required retinol for controlled aerobic ATP synthesis, whereas retinol withdrawal caused an acute energy crisis (13). In this report we present our analysis of the underlying mitochondrial signal pathway involved in this response. Mitochondria isolated from mouse liver (MLM), Jurkat human T cells, and MEFs were tested in vitamin A-free medium or in medium supplemented with 2 μM all-trans retinol for 10 min. Pyruvate- and malate-dependent respiration was significantly lower in vitamin A-deficient compared to vitamin A-containing mitochondria (Fig. 1A). Intact Jurkat and MEF cells that were serum (and vitamin A) starved (26) displayed similarly reduced respiration, which increased 1.6- or 1.3-fold, respectively, after addition of 4 μM retinol for 2 h (Fig. 1B, C). The rates of ATP synthase activity paralleled those of oxygen consumption. Isolated mitochondria of mouse liver, Jurkat, and MEF cells showed ATP synthase rates that were consistently increased after retinol addition (Fig. 1D–F).
Figure 1.
Retinol modulates respiration. A) Pyruvate/malate-dependent oxygen consumption in retinol-treated (Rol; 2 μM, 10 min) mouse liver mitochondria (MLM) is increased by 30% compared to untreated mitochondria (n=6) (n.t., not treated). B, C) Respiration in intact retinol-treated (4 μM, 2 h) Jurkat (B) or MEF (C) cells is increased by 60 and 30%, respectively, compared to nontreated cells (Jurkat cells, n=14; MEF cells, n=8). D–F) Pyruvate/malate-driven ATP synthase activity is increased in isolated MLM (D; n=12) or mitochondria of Jurkat cells (E; n=6) or MEF cells (F; n=6). Mitochondria were incubated for 10 min with 2 μM retinol, phorbol-myristoyl-acetate (PMA, 2 μg/ml), or the combination of Go6976 (5 μM) and retinol (2 μM). Retinol increased ATP synthesis in the 3 systems by 55% (MLM), 25% (Jurkat mitochondria), and 23% (MEF mitochondria). Retinol effect was mimicked by PMA, but was neutralized by the PKC inhibitor, Go6976. G) Comparison of retinol-mediated up-regulation of ATP synthase activity of MLM isolated from mice with differing vitamin A nutritional status. LRAT−/− mice were maintained on vitamin A-deficient or -sufficient diets. One group of vitamin A-deficient mice was reconstituted by IP injection of vitamin A 3 h before harvesting liver. MLM were tested for ATP synthetic rate before and after incubation with 2 μM retinol in organello for 10 min. Average relative increases (%) were plotted. Retinol stimulated ATP synthase activity more profoundly in LRAT−/− mitochondria of vitamin A-deprived mice (70%) than those of deficient (25%), reconstituted LRAT−/−, or wild-type mice (not shown). **P < 0.001; ***P < 0.0001.
Liver cells, and to a lesser degree, MEFs and T cells, store retinyl esters (11). To avoid interference by retinyl esters, or retinoids derived from the latter, we tested mitochondria from mice with genetic ablation of the lecithin:retinol acyl transferase (LRAT) gene, incapable of retinyl-ester synthesis and storage (19). LRAT−/− mice were maintained either on vitamin A-free or vitamin A-sufficient diets. One group of vitamin A-deficient mice was restored to sufficiency by intraparenteral injection of vitamin A 3 h prior to isolating their liver mitochondria. The relative responsiveness, as measured by the ratio of ATP synthase rates before and after in vitro stimulation for 10 min with 2 μM retinol, was highest in the stringently retinol depleted group (70% relative increase), followed by LRAT−/− mitochondria of mice maintained on retinol-sufficient diet (25% increase) and mice after short-term restoration of vitamin A sufficiency (Fig. 1G). The fact that the baseline levels of oxidative phosphorylation capacity dropped significantly during vitamin A deprivation but was restored to normal promptly after retinol administration suggested a true physiological function of vitamin A.
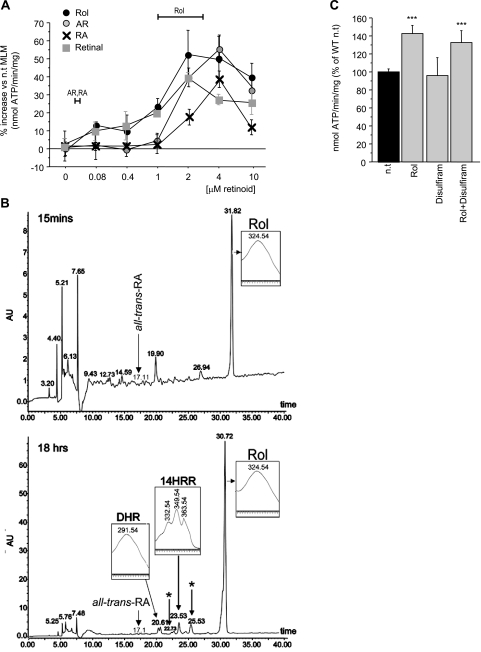
It is commonly assumed that, except for vision, vitamin A effects are mediated by the major retinol metabolite, retinoic acid, and that nuclear receptor-dependent gene transcription is the principal mechanism underlying vitamin A action (3, 4). Because retinol influenced the performance of isolated mitochondria, and because this effect occurred in a mere 10 min, transcriptional transactivation was an unlikely explanation. We argued, therefore, for a role of retinol in signal transduction. We needed to show that retinol was the only physiologically relevant retinoid to fulfill this function. While in a pharmacological setting retinal, retinoic acid and anhydroretinol were capable of up-regulating ATP synthase activity of isolated mitochondria with similar dose response characteristics as retinol (Fig. 2A), we found no evidence for their biosynthesis in mitochondria stimulated for 10 min with retinol (data not shown), nor did MEFs yield measurable amounts of retinol metabolites within 15 min (Fig. 2B, top panel). MEFs cultured for 18 h in the presence of 5 μM retinol produced the following nanomolar amounts of metabolites: two cis-trans isomers of 13,14-dihydroxy-retinol, 14-hydroxy-retro-retinol, an unidentified retro-retinoid, but no discernible amounts of retinal, retinoic acid, or anhydroretinol (Fig. 2B, bottom panel). Furthermore, the retinol-mediated increase in ATP synthase activity was not affected by disulfiram, a pharmacological blocker of retinal dehydrogenase (27), effectively excluding retinoic acid as potential response modifier (Fig. 2C). Given that enhancement of mitochondrial function required retinoid concentrations in the micromolar range, and that neither retinal, retinoic acid, anhydroretinol, nor any other retinol metabolite circulates in plasma, nor has ever been observed inside cells, at that level, whereas retinol does, it follows that retinol alone qualifies as response modifier of mitochondria.
Figure 2.
Capacity of natural retinoids to stimulate ATP synthase activity and their bioavailability in cells. A) Dose-response characteristics of retinol (Rol), retinal, retinoic acid (RA), and anhydroretinol (AR) are similar. However, only retinol bioavailability matches the dose optimum needed for synthase activation (horizontal bars). B) Retinoid profile, determined by high-pressure liquid chromatography. No retinol metabolites were found in MEFs incubated for 15 min with 5 μM retinol (top panel), whereas after 18 h, MEFs contained 13,14-dihydroxyretinol (DHR), 14-hydroxy-retro-retinol, and 2 unidentified retinoids (asterisk), but no discernible retinoic acid. Positive identification was by UV spectra (inserts) and mass spectrometry (not shown). C) Disulfiram does not block retinol-mediated ATP synthase stimulation.
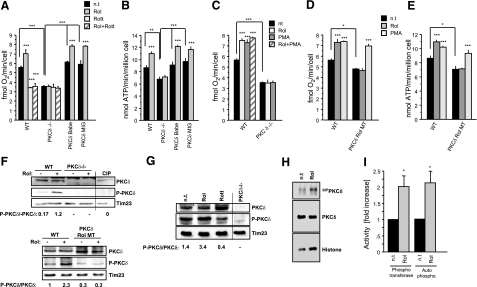
We previously reported that retinoids bind cRaf and PKC with high affinity (6, 7). Because several PKC isoforms localize to mitochondria, we tested the possibility that retinol engaged a PKC pathway. If this were the case, pharmacologic agonists of PKC were expected to mimic, and PKC antagonists to inhibit, the retinol effects. We found that the pan-PKC activator, phorbol-myristoyl-acetate, increased ATP output, whereas the specific PKC inhibitor, Go6976, neutralized the retinol enhancement effect (Fig. 1D–F). These findings suggested that activated PKC functioned as a key regulator of oxidative phosphorylation.
Three PKC isoforms have been physically and functionally associated with mitochondria, PKCδ, ε, and ζ (28, 29). We used pharmacological and genetic methods to identify the isoforms responsible for interaction with retinol in mitochondria. Among a panel of isoform-selective PKC inhibitors, rottlerin (18) was most potent in neutralizing the retinol effect (Fig. 3A), as measured by oxygen consumption. The identity of PKCδ so implicated was confirmed by measuring oxygen consumption and ATP synthase activity in MEFs derived from mice with germ-line deletions of PKCδ (30) (Fig. 3A, B). Although PKCδ−/− MEFs displayed reduced basal levels of respiration, the important observation was that these cells lost responsiveness to retinol, but regained it after complementation with retroviral vectors encoding full-length PKCδ (Fig. 3A, B). Because retinol effects were mimicked by PMA, it was important to show that PKCδ was the mediator in both cases. PKCδ−/− MEFs failed to show increased oxygen consumption when stimulated with either agent, nor were PMA and retinol effects additive in wild-type MEFs (Fig. 3C).
Figure 3.
PKCδ is a target for retinol action in mitochondria. A) Cellular respiration in wild-type (WT) MEFs (n=9), PKCδ−/−MEFs (n=9), or PKCδ−/− MEFs, where wild-type PKCδ was reintroduced by retroviral vectors, PKCδ rBabe (n=6), and PKCδ rMIG (n=6). Retinol (Rol) stimulated respiration in wild-type but not in PKCδ−/− MEFs. This stimulation was blocked by the PKCδ-specific inhibitor rottlerin (Rott). Retroviral reintroduction of full-length PKCδ in PKCδ−/− MEFs rescued responsiveness to retinol. B) Pyruvate/malate-driven ATP synthase activity in wild-type (n=10), PKCδ−/− (n=10), or PKCδ−/− reconstituted (n=6/cell line). Retinol stimulated ATP synthesis in wild-type but not in PKCδ−/− MEFs. Reintroduction of PKCδ in PKCδ−/− MEFs rescued the retinol response. C) Up-regulation of oxygen consumption was mimicked by PMA and was dependent on PKCδ. PMA and retinol effects were not additive. D, E) PKCδ requires intact retinol binding sites. PKCδ−/− MEFs reconstituted with full-length mutated PKCδ gene were unable to bind retinol (PKCδ Rol MT) and failed to restore responsiveness to retinol, as determined by respiration (D) and ATP synthase activity (E), respectively. F) Western blotting of wild-type MEF cell homogenates revealed that PKCδ was phosphorylated at Thr505 to higher degree when retinol was present, compared to its absence (top panel). MEFs expressing the retinol nonbinding mutant PKCδ did not yield appreciably phosphorylated PKCδ (bottom panel). Absence of immunoreactivity to PKCδ−/− MEF extracts indicated specificity of phosphoThr505-antibody reaction. Tim 23 was used as gel-loading control. Densitometry values of the intensity ratios P- PKCδ/ PKCδ are displayed beneath blots (n=3). G) PKCδ activation by retinol in isolated mitochondria. Stimulation of isolated wild-type mitochondria with retinol resulted in increased PKCδ phosphorylation. Rottlerin inhibited retinol-mediated PKCδ phosphorylation. PKCδ−/− MEFs are shown as specificity control. H) PKCδ kinase activity is stimulated by retinol. Wild-type MEFs were retinol deprived in serum-free medium. Aliquots were either left untreated or supplemented with 2 μM retinol. Mitochondria were isolated and lysed, and PKCδ activity was determined by immunoprecipitation/kinase assay (44). Phosphotransferase activity was revealed by PKC autophosphorylation (autoradiograph of top panel) and histone heterophosphorylation (autoradiograph of bottom panel). Images were analyzed by densitometry and normalized on amounts of PKC immunoprecipitated (Western blot of middle panel). I) Results from H presented as fold increases over basal PKC levels. One of 3 representative experiments is shown. *P < 0.01; **P < 0.001; ***P < 0.0001.
Although retinol was required for PKCδ activation, and although PKCδ harbors a binding site of 20 to 50 nM affinity for retinol in each of its 2 zinc-finger domains (7), it was necessary to show that occupancy of those sites was functionally important. We replaced the 2 PKCδ zinc-finger domains by the retinol nonbinding PKCα C1b domains (7) and expressed this chimeric molecule in PKCδ−/− MEFs. We failed to see enhanced oxygen consumption when tested for retinol responsiveness. However, the chimeric molecule responded to phorbol ester (Fig. 3D, E).
Classically, PKC is activated by the second messenger, diacylglycerol (31), but phospholipase C does not localize to mitochondria. Therefore, it has been suggested that mitochondrial PKC is activated by an alternate, oxidative stress pathway (32). Autophosphorylation of threonine residue 505 is commonly held to indicate kinase-competent PKCδ (33, 34). Using phospho-Thr505-specific antibody in Western blots, we confirmed that exposure of MEFs to retinol leads to PKC activation (Fig. 3F, G). Thr505 phosphorylation was not increased in MEF variants expressing retinol nonbinding PKCδ (Fig. 3F, bottom panel). That the entire signalosome was contained in mitochondria followed from experiments in isolated mitochondria showing that stimulation with retinol generated PKCδ kinase activity, leading to Thr505 phosphorylation. Blocking kinase activity with rottlerin abolished this effect (Fig. 3G). To further demonstrate that PKCδ converted to an active enzyme, phosphotransferase activity was analyzed with an extraneous substrate, histone, in PKCδ immunoprecipitated from mitochondrial extracts. As shown in Fig. 3H, I, mitochondria of resting, vitamin A-depleted MEFs contained less than half the phosphotransferase activity of those repleted with vitamin A. The autophosphorylation pattern of PKCδ (Fig. 3H, top panel) paralleled that of the phosphotransferase activity (Fig. 3H, bottom panel; I).
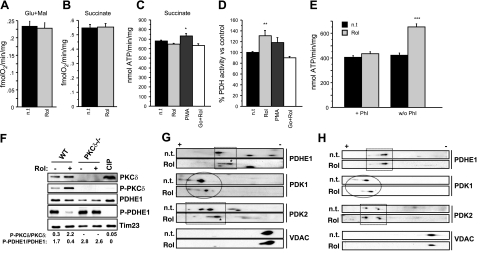
There are numerous points in the respiration pathway where PKC could theoretically have a regulatory effect, including the components of the electron transfer chain, enzymes of the tricarboxylic acid cycle, or the glycolytic pathways. We identified the PDH complex as the likely PKCδ target, based on the fact that pyruvate was required to drive retinol-dependent increases in oxygen consumption and ATP synthase rate (Fig. 1), whereas glutamate/malate and succinate were ineffective substrates (Fig. 4A–C). Because the PKC blocker Go6976 failed to prevent ATP synthase up-regulation, succinate appeared to bypass regulation by the PDH complex (Fig. 4C). Direct measurements revealed higher enzymatic activity of PDH in retinol-treated liver mitochondria compared to untreated controls (Fig. 4D), whereas we did not detect any increases in succinate dehydrogenase or COX activities (Supplemental Fig. 1).
Figure 4.
Retinol regulates PDH activity through PKCδ activation. A–C) Oxygen consumption driven by glutamate/malate (A) or succinate (n=6) (B), or ATP synthase activity driven by succinate (n=12) (C) in MLM were unchanged on retinol stimulation (2 μM, 10 min). D) PDH activity measured spectrophotometrically in MLM (n=9). Retinol stimulation increased PDH activity by 30%. Retinol effect was mimicked by PMA, but inhibited by Go6976. E) Enhanced pyruvate/malate-dependent ATP synthesis in MLM treated with retinol compared to untreated mitochondria was not observed when phosphatase inhibitors (Phi) were present, but was evident when Phi were absent (n=6). F) Western blots of MEF mitochondria revealed decreased PDHE1 phosphorylation as opposed to increased PKCδ phosphorylation at Thr505 when retinol was present in mitochondria of wild-type cells. Alkaline phosphatase treatment (CIP) of wild-type (WT) mitochondria was used to ascertain the specificity of the P-PKCδ and P-PDHE1 antibodies. Tim 23 was used as loading control. Intensity of bands was quantified by densitometry; ratio of phosphorylated/total products is displayed beneath blots (n=3). G, H) Two-dimensional gel electrophoresis revealed retinol-induced changes of phosphoproteome. Mitochondrial proteins were separated by IEF (7,8,9,10) followed by SDS-PAGE of wild-type (G) or PKCδ−/− (H) MEF cells. PDHE1 was less phosphorylated in wild-type retinol-treated than untreated (n.t.) mitochondria (G, top blot). PDHE1 phosphorylation state remained unchanged in PKCδ−/− mitochondria, when treated with retinol (H, top blot). PDK1 lost phosphate groups in both wild-type and PKCδ−/− mitochondria on retinol exposure (G, H; second blot from top). PDK2 reduced its phosphorylation state in retinol-treated wild-type mitochondria, compared to untreated wild-type mitochondria and retinol-treated or untreated PKCδ−/− mitochondria (G, H; third from top). VDAC was used as a reference marker to position the blots. (See also Supplemental Fig. 2.) Blots are representative of 3 independent experiments. *P < 0.01; **P < 0.001; ***P < 0.0001.
PDH is dually regulated by a set of 4 serine kinases: pyruvate dehydrogenase kinases (PDK) 1–4 (35, 36), and 2 serine phosphatases, pyruvate dehydrogenase phosphatase (PDP) 1 and 2 (37, 38). Phosphorylation of the E1 subunit of PDH by any of the 4 PDKs dampens acetyl-CoA production, whereas PDPs undo this negative regulation (14). The observed reduction in PDH enzyme activity demanded intervention by a phosphatase, not a kinase. This surmise was supported by findings that blocking phosphatases in intact cells with pharmacological inhibitors attenuated the retinol-induced increase in ATP synthesis (Fig. 4E). To obtain direct evidence that reduced PDH activity corresponded with a change in phosphorylation status, we probed the PDHE1 subunit by Western blot with a phospho-Ser-293-specific antibody. As expected, a marked decrease in PDHE1 phosphorylation occurred after retinol stimulation of wild-type MEFs, but not of PKCδ−/− MEFs (Fig. 4F). Note that phosphorylation patterns of PKCδ and PDHE1 are reciprocal. Independent confirmation of a reduction in phosphorylation was obtained by 2-dimensional gel electrophoresis. Mouse liver mitochondria treated with retinol, compared to those obtained from untreated mitochondria, revealed a shift to PDHE1 species with a higher isoelectric point (Fig. 4G), indicating decreased phosphorylation, consistent with the observed increase in PDH enzyme activity (Fig. 4D). When treated with calf intestinal alkaline phosphatase, the PDHE1 species with a low isoelectric point observed in untreated controls converted to reduced electronegativity, matching retinol-stimulated wild-type PDHE1 (Supplemental Fig. 2B). The shift in isoelectric point was therefore attributable to the loss of phosphate groups. The dephosphorylation of PDHE1, induced by retinol treatment, was seen in wild-type MEFs (Fig. 4G) but was not observed in PKCδ−/− MEFs (Fig. 4H), suggesting that PKCδ is instrumental for PDHE1 regulation. We probed for changes in phosphorylations of PDP1, PDP2, and PDK1–4 and identified among these decreased phosphorylations of PDK1 and PDK2, paralleling those of PDHE1 (Fig. 4G and Supplemental Fig. 2). As PDK2 dephosphorylation was dependent on PKCδ, whereas PDK1 dephosphorylation was independent (Fig. 4H), PDK2 emerged as possible downstream effector of retinol stimulation. A required phosphatase that is activated by PKCδ and dephosphorylates PDK2 has not been identified.
DISCUSSION
The accumulated evidence presented in Figs. 1234 indicates the existence of a signal pathway anchored by the complex of PKCδ and retinol that controls pyruvate utilization by the PDH complex. The capacity of retinol to prime PKCδ for activation by redox action is strongly supported by the evidence that retinol must be present in the medium and must physically bind PKCδ. Mutation of the retinol binding sites located in the zinc-finger domains (39) silences the PKCδ signal pathway. Of considerable interest, although at first glance counterintuitive, is the lack of selectivity of PKC for retinoids, allowing several natural retinol metabolites to act as coactivators as well. Their binding was predictable because the affinity of PKCδ zinc-finger domains for retinol, retinoic acid, and anhydroretinol was known to be similar in magnitude (7). However, functional selectivity is established by bioavailability, retinol being the only retinoid systemically available at the concentration needed to drive PKCδ activation. Retinol circulates in plasma at a constant concentration of 1 to 2 μM, matching the effective dose of 2 μM required for PKC coactivation. Other retinoids (e.g., retinoic acid) circulate at concentrations 3 orders of magnitude lower or not at all (anhydroretinol and retinal) (40). Further evidence that retinol is active in the PKCδ signal path, regardless of whether other retinoids can mimic retinol in pharmacological experiments, was the responses of isolated mitochondria in short-term experiments when reaction times were too brief to permit appreciable metabolism of retinol. In fact, no metabolites were detected in isolated mitochondria or MEFs incubated for 15 min with retinol (Fig. 2B). It is also important to recall that retinol functions as a survival factor (26, 41). In contrast, despite initial stimulation of the PDH complex, the exposure of cells to anhydroretinol for longer than 2 h causes a fatal energy crisis (13).
Whether cells are propagated in culture or harvested from the liver, they come from a vitamin A-rich environment. To minimize the carry-over of retinoids, we used the LRAT−/− mouse strain that lacks the capacity to store vitamin A and becomes rigorously depleted when maintained on a vitamin A-free diet. Mitochondria from the livers of such mice have abnormally low baseline levels of oxygen consumption, but up-regulate the PDH pathway within 3 h of supplementation with retinol in vivo or 10 min in organello. Nutritional supplementation of these mice with retinol markedly decreases the in vitro dependence of their mitochondria on retinol (Fig. 1G): the higher the likelihood that PKCδ is loaded with retinol in a vitamin A-rich environment, the lower the margin of up-regulation, and vice versa. Because the levels of responsiveness reflect the differences in vitamin A statuses of the animals from which mitochondria are harvested, a physiological capacity of retinol to regulate energy metabolism was suggested. Future experiments will explore the full physiological significance of vitamin A in the mitochondrion.
The biochemical role of retinol as PKC coactivator is not fully understood. In previous reports we suggested that retinol binding did not per se suffice for functional activation, but primed PKC for activation be oxidative stress (7). The activation of PKC by redox action has been reported (32). The generation of activated PKCδ in mitochondria was dependent on retinol and was demonstrable by autophosphorylation of Thr505 (32) and increased phosphotransfer capacity. Mitochondria are not known to produce diacyl glycerol (DAG). Furthermore, in Jurkat T cells with natural deletion of PLCγ, the principal source of DAG, mitochondrial energy regulation by retinol appears intact (unpublished results). We suggest that the PKCδ/retinol complex functions as redox sensor, actuating a feed-forward loop for the adjustment of fuel flux through the Krebs cycle. The presented data are consistent with regulation of acetyl-CoA production by the PKCδ/retinol signal system.
Although firm evidence points to the PDH complex as the target of PKCδ, it is unlikely to be the direct substrate. We hypothesize that PKCδ activates a phosphatase, the nature of which is still elusive. Our analysis of retinol-induced changes in the phosphoproteome of mitochondria excluded both PDP1 and 2 as targets (Supplemental Fig. 2A), but revealed that PDK1 and PDK2 lost phosphate groups, and hence became less active, in the course of cell activation by retinol (Fig. 4G, Supplemental Fig. 2A). Although reduced PDK activity explained the observed higher PDH activity of retinol-sufficient cells, loss of phosphorylation was inconsistent with the direct action of PKCδ on PDK1 and 2 and suggested instead the action of a phosphatase controlled by PKCδ. Because PDK2 dephosphorylation was not observed in PKCδ−/− MEFs, this isoform appears to be a legitimate indirect target of PKCδ (Fig. 4H). On the other hand, PDK1 lost phosphate groups independently of whether PKCδ was present (Fig. 4F, G), suggesting that the PDK1 axis is regulated by another, still to be identified, kinase/phosphatase pair.
CONCLUSIONS
Vitamin A and retinoic acid are frequently used as synonyms, although they are chemically different and have distinct physiological functions. We provide compelling evidence that vitamin A itself functions as a vital component in a novel mitochondrial signal pathway regulating oxidative phosphorylation. During the heyday of nutritional vitamin research, it did not go unnoticed that many pathophysiological manifestations of vitamin A deficiency in animals and humans could not be corrected with retinoic acid but required vitamin A to restore health. Although several organs were affected (42), no organelle or molecular targets were recognized until researchers who explored the potential of retinoids for apoptosis induction focused on the mitochondrion. Most often the proapoptotic or necrotic outcomes were linked to the extended production of damaging levels of reactive oxygen species (9, 13, 43). Although the clinical potential of synthetic retinoids for cancer therapy was recognized, receptor sites interacting with these synthetic retinoids remained obscure. Conceivably, these are the same as those identified for PKC and cRaf (6), which bind natural retinoids for growth regulation (25, 41, 44). But owing to differences in chemical structure, synthetic retinoids, such as the prototypic fenretinide (45), as well as the natural retinol antagonist, anhydroretinol (10), may be defective in the ability to coactivate PKCδ (7) and hence may interfere with catabolic or, more important, anabolic intermediary metabolism.
Bioenergetics is fundamental to all cells (46). In view of this tenet, it is puzzling why metabolic regulation by the pathway described in this report depends on retinol that vertebrates cannot synthesize de novo. In limiting vitamin A to nutritional sources, there must be an evolutionary advantage of such import as to override the physiological needs for vitamin A in vision and retinoic acid-dependent transcription. The answer may lie in the scenario that finite amounts of vitamin A are subject to depletion during periods of severe starvation when an organism is forced to conserve energy. Our observation that in the absence of vitamin A energy generation by respiration adapts downwards appears relevant in this context. Accumulation of triglycerides in the livers of vitamin A-deficient mice (47) may also signify a metabolic switch to fat for energy generation to offset limited utilization of pyruvate from glycolytic sources. It is also predictable that chronic deviations of vitamin A transport will lead to metabolic disease. Recent observations that the circulating levels of retinol binding protein 4, the major transporter of vitamin A in plasma, are elevated in obesity illuminate this point (48).
Supplementary Material
Acknowledgments
The authors thank Anatoli Starkov and Gregg Duester for helpful discussions and Ouathek Ouerfelli and Alice Cao for their expertise in analytical chemistry. This work was supported by grants from the National Institutes of Health: DK069384 (to U.H.); CA089362 (to U.H.); DK079221 (to W.S.B.); and K02NS047306 (to G.M.). R.A.-P. thanks the Spanish Ministry of Education for a Fulbright fellowship.
References
- McCollum E V, Davis M. The necessity of certain lipins in the diet during growth. J Biol Chem. 1913;15:167–175. [Google Scholar]
- Osborne T B, Mendel L B. The relation of growth to the chemical constitutents of the diet. J Biol Chem. 1913;145:311–326. [Google Scholar]
- Green S, Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988;4:309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Evans R M, Hollenberg S M. Zinc-fingers: guilt by association. Cell. 1988;52:1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Wald G. Molcular basis of visual excitation. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- Hoyos B, Imam A, Chua R, Swenson C, Tong G-X, Levi E, Noy N, Hammerling U. The cysteine-rich regions of the regulatory domains of Raf and protein kinase C as retinoid receptors. J Exp Med. 2000;192:835–845. doi: 10.1084/jem.192.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam A, Hoyos B, Swenson C, Chua R, Levi E, Viriya E, Hammerling U. Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J. 2001;15:29–30. doi: 10.1096/fj.00-0329fje. [DOI] [PubMed] [Google Scholar]
- Korichneva I, Hammerling U. F-actin as a functional target for retro-retinoids: a potential role in anhydroretinol-triggered cell death. J Cell Sci. 1999;112:2521–2528. doi: 10.1242/jcs.112.15.2521. [DOI] [PubMed] [Google Scholar]
- Korichneva I, Waka J, Hammerling U. Regulation of the cardiac mitochondrial membrane potential by retinoids. J Pharm Exp Ther. 2003;305:426–433. doi: 10.1124/jpet.103.048900. [DOI] [PubMed] [Google Scholar]
- Buck J, Grun F, Kimura S, Noy N, Derguini F, Hammerling U. Anhydroretinol: a naturally occurring inhibitor of lymphocyte physiology. J Exp Med. 1993;178:675–680. doi: 10.1084/jem.178.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger T M, Buck J, Hammerling U. Growth control or terminal differentiation: endogenous production and differential activities of vitamin A metabolites in HL-60 cells. J Exp Med. 1993;178:1995–2005. doi: 10.1084/jem.178.6.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M, Chua R, Hoyos B, Buck J, Chen C Q, Derguini F, Hammerling U. Retro-retinoids in regulated cell growth and death. J Exp Med. 1996;184:549–555. doi: 10.1084/jem.184.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H-J, Fischman D A, Hammerling U. Vitamin A-depletion causes oxidative stress, mitochondrial dysfunction and PARP-1-dependent energy deprivation. FASEB J. 2008;22:3738–3787. doi: 10.1096/fj.08-112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Korotchinka L. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- Patel M S, Roche T E. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4:3224–3233. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- Ross A C, Hammerling U. Retinoids and the immune system. Sporn M B, Roberts A B, Goodman D S, editors. New York: Raven Press; The RetinoidsBiology, Chemistry, and Medicine. (2nd ed.) 1994:521–544. [Google Scholar]
- Livera G, Rouiller-Fabre V, Pairault C, Levacher C, Habert R. Regulation and perturbation of testicula functions by vitamin A. Reproduction. 2002;124:173–180. [PubMed] [Google Scholar]
- Gschwend M, Muller H J, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- O'Byrne S M, Wongsiriroy N, Libien J, Vogel S, Goldberg I J, Baehr W, Palczewski K, Blaner W S. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg I J, Johnston T P, Li E, Blaner W S. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vizarra E, Lopez-Perez M J, Enriquez J A. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods. 2002;26:292–297. doi: 10.1016/S1046-2023(02)00034-8. [DOI] [PubMed] [Google Scholar]
- Hofhaus G, Johns D R, Hurko O, Attardi G, Chomyn A. Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber’s hereditary optic neuropathy. J Biol Chem. 1996;271:13155–13161. doi: 10.1074/jbc.271.22.13155. [DOI] [PubMed] [Google Scholar]
- Birch-Machin M A, Turnbull D M. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Yang L, Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 2007;80:155–171. doi: 10.1016/S0091-679X(06)80007-5. [DOI] [PubMed] [Google Scholar]
- Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by l4-hydroxy-retro-retinol. Science. 1991;254:1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- Buck J, Ritter G, Dannecker L, Katta V, Cohen S L, Chait B T, Hammerling U. Retinol is essential for growth of activated human B cells. J Exp Med. 1990;171:1613–1624. doi: 10.1084/jem.171.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Carmine A, Buervenich S, Duester G, Olsen L. Distribution of class I, III and IV alcohol dehydrogenase mRNAs in the adult rat, mouse and human brain. Eur J Biochem. 2003;270:1316–1326. doi: 10.1046/j.1432-1033.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- Pinton P, Leo S, Wieckowski M R, Di Benedetto G, Rizzuto R. Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol. 2004;2004:1–10. doi: 10.1083/jcb.200311061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P K, Mishra N C, Sun X, Bharti A, Kharbanda S, Saxena S, Kufe D. Targeting of protein kinase C δ to mitochondria in the oxidative stress response. Cell Growth Differ. 2001;12:465–470. [PubMed] [Google Scholar]
- Leitges M, Gimborn K, Elis W, Kalesnikoff J, Hughes M R, Krystal G, Huber M. Protein kinase C-δ is a negative regulator of antigen-induced mast cell degranulation. Mol Cell Bio. 2002;22:3970–3980. doi: 10.1128/MCB.22.12.3970-3980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase Cδ (PKCδ): activation mechanisms and functions. J Biochem. 2002;132:831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- LeGood J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Proteinkinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Gudi R, Bowker-Kinkley M M, Kedishvili N Y, Zhao Y, Popov K M. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989–28994. doi: 10.1074/jbc.270.48.28989. [DOI] [PubMed] [Google Scholar]
- Rowles J, Scherer S W, Xi T, Majer M, Nickle D, Riommens J M, Popov K M, Harris R A, Riebow N L, Xia J, Tsui L C, Bogardus C, Prochazka M. Cloning and characterization of PDK4 on 7q21.3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. J Biol Chem. 1996;271:22376–22382. doi: 10.1074/jbc.271.37.22376. [DOI] [PubMed] [Google Scholar]
- Bowker-Kinley M M, Davis W I, Wu P, Harris R A, Popov K M. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Gudi R, Wu P, Harris T A, Hamilton J, Popov K M. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem. 1998;273:17680–17688. doi: 10.1074/jbc.273.28.17680. [DOI] [PubMed] [Google Scholar]
- Hoyos B, Jiang S, Hammerling U. Location and functional significance of retinol binding sites on the serine/threonine kinase, cRaf. J Biol Chem. 2005;280:6872–6878. doi: 10.1074/jbc.M412695200. [DOI] [PubMed] [Google Scholar]
- Mao G E, Collinds M D, Derguini F. Teratogenicity, tissue distribution and metabolism of retinoids, 14-hydroxy-retro-retinol, and anhydroretinol in the C57BL/6J mouse. Toxicol Appl Pharmacol. 2000;163:38–49. doi: 10.1006/taap.1999.8828. [DOI] [PubMed] [Google Scholar]
- Garbe A, Buck J, Hammerling U. Retinoids are important cofactors in T cell activation. J Exp Med. 1992;176:109–117. doi: 10.1084/jem.176.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach S B, Howe P R. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Buck J, Derguini F. Anhydroretinol induces oxidative stress and cell death. Cancer Res. 1999;59:3985–3990. [PubMed] [Google Scholar]
- Hoyos B, Imam A, Korichneva I, Levi E, Chua R, Hammerling U. Activation of c-Raf kinase by ultraviolet light. J Biol Chem. 2002;277:23949–23947. doi: 10.1074/jbc.M110750200. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Chakravarti N, Oridate N, Choe C, Claret F-X, Lotan R. N-(4-Hydroxyphenyl)retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene. 2006;25:2785–2794. doi: 10.1038/sj.onc.1209303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis R J, Lum J J, Hatzivassiliou G, Thompson C B. The biology of cancer: metabolic programming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kang H W, Bhimidi G R, Odom D P, Brun P-J, Fernandez M-L, McGrane M M. Altered lipid catabolism in the vitamin A deficient liver. Mol Cell Endocrinol. 2007;271:18–27. doi: 10.1016/j.mce.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham T E, Mody N, Pretriner F, Peroni O D, Zabolotny J M, Kotani K, Kahn B B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type II diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.