Abstract
Recent studies have shown that the rate of aging can be modulated by diverse interventions. Dietary restriction is the most widely used intervention to promote longevity; however, the mechanisms underlying the effect of dietary restriction remain elusive. In a previous study, we identified two novel genes, nlp-7 and cup-4, required for normal longevity in Caenorhabditis elegans. nlp-7 is one of a set of neuropeptide-like protein genes; cup-4 encodes an ion-channel involved in endocytosis by coelomocytes. Here, we assess whether nlp-7 and cup-4 mediate longevity increases by dietary restriction. RNAi of nlp-7 or cup-4 significantly reduces the life span of the eat-2 mutant, a genetic model of dietary restriction, but has no effect on the life span of long-lived mutants resulting from reduced insulin/IGF-1 signaling or dysfunction of the mitochondrial electron transport chain. The life-span extension observed in wild-type N2 worms by dietary restriction using bacterial dilution is prevented significantly in nlp-7 and cup-4 mutants. RNAi knockdown of genes encoding candidate receptors of NLP-7 and genes involved in endocytosis by coelomocytes also specifically shorten the life span of the eat-2 mutant. We conclude that two novel pathways, NLP-7 signaling and endocytosis by coelomocytes, are required for life extension under dietary restriction in C. elegans.—Park, S.-K., Link, C. D., Johnson, T. E. Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans.
Keywords: CUP-4, longevity, regulation of aging, signal transduction, modulation of aging
The first evidence that limiting food consumption can retard aging and increase life span was obtained in rats (1). Since then, similar observations have been reported in a broad spectrum of model organisms. Dietary restriction (DR) is certainly the best studied and still the most commonly used method of retarding senescence and reducing mortality in mammals. In mice, a 40% decrease in food intake extends life span up to 43% (2). Diet-restricted mice also show increased resistance to external stressors, including heat and oxidative stress (3). Similar life-span extension by DR is observed in Drosophila melanogaster by reducing availability of live yeast (4) and by dilution of nutrients in the medium (5).
The life-extending effect of DR is also observed in the nematode Caenorhabditis elegans, where several distinct methods of imposing DR have been found. DR using decreased bacterial concentration causes life extension in C. elegans both in liquid culture and on solid plates (6, 7). Reduced bacterial concentration on agar plates by decreasing amount of bactopeptone (peptone) extends life span without reduction of reproductive capacity or body volume (6). eat mutants are a genetic model of DR in C. elegans and have a reduced pumping rate and increased life span (8). Worms grown in synthetic (axenic) medium in the absence of bacteria have slower development and a 2-fold increase in life span (9). Chemically defined liquid medium (CDLM) also increased the life span of C. elegans, with slowed development and reduced fecundity (10). Several laboratories have reported that complete starvation (with reproduction blocked) results in life extension (11,12,13) and that the heat-shock transcription factor hsf-1 is required for the longevity conferred by starvation (14). Despite intensive studies focused on the underlying mechanisms of DR, the molecular basis of the effects of DR on longevity remains unknown.
One common strategy in C. elegans is to look for genes that interact with and/or epistatically block this life extension (15). Greer et al. (16) reported that increased longevity with reduced bacterial concentration on solid plate was mediated by an AMP-activated protein kinase (AMPK) and dependent on DAF-16. The hypoxia inducible factor HIF-1, one of the downstream targets of the target of rapamycin (TOR) pathway in mammalian cells, mediates life extension by DR using bacterial dilution; a mutation in hif-1 extends the life span of worms grown in normal conditions, but fails to increase the life span of diet-restricted worms further (17). Recently, two transcription factors mediating DR-induced longevity of C. elegans have been reported. PHA-4, the worm ortholog of the human Foxa family of transcription factors, is required for multiple forms of DR response (18). PHA-4 is known to be required in embryogenesis for development of the pharynx (19). In adult worms, loss of pha-4 completely blocks the life-extending effect of DR but is not required for the longer life span of mutants having reduced insulin/IGF-like signaling (IIS) or reduced mitochondrial electron transport chain (ETC) activity (18). Bishop and Guarente (20) found that SKN-1 expressed in the two ASI neurons mediates the longevity resulting from bacterial-dilution-mediated DR in C. elegans. SKN-1 is a transcription factor that is critical in intestinal development (21). In adult worms, SKN-1 is expressed only in the intestine and in two ASI neurons; intestinal SKN-1 is required for the response to oxidative stress (22). In diet-restricted worms, SKN-1 is activated in the two ASI neurons that are critical in translating information about food availability into endocrine signals and also influence the dauer decision during development (23). The activation of SKN-1 in head neurons signals peripheral tissues to increase metabolic activity. The increased respiration rate observed under DR is dependent on SKN-1 (20). In addition, drugs inhibiting electron transport prevent DR-induced longevity increases (20). However, the downstream mechanisms by which SKN-1 mediates these DR-induced longevity increases are unknown.
Through genome-wide transcriptional profiling, we identified downstream targets of SKN-1 that are induced in response to oxidative stress (24). Among them, knockdown of two downstream targets of SKN-1, nlp-7 and cup-4, reduced the life span of wild-type N2 worms and lowered resistance to oxidative stress (24). Based on these findings, we hypothesized that NLP-7 and CUP-4 may function downstream of SKN-1 to modulate aging in C. elegans. Using RNAi knockdown and DR with bacterial dilution, we show that nlp-7 and cup-4 mediate DR-induced longevity. NLP-7 is a neuropeptide-like protein expressed in neurons (25), and CUP-4 is a ligand-gated ion channel essential for endocytosis by coelomocytes (26). We also find that other genes involved in NLP-7 signaling and endocytosis by coelomocytes are necessary for life extension by DR. This study suggests that neuronal signaling, involving NLP-7, and coelomocyte function are required for DR-induced longevity in C. elegans.
MATERIALS AND METHODS
Worm strains and generation of transgenic lines
The wild-type N2 CGCb strain was used as a control for all experiments. Two deletion mutants of nlp-7 (tm2984 and tm2990) were obtained from S. Mitani (Tokyo Women’s Medical University, Tokyo, Japan). The deletion mutant of cup-4(ok837) was constructed by the Gene Knockout Consortium. All strains were grown at 20°C on NGM plates (1.7% agar; 2.5 g/l peptone; 25 mM NaCl; 50 mM KH2PO4, pH 6.0; 5 μg/ml cholesterol; 1 mM CaCl2; 1 mM MgSO4) with fresh Escherichia coli OP50 as a food source. Synchronous populations of hermaphrodites were established by placing five young adults onto a fresh NGM plate and permitting eggs to be laid for 4 h at 20°C (27).
The vector pCL66 containing the promoter element of hsp-16.2 was used for the construction of the expression vector of nlp-7 or cup-4. pCL66 was constructed by replacing the KpnI/EcoRI GFP fragment of pCL25 (28) with the KpnI/EcoRI GFP variant fragment. The coding regions of nlp-7 and cup-4 were amplified using polymerase chain reaction (PCR) and inserted next to the hsp-16.2 promoter in pCL66 vector. We also amplified nlp-7 and cup-4 genes including a region 1-kb upstream from the start codon and inserted to the same vector. However, we failed to get a clone with cup-4 containing the 1-kb upstream region. Each expression vector (100 ng/μl) was coinjected to the gonad of young hermaphrodite N2 worms with pRF4 (dominant rol-6 marker). In addition to wild-type N2, CH1035, an N2 transgenic carrying pRF4, was included as a control in life-span assays.
Life-span assay
Longevity assessments were performed as described previously (24). All life-span assays were conducted on NGM plates containing 0.5 mg/ml 5-fluoro-2′-deoxyuridine at 20°C. Sixty age-synchronized hermaphrodites were picked 3 d after hatching and were transferred to a fresh plate daily during the egg-laying period. Thereafter, worms were transferred every 2–3 d until all worms were dead. An animal was scored as dead when it did not respond to mechanical stimulation. P values were calculated using the log-rank test. In transgenic worms carrying the target gene under the hsp-16.2 promoter, single or multiple heat shocks were used to induce expression. For single heat shock, plates were heated at 35°C for 2 h when worms were 3 d old. For multiple heat shocks, plates were heated at 35°C for 1 h every other day throughout the assay.
RNAi feeding
All RNAi clones studied were contained in the Ahringer RNAi library (29) and verified by sequencing. Worms were fed RNAi from larval stages unless otherwise stated. Five L4/young adult worms cultured on NGM plates were transferred to a fresh NGM plate containing 100 μg/ml ampicillin, 12.5 μg/ml tetracycline, 0.4 mM IPTG, and 0.5 mg/ml 5-fluoro-2′-deoxyuridine and spotted with bacteria expressing each double-strand RNA (dsRNA). After laying eggs for 4 h, all five adult worms were removed. Sixty young adults were picked 3 d after hatching and were transferred to a fresh plate with dsRNA expressing bacteria.
DR
Age-synchronized young adult worms grown on NGM plates seeded with OP50 bacteria were transferred to a new NGM plate containing 100 μg/ml ampicillin to inhibit bacterial growth and 0.5 mg/ml 5-fluoro-2′-deoxyuridine to prevent progeny hatching. Different concentrations of bacterial solution were made using serial dilutions from 1 × 1010 bacteria/ml. Life span was compared in bacterial concentrations of 1 × 1010, 5 × 109, 1 × 109, 5 × 108, 1 × 108 bacteria/ml. Bacteria culture (200 μl) of each concentration was spotted on small NGM plates (50 mm diameter), and 30 worms were grown on one plate. For quantitative RT-PCR assay, two different concentrations of OP50, 1×1010 and 5×108 bacteria/ml [ad libitum (AL) and DR groups, respectively], were used, and 2 ml of bacteria culture of each concentration was spread on large NGM plates (100 mm diameter).
Resistance to oxidative stress
To assess the resistance to oxidative stress, paraquat was used as an oxidative-stress inducer, as described previously (24). Age-synchronized young adult worms were transferred to NGM plates containing 20 mM paraquat and 0.5 mg/ml 5-fluoro-2′-deoxyuridine and spotted with OP50. Dead worms were counted 3×/d until all worms were dead.
Fertility assay
Single L4-stage worms grown at 20°C were transferred to fresh plates every day until they stopped laying eggs. All progeny plates were incubated at 20°C for 2 d, and the number of progeny developed was counted for every plate.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from ∼1000 worms using RNeasy Mini kit and RNase-free DNase (Qiagen, Valencia, CA, USA). Total RNA (1 μg) was then converted to cDNA using the Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Quantitative PCR was performed as described previously (24). SYBR Green 2X Mater Mix (Applied Biosystems, Foster City, CA, USA) was used for PCR reaction, and the real-time amplification was detected by ABI Prism 7000 Sequence Detection System (TaqMan) (Applied Biosystems). The primer sets used are shown in Supplemental Table S1. ama-1 was used as a control gene for normalization.
RESULTS
NLP-7 and CUP-4 are required specifically for DR-induced longevity
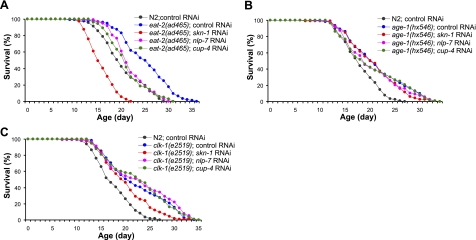
NLP-7 and CUP-4 have been shown to be required for normal life span and resistance to oxidative stress in wild-type N2 worms (24). We asked whether NLP-7 and/or CUP-4 are involved in other known pathways modulating life span in C. elegans. The eat-2(ad465) mutant, having reduced pharyngeal pumping rate and increased life span, is a much-studied genetic model of DR. RNAi knockdown of nlp-7 or cup-4 significantly reduced the extended life span of eat-2(ad465) (Fig. 1A and Supplemental Table S2). eat-2 lived 6 d longer than wild-type, and RNAi of both nlp-7 and cup-4 cut this life extension in half. However, the effect of RNAi of nlp-7 or cup-4 was smaller than that of skn-1 RNAi.
Figure 1.
Effect of RNAi of skn-1, nlp-7, or cup-4 on life span of long-lived mutants. A) Longevity of eat-2(ad465) mutant was significantly (P<0.01) suppressed by skn-1, nlp-7, or cup-4 knockdown. Mean life span of N2 and eat-2(ad465) was 19.3 and 24.1 d, respectively. Both mean and maximum life span of the eat-2(ad465) mutant were significantly shortened by RNAi of skn-1 (mean life span 14.8 d), nlp-7 (mean life span 20.9 d), or cup-4 (mean life span 20.2 d) (Supplemental Table S2). B, C) Life-span analysis of age-1(hx546) (B) and clk-1(e2519) (C) mutants fed with dsRNA of skn-1, nlp-7, or cup-4. Data are means of duplicates.
Reduced IIS or decreased mitochondrial ETC activity increases longevity. We examined the effect of RNAi of nlp-7 or cup-4 on age-1(hx546), a central component of IIS, and clk-1(e2519), a gene necessary for biosynthesis of ubiquinone in mitochondria. RNAi of both nlp-7 and cup-4 failed to block the life extension of both long-lived mutants (Fig. 1B, C and Supplemental Table S2). We thus conclude that both nlp-7 and cup-4 are required specifically for the life-extension effect of DR.
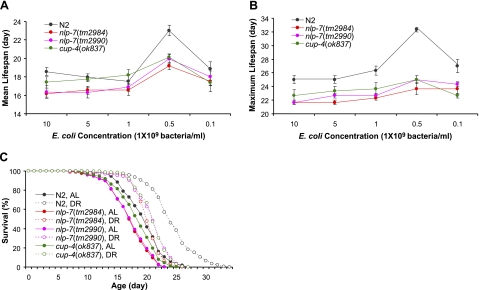
We then asked whether nlp-7 and cup-4 are involved in other DR life-extension models in C. elegans. We used bacterial dilution on solid plates (sDR) (16). Wild-type N2 worms fed with 5 × 108 bacteria/ml showed significant increases in both mean and maximum life span, compared to worms fed with 1 × 1010 bacteria/ml (Fig. 2 and Supplemental Table S3). Mean and maximum life span of N2 with DR (5×108 bacteria/ml) were significantly increased to 23.0 ± 0.6 d (mean±se, 24%) and 32.3 ± 0.3 d (29%), respectively, compared to those of N2 with AL feeding (1×1010 bacteria/ml) (18.6±0.4 d for mean life span and 25.0±0.6 d for maximum life span). In contrast, life span of mutants nlp-7(tm2984), nlp-7(tm2990), and cup-4(ok837) were increased only moderately by this sDR. Two deletion alleles of nlp-7 decreased the life span compared to wild-type control worms, and the life span of the cup-4(ok837) mutant was also shorter than that of N2 (Fig. 2). Under DR, only small increases of maximum life span of nlp-7 and cup-4 mutants (9–15%) were found. Mean life span was also extended partially by sDR in those mutants [19, 22, and 16% in nlp-7(tm2984), nlp-7(tm2990), and cup-4(ok837), respectively] (Fig. 2 and Supplemental Table S3).
Figure 2.
Effect of DR on life span using bacterial dilution. A, B) Serial dilution of OP50 showed significant increases in both mean (A) and maximum (B) life span of wild-type N2 worms. However, maximum life span of nlp-7 and cup-4 mutants was not affected by DR, and mean life span of these mutants was only moderately increased in worms with 5 × 108 bacteria/ml. Data are means ± se of 3 independent experiments. C) Life-span curves of N2, nlp-7(tm2984), nlp-7(tm2990), and cup-4(ok837) mutants were compared between AL (1×1010 bacteria/ml) and DR (5×108 bacteria/ml).
NLP-7 and CUP-4 work in the same signaling pathway
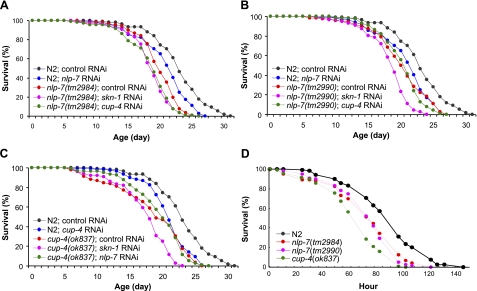
To determine the interaction of nlp-7, cup-4, and skn-1, we undertook an epistatic analysis using RNAi knockdown in genetic deletion backgrounds. Inactivation of cup-4 had no effect on life span of nlp-7(tm2984) or nlp-7(tm2990) mutants (Fig. 3A, B and Supplemental Table S4). RNAi of nlp-7 also failed to show a significant effect on life span of cup-4(ok837) (Fig. 3C and Supplemental Table S4). However, an additional decrease was found in both mean and maximum life span by skn-1 RNAi in nlp-7(tm2990) and cup-4(ok837) mutants (Fig. 3B, C and Supplemental Table S4). These findings indicate that NLP-7 and CUP-4 function in the same pathway under DR. Other SKN-1-dependent pathways might be activated by DR.
Figure 3.
Knockout of nlp-7 or cup-4 reduces life span and resistance to oxidative stress. A, B) Life span of nlp-7(tm2984) (A) and nlp-7(tm2990) (B) (P<0.01) decreased significantly compared with wild-type N2 worms. RNAi of cup-4 on nlp-7 mutants showed no effect on life span of either nlp-7 mutant. However, a further decrease in life span of nlp-7(tm2990) (B) by RNAi of skn-1 (Supplemental Table S5) was evident. C) Mean life span of cup-4(ok837) (17.5 d) was shorter than that of N2 (22.1 d). skn-1 RNAi (mean life span 16.3 d) further decreased the life span of cup-4(ok837), while nlp-7 RNAi had no effect on the life span of cup-4(ok837) (Supplemental Table S5). D) Paraquat-resistance assay of nlp-7(tm2984), nlp-7(tm2990), and cup-4(ok837). Resistance to oxidative stress caused by paraquat was reduced in nlp-7 or cup-4 mutants. Data are means of duplicates.
We also examined the resistance to oxidative stress of nlp-7 and cup-4 mutants. Paraquat (methyl viologen dichloride hydrate) was used as an oxidative-stress inducer. All mutant strains, nlp-7(tm2984), nlp-7(tm2990), and cup-4(ok837), showed significantly decreased resistance to oxidative stress compared to wild-type (Fig. 3D): The average mean survival of two independent experiments was 80 h in N2, 63 h in nlp-7(tm2984) and nlp-7(tm2990), and 54 h in cup-4(ok837) (Supplemental Table S5).
Overexpression of nlp-7 and cup-4
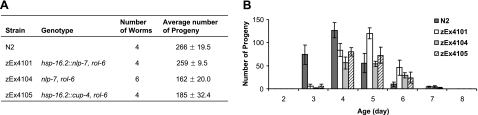
We next tested the effect of overexpression of nlp-7 or cup-4 on life span and resistance to oxidative stress. Three transgenic strains were generated; two expressed nlp-7 or cup-4 under the hsp-16.2 promoter and one expressed nlp-7 under its native promoter: zEx4101 (phsp-16.2::nlp-7, rol-6), zEx4105 (phsp-16.2::cup-4, rol-6), and zEx4104 (nlp-7, rol-6). The life span of zEx4101 was not different from that of wild-type N2. However, zEx4104 and zEx4105 had significantly reduced life span compared to wild-type N2 (Table 1). Single or multiple heat shock at 35°C was used to induce the expression of nlp-7 or cup-4 under the hsp-16.2 promoter in zEx4101 and zEx4105 but showed no significant difference in life span compared to non-heat-shock worms. zEx4101 exhibited no significant difference in resistance to oxidative stress, while zEx4104 and zEx4104 had decreased resistance to oxidative stress compared to wild-type controls (Table 1). Fertility showed the same pattern observed in life span and resistance to oxidative stress: No difference in zEx4101 and fewer progeny in zEx4104 and zEx4105 compared to wild-type N2, 162 ± 20.0 and 185 ± 32.4, respectively (Fig. 4A). Interestingly, all transgenic strains had a delayed egg-laying period compared to wild-type N2 (Fig. 4B). These results show that increased expression of nlp-7 or cup-4 causes deleterious effects on survival and fertility and suggest that nlp-7 and cup-4 are required for DR-induced life extension but cannot cause the DR response.
TABLE 1.
Life span and resistance to oxidative stress of nlp-7 orcup-4 transgenic worms
| Strain | Genotype | HS | Life span (d) | Resistance to oxidative stress (h)a |
|---|---|---|---|---|
| N2 | 18.4/18.5 | 59.1/56.6 | ||
| N2 | √ | 18.2b/19.0c | ND | |
| CH1035 | rol-6 | 18.3/17.1 | 53.9/49.8 | |
| zEx4101 | hsp-16.2::nlp-7, rol-6 | 17.1* /17.7 | 52.9/51.9 | |
| zEx4101 | hsp-16.2::nlp-7, rol-6 | √ | 16.9b/16.6c | ND |
| zEx4104 | nlp-7, rol-6 | 11.3* /13.2* | 36.9† /28.3† | |
| zEx4105 | hsp-16.2::cup-4, rol-6 | 15.6* /14.2* | 53.8† /49.0 | |
| zEx4105 | hsp-16.2::cup-4, rol-6 | √ | 13.4b*† /14.4c* | ND |
Data are expressed as means of experiment 1/experiment 2. HS, heat shock; ND, not determined.
Data expressed as mean survival.
Single heat shock of 2 h at 35°C on 3-d-old worms.
Heat shock of 1 h at 35°C every other day throughout the assay.
P< 0.05 vs. N2 with the same heat-shock treatment;
P< 0.05 vs. same strain without heat shock;
P < 0.05 vs. wild-type N2.
Figure 4.
Fertility of nlp-7 and cup-4 transgenic worms. A) Total number of progeny produced by each strain. Fertility of zEx4101 carrying nlp-7 under hsp-16.2 promoter was similar to that of wild-type N2. However, zEx4104 carrying nlp-7 with its native promoter and zEx4105 carrying cup-4 with hsp-16.2 promoter produced less progeny compared with wild-type N2. B) Time-course distribution of number of progeny produced. Compared to wild-type N2, the curve was shifted to left in all transgenic strains tested, which suggests delayed production of progeny in nlp-7 and cup-4 transgenic worms. Data are means ± se.
Effect of inhibition of NLP-7 signaling on life span
We asked whether additional genes involved in NLP-7 processing and signaling are also specifically required for the extended life span by DR using the eat-2(ad465) mutant. NLP7 is expressed as a proprotein and is processed by peptidase EGL-3 (30). The 122-aa NLP-7 proprotein contains four cleavage sites for the EGL-3 peptidase, which is specific for dibasic sites (KK, KR, RR, and RK). We tested the effect of knockdown of egl-3 on the life span of N2, eat-2, age-1, and clk-1. RNAi of egl-3 failed to affect the life span of all strains tested.
The C. elegans genome has two genes encoding putative NLP-7 receptors, ckr-1 and ckr-2 (31). CKR-1 is localized mainly to nerve ring neurons (32), and the anatomic expression pattern of CKR-2 in worms remains unknown. The knockdown of ckr-1 or ckr-2 using RNAi clones found in the Ahringer RNAi library significantly reduced the life span of eat-2 but not that of age-1 nor clk-1 (Table 2). These observations suggest that CKR-1 and CKR-2 are also specifically required for life extension under DR.
TABLE 2.
Effect of RNAi of genes involved in NLP-7 signaling or endocytosis by coelomocytes on life span of N2 and long-lived mutants
| RNAia | Function | Lifespan (d)
|
|||
|---|---|---|---|---|---|
| N2 | age-1(hx546) | clk-1(e2519) | eat-2(ad456) | ||
| EV | 18.4/19.3 | 21.9/21.9 | 22.1/24.4 | 26.9/26.4 | |
| nlp-7 | Neuropeptide-like protein 7 | 16.3* /17.3* | 20.6/22.9 | 22.8/24.1 | 24.5* /25.0 |
| egl-3 | Processing of NLP-7 | 18.7/20.1 | 24.6/24.1 | 22.8/25.5 | 27.1/26.3 |
| ckr-1 | Candidate receptor for NLP-7 | 17.2/18.3 | 21.2/21.4 | 21.9/24.5 | 24.7* /24.5* |
| ckr-2 | Candidate receptor for NLP-7 | 18.0/19.0 | 21.5/22.2 | 21.8/24.3 | 23.4* /25.0 |
| cup-4 | Coelomocyte-specific ion channel | 16.3* /17.7* | 21.0/22.2 | 21.7/24.1 | 25.8* /24.8* |
| lgc-26 | Coelomocyte-specific ion channel | 17.5/19.6 | 18.6* /22.2 | 23.1/24.9 | 24.1* /24.1* |
| rab-5b | Early endosome formation | 10.7* /ND | 10.7* /ND | 12.5* /ND | 14.6* /12.9* |
| chc-1b | Endocytosis by coelomocyte | 14.2* /ND | 16.3* /ND | 16.4* /ND | 23.7* /24.1* |
| cup-5 | Lysosome biogenesis | 17.4/18.5 | 21.4/22.3 | 22.5/23.9 | 25.1* /24.6* |
Data are expressed as means of experiment 1/experiment 2. ND, not determined.
Eggs were produced in RNAi plates.
RNAi was started from 3-d-old young adults.
P< 0.05 vs. EV control.
Knockdown of genes involved in endocytosis by coelomocytes
CUP-4 is a coelomocyte-specific ion channel involved in endocytosis (26). Many other proteins besides CUP-4 are known to be involved in endocytosis by coelomocytes. CHC-1, the C. elegans clathrin heavy chain ortholog (33), is involved in recruitment of the machinery for endocytosis to the endocytic site (26). LGC-26 is an ion channel expressed in coelomocytes and suggested to act together with CUP-4 to regulate coelomocyte endocytosis (26). RAB-5 activates endosome fusion (34), and CUP-5 is required for lysosome biogenesis in C. elegans (35). We tested the effect of RNAi of these four individual genes involved in different steps of endocytosis (lgc-26, rab-5, cup-5, and chc-1) on the life span of N2 and long-lived mutants. RNAi of lgc-26 or cup-5 showed a significant decrease in life span of eat-2 specifically, as did RNAi of cup-4 (Table 2): The life span of eat-2 was reduced significantly by RNAi of lgc-26 or cup-5, whereas the life span of age-1 or clk-1 was not affected. However, survival of all strains tested, including N2, was impaired significantly by the inactivation of rab-5 or chc-1. These results show that endocytosis by coelomocytes is required for DR-induced longevity in C. elegans.
nlp-7, cup-4, and pha-4 are induced under DR
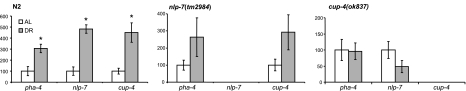
The expression of nlp-7 is induced under DR using a chemically defined axenic medium (10). Thus, we asked whether the expression of nlp-7 and cup-4 was altered in the sDR method of imposing DR. We also examined the expression level of pha-4 under sDR; PHA-4 is a transcription factor required for the increased longevity by DR but not by other genetic pathways including IIS and mitochondrial ETC (18). In adult worms, the expression of pha-4 is found in the intestine and is increased up to 80% in eat-2 mutants, compared with wild-type animals (18). We used Q-PCR to examine the expression of nlp-7, cup-4, and pha-4 in N2 under AL or DR conditions. The expression of all three genes increased significantly by sDR: 3.1-fold increase in pha-4, 4.8-fold increase in nlp-7, and 4.5-fold increase in cup-4 (Fig. 5A). We next asked whether induction of any of these genes was impaired in nlp-7(tm2984) or cup-4(ok837) under sDR. In nlp-7(tm2984), both pha-4 and cup-4 were induced by sDR, 2.6- and 2.9-fold, respectively. Surprisingly, we found no increase in expression of pha-4 and nlp-7 in response to sDR in cup-4(ok837) (Fig. 5B, C). Based on these findings, we conclude that CUP-4 acts upstream of PHA-4 and NLP-7 under sDR. Since RNAi of pha-4 completely blocked the effect of multiple forms of DR on longevity while RNAi of cup-4 or nlp-7 partially reduced the extended life span of eat-2(ad465), other mechanisms might activate PHA-4 under other DR conditions independent of CUP-4.
Figure 5.
Quantitative RT-PCR of pha-4, nlp-7, and cup-4. Relative expression of each gene under DR was calculated compared to the expression in AL (percentage of AL). ama-1 was used a control for data normalization. Values are means ± se; n = 5–6. *P < 0.05.
DISCUSSION
This study identifies two novel pathways mediating DR response in C. elegans. Previously, we identified two downstream targets of SKN-1 (nlp-7 and cup-4) that were required for normal longevity in C. elegans (24). RNAi of nlp-7 or cup-4 shortened the life span of the eat-2(ad465) mutant but had no effect on the extended life span of age-1(hx546) and clk-1(e2519) mutants. In addition, DR-induced longevity observed in wild-type N2 using bacterial dilution method is partially inhibited in nlp-7 and cup-4 mutants. These observations indicate that nlp-7 and cup-4 mediate DR-induced longevity in C. elegans but are not required for the extended life span by reduced IIS or decreased mitochondrial ETC activity. Since the knockout of skn-1 completely blocks the response to DR (20), other pathways should be activated by SKN-1 and required for DR-induced longevity besides the pathway involving nlp-7 and cup-4. Our observation of a further decrease in life span by skn-1 RNAi in both nlp-7 and cup-4 mutants supports this hypothesis.
NLP-7 is one of the neuropeptide-like proteins predicted from the genome sequence of C. elegans (36). However, little is known about the biological functions of different neuropeptides. Previous microarray data analysis revealed that expression of four NLPs, nlp-6, nlp-7, nlp-14, and nlp-16, were induced by oxidative stress, but the expression of only nlp-7 was dependent on SKN-1 activation (24). Life-span analysis with knockdown of these four nlp genes also shows that only inhibiting nlp-7 can specifically reduce the longevity of the eat-2(ad465) mutant (Supplemental Table S6). NLP-7 is expressed as a proprotein and processed by EGL-3 (30). Mature NLP-7 belongs to the MSFamide family characterized by a carboxy-terminal-SFamide motif (25). The mouse homologue of NLP-7 is likely to be cholecystokinin (CCK), which also ends in the MSFamide motif and is found in both the brain and the gastrointestinal tract (37). CCK plays a central role in initiating satiety and modulating food intake. CCK regulates food intake by releasing from enteroendocrine cells in response to the presence of lipid or protein in the gut postprandially (37). Exogenous administration of CCK decreases food intake in rats (38) and humans (39). However, mice lacking the gene for CCK have normal body weight, body mass, and fat absorption as compared with wild-type littermate controls (40); differences in longevity were not examined. Food-restricted rats showed increased plasma CCK, which suggests a physiological role for CCK in the adaptation to reduced food ingestion (41). Interestingly, a recent study demonstrated that the expression of nlp-7 is also up-regulated in long-lived C. elegans under DR (10). Two distinct receptor proteins, CCK1R and CCK2R, mediate the action of CCK in mice, and both receptors play a regulatory role in the control of food intake (42, 43). Two potential homologs of CCK receptors are found in the C. elegans genome, ckr-1 and ckr-2 (31). Recently, Janssen et al. (31) found good evidence that NLP-12 is a probable ligand for ckr-2, but they failed to find a ligand for ckr-1. Interestingly, RNAi knockdown of ckr-1 or ckr-2 also decreases the longevity of the eat-2 mutant significantly but has no effect on the life span of age-1(hx546) and clk-1(e2519) mutant. These results suggest that NLP-7 signaling in neurons and nerve ring play an important role in life extension under DR, possibly by means of the adaptation to restricted food intake in C. elegans.
In this study, we demonstrate that cup-4, a gene required for efficient endocytosis by C. elegans coelomocytes, is specifically required for DR-induced longevity. cup-4 encodes a protein that is homologous to ligand-gated ion channels (26). Another coelomocyte-specific ligand-gated ion-channel protein, LGC-26, is also required for endocytosis by coelomocytes and predicted to act with CUP-4 as a complex to regulate function of coelomocytes (26). CUP-5, a protein required for lysosome biogenesis, functions downstream of CUP-4 (26). We observe that knockdown of lgc-26 or cup-5 causes the same eat-2-specific decrease in life span as observed with RNAi of cup-4. These findings suggest that increased endocytosis by coelomoctyes is an essential mechanism for DR-induced longevity. Coelomocytes are the scavenger cells that continually and nonspecifically endocytose fluid from body cavity (26). Endocytosis is a basic function required for the internalization of fluid from the extracellular medium, nutrient uptake, and the recycling of membrane components. Our results raise the possibility that C. elegans coelomocytes may exert beneficial effects of DR through the activation of endocytosis. Under starved conditions, worms recycle their own proteins and organelles through a process called autophagy. Many recent studies have shown that autophagy is associated with the aging mechanisms in C. elegans. DR activates autophagy in C. elegans and RNAi of autophagy-associated genes, bec-1 and avsp-34, shortened the life span of eat-2 mutants (44). The longevity phenotype of daf-2 mutants also requires autophagy genes, such as bec-1, atg-7, and atg-12 (45, 46). In Drosophila, Atg7 (autophagy-related 7) mutants are hypersensitive to oxidative stress and short lived (47), while overexpression of Atg8a (autophagy-related 8a) in the brain increases the resistance to oxidative stress and extends the average life span up to 56% (48).
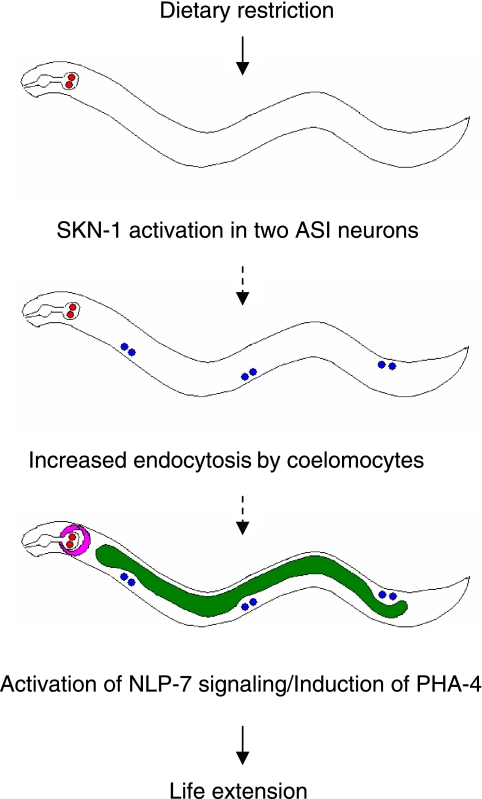
The knockdown of cup-4 in the nlp-7 mutant or vice versa does not reduce the life span of the mutant, suggesting that nlp-7 and cup-4 work in the same signaling pathway. Quantitative RT-PCR analysis reveals that cup-4 works upstream of nlp-7 under DR; DR in the nlp-7 mutant increases the expression of cup-4, while DR of the cup-4 mutant fails to induce nlp-7. The expression of pha-4, a Foxa transcription factor mediating DR-induced longevity in C. elegans (18), is also induced by DR in both N2 and nlp-7 but not in cup-4. Based on these findings, we propose a mechanism for signaling DR-induced longevity: SKN-1 in two ASI neurons senses DR and signals to coelomocytes to promote endocytosis. Increased endocytosis by coelomocytes, then, leads to the activation of NLP-7 signaling in neurons and the induction of pha-4 in the intestine, resulting in life-span extension in response to DR (Fig. 6). Future work identifying the mechanisms and other factors involved in increased endocytosis by coelomocytes and NLP-7 signaling under DR will be important for elucidating underlying mechanisms of DR for life extension.
Figure 6.
Possible mechanisms of DR-induced longevity in C. elegans. DR activates SKN-1 in two ASI neurons, which signals to coelomocytes to increase endocytosis required for proteins/organelle recycling under DR. Then, the neuronal signaling involving NLP-7, a worm ortholog of mammalian satiety factor, and the expression of PHA-4, a transcription factor mediating DR-induced longevity in C. elegans, are induced to extend life span in diet-restricted worms.
We have developed transgenic worms of nlp-7 or cup-4 and shown that the overexpression of nlp-7 or cup-4 failed to increase the life span. These findings suggest that both nlp-7 and cup-4 are necessary for DR-induced longevity but are not sufficient to cause increased longevity alone. Transgenic worms overexpressing nlp-7 with its native promoter showed decreased life span compared to N2. These animals also have reduced resistance to oxidative stress and produce less progeny than wild-type N2. Interestingly, the egg-laying period is also delayed in transgenic worms. However, overexpression of nlp-7 with hsp-16.2 promoter shows no effect on survival; no decrease in life span and no change in resistance to oxidative stress. The number of progeny produced by the transgenic worms overexpressing nlp-7 under hsp-16.2 promoter is also similar to that of wild-type N2. These findings suggest that neuronal overexpression of nlp-7 causes an increased sensitivity to stress and developmental defects in the germ line, leading to a reduced life span, but ectopic expression of nlp-7 has no effect on survival and fertility. In contrast, the ectopic overexpression of cup-4 causes detrimental effects on survival. Transgenic worms overexpressing cup-4 with the hsp-16.2 promoter show defects in resistance to oxidative stress and fertility and have shortened life span. However, we cannot rule out the possibility that overexpression of cup-4 in coelomocytes may have different effects on survival and fertility, since we studied only the effect of overexpression of cup-4 with the hsp-16.2 promoter but not with native promoter. The other possible interpretation for the reduced life span in transgenic worms is that the overexpression of nlp-7 or cup-4 alone may not be sufficient to elicit a life extension by mimicking the effect of DR and other genes may be also required for nlp-7 or cup-4 to extend life span. Our observation that RNAi of two putative receptors of nlp-7 and knockdown of lgc-26 and cup-5 also specifically decrease the life span of eat-2 supports this hypothesis.
We report the first findings about the involvement of coelomocytes and neuronal signaling of NLP-7 in DR-induced longevity. Further studies concerning the role of these pathways in different DR methods used in C. elegans and the relationship with other known DR-mediating proteins should help us understand the mechanism of DR effects on the aging process. It will be also interesting to test whether mammalian CCK signaling is required for DR-mediated longevity in mammals.
Supplementary Material
Acknowledgments
We thank the members of the T.E.J. and C.D.L. laboratories for helpful discussion. We also thank Shohei Mitani (Tokyo Women’s Medical University, Tokyo, Japan) for providing strains of nlp-7 deletion mutants. This research was supported by the U.S. National Institute on Aging (grant AG016219).
References
- McCay C M, Crowell M F, Maynard L A. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition. 1935;5:155–171. [PubMed] [Google Scholar]
- Sohal R S, Ku H H, Agarwal S, Forster M J, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal R S, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale A K, Leroi A, Kim S B, Rose M R. Phenotypic plasticity and selection in Drosophila life history evolution. 1. Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–193. [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Hosono R, Nishimoto S, Kuno S. Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp Gerontol. 1989;24:251–264. doi: 10.1016/0531-5565(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Klass M R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman B P, Johnson T E, Vanfleteren J R. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Szewczyk N J, Udranszky I A, Kozak E, Sunga J, Kim S K, Jacobson L A, Conley C A. Delayed development and life span extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J Exp Biol. 2006;209:4129–4139. doi: 10.1242/jeb.02492. [DOI] [PubMed] [Google Scholar]
- Cypser J R, Tedesco P, Johnson T E. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein T L, Smith E D, Tsuchiya M, Welton K L, Thomas J H, Fields S, Kennedy B K, Kaeberlein M. Life span extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Lee G D, Wilson M A, Zhu M, Wolkow C A, de Cabo R, Ingram D K, Zou S. Dietary deprivation extends life span in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus K A, Smith E D, Davis C, Carr D, Pendergrass W R, Sutphin G L, Kennedy B K, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T E, Friedman D B, Foltz N, Fitzpatrick P A, Shoemaker J E. Genetic variants and mutations of Caenorhabditis elegans provide tools for dissecting the aging processes. Harrison D E, editor. Caldwell, UK: Telford Press; 1990:101–126. [Google Scholar]
- Greer E L, Dowlatshahi D, Banko M R, Villen J, Hoang K, Blanchard D, Gygi S P, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Thomas E L, Kapahi P. HIF-1 modulates dietary restriction-mediated life span extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski S H, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Mango S E, Lambie E J, Kimble J. The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development. 1994;120:3019–3031. doi: 10.1242/dev.120.10.3019. [DOI] [PubMed] [Google Scholar]
- Bishop N A, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bowerman B, Eaton B A, Priess J R. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992;68:1061–1075. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- An J H, Blackwell T K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C I, Horvitz H R. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Park S K, Tedesco P M, Johnson T E. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8:258–269. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathoo A N, Moeller R A, Westlund B A, Hart A C. Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc Natl Acad Sci U S A. 2001;98:14000–14005. doi: 10.1073/pnas.241231298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton A, Knuth S, Schaheen B, Dang H, Greenwald I, Fares H. Endocytosis function of a ligand-gated ion channel homolog in Caenorhabditis elegans. Curr Biol. 2005;15:1045–1050. doi: 10.1016/j.cub.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J. Methods. Wood W B, editor. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; The Nematode Caenorhabditis elegans. 1988:587–606. [Google Scholar]
- Link C D, Cypser J R, Johnson C J, Johnson T E. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R S, Fraser A G, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman D P, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Husson S J, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- Janssen T, Meelkop E, Lindemans M, Verstraelen K, Husson S J, Temmerman L, Nachman R J, Schoofs L. Discovery of a cholecystokinin-gastrin-like signaling system in nematodes. Endocrinology. 2008;149:2826–2839. doi: 10.1210/en.2007-1772. [DOI] [PubMed] [Google Scholar]
- McKay R M, McKay J P, Suh J M, Avery L, Graff J M. Tripeptidyl peptidase II promotes fat formation in a conserved fashion. EMBO Rep. 2007;8:1183–1189. doi: 10.1038/sj.embor.7401086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener T, Grant B, Zhang Y, Wu X, Greene L E, Hirsh D, Eisenberg E. Caenorhabditis elegans auxilin: a J-domain protein essential for clathrin-mediated endocytosis in vivo. Nat Cell Biol. 2001;3:215–219. doi: 10.1038/35055137. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton R G, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh B M, Hartwieg E, Horvitz H R. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc Natl Acad Sci U S A. 2002;99:4355–4360. doi: 10.1073/pnas.062065399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitology. 2005;131:S109–S127. doi: 10.1017/S0031182005009376. [DOI] [PubMed] [Google Scholar]
- Moran T H. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16:858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young R C, Smith G P. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Kissileff H R, Pi-Sunyer F X, Thornton J, Smith G P. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr. 1981;34:154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- Lo C M, Samuelson L C, Chambers J B, King A, Heiman J, Jandacek R J, Sakai R R, Benoit S C, Raybould H E, Woods S C, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol. 2008;294:R803–R810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- Chowdhury P, Rayford P L. Effect of food restriction on plasma cholecystokinin levels and exocrine pancreatic function in rats. Ann Clin Lab Sci. 2001;31:376–382. [PubMed] [Google Scholar]
- Clerc P, Coll Constans M G, Lulka H, Broussaud S, Guigne C, Leung-Theung-Long S, Perrin C, Knauf C, Carpene C, Penicaud L, Seva C, Burcelin R, Valet P, Fourmy D, Dufresne M. Involvement of cholecystokinin 2 receptor in food intake regulation: hyperphagia and increased fat deposition in cholecystokinin 2 receptor-deficient mice. Endocrinology. 2007;148:1039–1049. doi: 10.1210/en.2006-1064. [DOI] [PubMed] [Google Scholar]
- Donovan M J, Paulino G, Raybould H E. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav. 2007;92:969–974. doi: 10.1016/j.physbeh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Chandra A, Mitic L L, Onken B, Driscoll M, Kenyon C. A role for autophagy in the extension of life span by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hars E S, Qi H, Ryazanov A G, Jin S, Cai L, Hu C, Liu L F. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3:93–95. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen E L, Hall D H, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Erdi B, Sass M, Neufeld T P. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007;21:3061–3066. doi: 10.1101/gad.1600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming R C, Brech A, Isakson P, Schubert D R, Finley K D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.