Abstract
Although the lipid mediator prostaglandin E2 (PGE2) exerts antifibrotic effects by inhibiting multiple fibroblast functions, its ability to regulate fibroblast survival is unknown. Here, we examined the effects of this prostanoid on apoptosis and apoptosis pathways in normal and fibrotic lung fibroblasts. As compared to medium alone, 24 h of treatment with PGE2 increased apoptosis of normal lung fibroblasts in a dose-dependent manner (EC50∼50 nM), as measured by annexin V staining, caspase 3 activity, cleavage of poly-ADP-ribose polymerase, and single-stranded DNA levels. PGE2 also potentiated apoptosis elicited by Fas ligand plus cycloheximide. These proapoptotic actions were dependent on signaling through the EP2/EP4 receptors and by downstream activation of both caspases 8 and 9. Silencing and gene deletion of PTEN demonstrated that the effects of PGE2 involved decreased activity of the prosurvival molecule Akt. PGE2 also down-regulated expression of survivin, an inhibitor of apoptosis, and increased expression of Fas. Fibroblasts from patients with pulmonary fibrosis exhibited resistance to the apoptotic effects of PGE2. These findings show for the first time that, in contrast to its effects on many other cell types, PGE2 promotes apoptosis in lung fibroblasts through diverse pathways. They provide another dimension by which PGE2 may inhibit, and perhaps even reverse, fibrogenesis in patients with interstitial lung disease.—Huang, S. K., White, E. S., Wettlaufer, S. H., Grifka, H., Hogaboam, C. M., Thannickal, V. J., Horowitz, J. C., Peters-Golden, M. Prostaglandin E2 induces fibroblast apoptosis by modulating multiple survival pathways.
Keywords: PTEN, survivin, Fas, Akt, idiopathic pulmonary fibrosis
A proper balance between cell survival and death is critical for maintaining normal tissue homeostasis, and many diseases are characterized by a disruption in this balance (1). For example, both excess epithelial cell apoptosis (2,3,4) and enhanced fibroblast survival (5,6,7,8) are believed to be central to the pathogenesis of pulmonary fibrotic disorders. While fibroblasts are critical to normal wound healing, their apoptosis is ultimately necessary to prevent excessive matrix deposition and scarring (9). Fibroblasts from patients with pulmonary fibrosis have been shown to exhibit resistance to apoptosis (10, 11). Thus, identifying mediators that modulate fibroblast survival may not only shed light on disease pathogenesis, but also provide targets for the development of novel therapies that may limit or even reverse the progression of this deadly disease. Although one approach is to interrupt the actions of mediators that promote fibroblast survival, such as transforming growth factor (TGF)-β, another approach is to augment pathways that promote fibroblast apoptosis.
Prostaglandin E2 (PGE2) is an antifibrotic lipid mediator derived from the metabolism of arachidonic acid by cyclooxygenase. Human idiopathic pulmonary fibrosis (IPF) has been shown to be characterized by a relative deficiency of PGE2 production (12,13,14) and responsiveness (15, 16), and the latter has also been observed in animal models of lung fibrosis (13). Elaborated from lung epithelial cells (17), fibroblasts (14), and macrophages (18, 19), PGE2 inhibits fibroblast proliferation (18, 19), collagen synthesis (20), migration (21, 22), and differentiation into myofibroblasts (23). However, PGE2 has also been shown to be a potent antiapoptotic/prosurvival mediator in normal epithelial cells (24, 25), cancer cells (26, 27), T cells (28, 29), and adipocytes (30). Whether PGE2 is also antiapoptotic in normal lung fibroblasts, or instead promotes apoptosis, which would be consistent with its other antifibrotic actions, is unknown.
The purpose of our study was to delineate the effects of PGE2 on lung fibroblast survival and the intracellular mechanisms that mediate them. Our results show for the first time that PGE2 promotes fibroblast apoptosis through activation of multiple apoptotic pathways. This is dependent on signaling through the E prostanoid (EP) 2 and 4 receptors and activation of phosphatase and tensin homologue on chromosome 10 (PTEN) with downstream inhibition of protein kinase B/Akt, a known prosurvival signaling mediator. Interestingly, fibroblasts from some patients with IPF are resistant to the proapoptotic effect of PGE2. These findings reveal another dimension by which PGE2 may act as an antifibrotic mediator and contrast with the well-recognized role of PGE2 as a prosurvival mediator in other cell types.
MATERIALS AND METHODS
Reagents
PGE2, PGD2, the prostacyclin analog iloprost, the thromboxane A2 analog U-4419, and the EP2-specific agonist butaprost free acid were purchased from Cayman Chemical (Ann Arbor, MI, USA). The specific caspase 8 inhibitor Z-IETD-FMK, caspase 9 inhibitor Z-LEHD-FMK, and camptothecin (CPT) were purchased from Calbiochem (San Diego, CA, USA). Activating antibody for the Fas (CD95) receptor (FasL/CD95L/APO-1) was obtained from Millipore (Temecula, CA, USA). Cycloheximide (CHX) was obtained from Sigma-Aldrich (St. Louis, MO, USA). The EP3-specific agonist ONO-AE3–248 and the EP4-specific agonist ONO-AE1–329 were generously provided by Ono Pharmaceuticals (Osaka, Japan). Antibodies for poly-ADP-ribose polymerase (PARP), Akt, phospho-Akt, PTEN, and survivin were obtained from Cell Signaling (Danvers, MA, USA); α-tubulin was obtained from Sigma-Aldrich.
Cell culture
Primary normal fetal lung fibroblasts (IMR-90) were obtained from the Coriell Institute for Medical Research (Camden, NJ, USA). Fibroblasts from patients with usual interstitial pneumonia (UIP), the characteristic histological abnormality of IPF, were grown from lung explants derived from biopsy specimens obtained during diagnostic evaluation. Normal adult control fibroblasts were grown from histologically normal margins of tissue resected for lung cancer and from cell lines (CCL201) obtained from American Type Culture Collection (Manassas, VA, USA). All patient-derived cells were isolated as described previously (31). This study was approved by the University of Michigan Institutional Review Board, with all patients providing informed consent. Murine embryonic PTEN−/− and embryonic C57BL/6 wild-type fibroblasts were isolated as described previously (21). All cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (Hyclone, Logan, UT, USA), and 1% penicillin/streptomycin (Invitrogen). Cells were studied between passages 5–10.
Annexin V-FITC/propidium iodine (PI) staining
Cells were treated for 24 h in serum-free medium under the indicated conditions. Detached cells were collected by centrifugation and combined with adherent cells; cells were then resuspended in Annexin V binding buffer (BD Biosciences, San Diego, CA, USA). Cells were then incubated with 5 μl of Annexin V-FITC (which binds to phosphatidylserine, present only on the outer membrane of apoptotic cells) and 5 μl of PI (which binds to DNA) for 15 min at room temperature. Cells were analyzed and quantitated by flow cytometry.
PARP cleavage
PARP is a target for cleavage by caspases, and increases in cleaved PARP are indicative of apoptotic cells. Both intact and cleaved PARP was detected by immunoblotting. Cells (5×105) grown in 6-well plates were treated under the indicated conditions for 24 h in serum-free medium before being collected in lysis buffer (phosphate-buffered saline with 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS). Any nonadherent cells were centrifuged and combined with the adherent cells for preparation of lysates. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and subjected to immunoblot analysis using anti-PARP antibody (1:1000).
Single-stranded DNA assay
Denaturation of DNA results in single-stranded DNA (ssDNA) that is selectively found in condensed chromatin of apoptotic cells. We measured ssDNA by immunoassay, as described previously (32). Cells (5×103) grown in 96-well plates were treated for 24 h in serum-free medium. DNA was then denatured with formamide, and ssDNA was detected by a monoclonal antibody (Chemicon, Billerica, MA, USA). After washing, the bound antibody was detected by ABTS chromophore (Calbiochem) and quantitated on an absorbance plate reader, with values corrected for cell count.
Caspase 3, 8, and 9 assays
Caspase 3 activity was measured in cell lysates using the Caspase-3 Fluorometric Assay Kit (Assay Designs, Ann Arbor, MI, USA), according to manufacturer’s protocol. All cells were treated under the indicated condition in serum-free medium for 24 h before being collected for assay. Caspase 8 and 9 activities were measured in lysates using the Caspase 8 and Caspase 9 Fluorometric Assay Kits (R&D Systems, Minneapolis, MN, USA), performed according to manufacturer’s protocol. All values were normalized for protein concentration.
PTEN siRNA
IMR-90 cells were transfected using Lipofectamine LTX (Invitrogen) with either 100 nM of PTEN siRNA (Cell Signaling) or nonspecific scrambled siRNA (Cell Signaling). Cells were studied at 48 h after transfection.
Immunoblotting
Proteins from cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting, as described previously (33). Antibodies for Akt, phospho-Akt, PTEN, survivin, and α-tubulin were used at 1:1000; antibody for Fas receptor was used at 1:500. Bound primary antibodies were visualized with appropriate secondary antibody conjugated to horseradish peroxidase and developed with enhanced chemiluminescence reagent (GE Healthcare, Piscataway, NJ, USA). Densitometric analysis was performed using Scion Image (U.S. National Institutes of Health, Bethesda, MD, USA).
Data analysis
Statistical analysis was performed using either ANOVA or Student’s t test, as appropriate, using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
PGE2 elicits a dose-dependent increase in fibroblast apoptosis
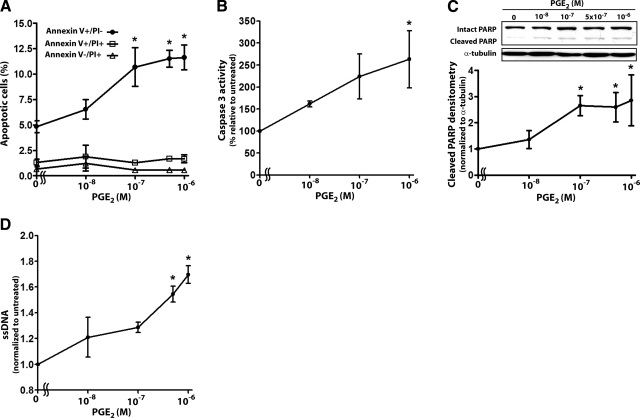
To determine the effects of PGE2 on lung fibroblast survival, we treated IMR-90 cells for 24 h with increasing doses of PGE2 (10–1000 nM) and measured cellular apoptosis by annexin V staining, caspase 3 activity, cleavage of PARP, and ssDNA levels (Fig. 1). In all assays, PGE2 elicited a dose-dependent increase in apoptosis (EC50∼50 nM), with maximal effects seen at 500–1000 nM. Vehicle control consisting of medium with 0.01% dimethyl sulfoxide (which corresponds to the amount of dimethyl sulfoxide in the 1000 nM PGE2 treatment condition) elicited no significant increase in apoptosis over that observed in untreated cells. PGE2 treatment resulted in an increase in annexin V+/PI− cells (early apoptosis), but no change in annexin V+/PI+ (late apoptosis/necrosis) or annexin V−/PI+ cells (necrosis).
Figure 1.
PGE2 dose-dependently increases fibroblast apoptosis. IMR-90 fibroblasts were treated for 24 h with varying concentrations of PGE2 and apoptosis was measured by annexin V/PI staining (n=3) (A), caspase 3 activity (n=3) (B), cleavage of PARP (n=4) (C), and ssDNA levels (n=3) (D), as described in Materials and Methods. *P < 0.05 vs. untreated cells.
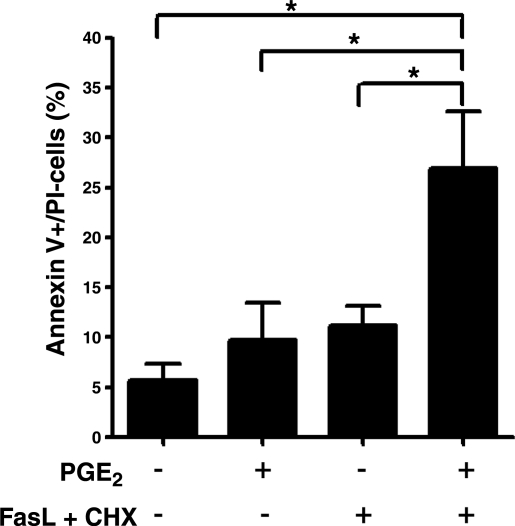
We next examined the effects of PGE2 on fibroblast survival in the presence of another apoptotic stimulus. FasL plus cycloheximide have been shown to induce fibroblast apoptosis, and impaired susceptibility to FasL-induced apoptosis has been observed in cells from patients with pulmonary fibrosis (10, 11). Combined treatment with PGE2 and FasL/cycloheximide led to more apoptosis than did either alone (Fig. 2). This finding shows that PGE2 augments fibroblast apoptosis in the presence of another known apoptotic stimulus.
Figure 2.
PGE2 potentiates FasL-induced apoptosis of fibroblasts. IMR-90 fibroblasts were treated in the presence or absence of PGE2 (0.1 μM) and FasL (100 ng/ml) plus CHX (0.5 μg/ml) for 24 h, and cellular apoptosis was measured by annexin V/PI staining (n=3). Results are expressed as the percentage of annexin V+/PI− (early apoptosis) cells. *P < 0.05.
PGE2 induces apoptosis via EP2-EP4 receptor signaling
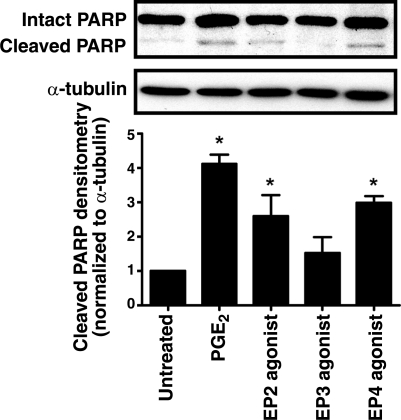
PGE2 can signal through 4 distinct G-protein-coupled EP receptors (EP1–4). In lung fibroblasts, the Gαs-coupled EP2 is the most abundantly expressed (33) and mediates the signaling necessary for inhibition of collagen synthesis, cell proliferation (33), and fibroblast migration (21). To determine which EP receptors are responsible for PGE2-induced apoptosis, we treated cells with specific EP2, EP3, and EP4 agonists. An increase in apoptosis was observed with agonists for both of the Gαs-coupled EP2 and EP4 receptors but not with the Gαi-coupled EP3 agonist (Fig. 3). Interestingly, among other prostanoids tested, PGD2, which is produced predominantly by mast cells and signals through the Gαs-coupled DP receptor, was also able to elicit apoptosis. A stable analog of thromboxane A2, which signals through the Gαi-coupled TP receptor, did not. Iloprost, a stable analog of prostacyclin, which signals through the Gαs-coupled IP receptor, elicited a degree of apoptosis that was slightly greater than untreated control cells. (Supplemental Fig. 1).
Figure 3.
PGE2 elicits apoptosis via EP2/EP4 signaling. IMR-90 cells were treated for 24 h with PGE2 (500 nM), the EP2-specific agonist butaprost free acid (BFA; 500 nM), the EP3-specific agonist ONO-AE3–248 (100 nM), or the EP4-specific agonist ONO-AE1–329 (100 nM), and apoptosis was measured by cleavage of PARP. Top panel: representative blot. Bottom panel: densitometric analysis of cleaved PARP relative to α-tubulin (n=3). *P < 0.05 vs. untreated control.
Both intrinsic and extrinsic pathways mediate PGE2-induced apoptosis
The development of apoptosis can proceed through various pathways (34). The intrinsic, or mitochondrial, pathway is activated under conditions of metabolic stress; cytochrome c is released from the mitochondria with activation of the apoptosome complex containing caspase 9, which leads to further cleavage of downstream effector caspases 3, 6, and 7 (35). The extrinsic, or death receptor, pathway involves the binding of extracellular signals (i.e., FasL, TNF-α) to death receptors (i.e., Fas), which, in turn, triggers the formation of the death-induced signaling complex, which leads to activation of caspase 8 (36, 37); activated caspase 8 further cleaves downstream effector caspases.
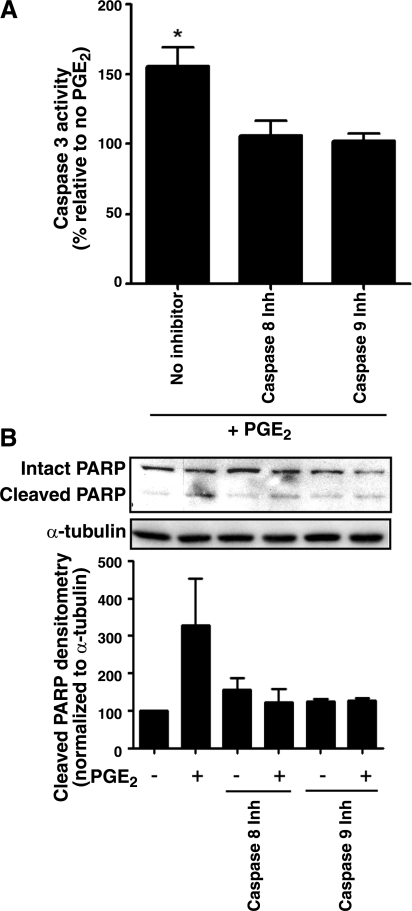
To determine which pathway might be responsible for PGE2-induced apoptosis, we first measured the activities of both caspases 8 and 9 in cells treated with PGE2. PGE2 induced a 25 ± 7.8% increase in caspase 8 activity and a 48 ± 10.9% increase in caspase 9 activity (P<0.05 for both). Furthermore, both the caspase 8 inhibitor Z-IETD-FMK and the caspase 9 inhibitor Z-LEHD-FMK (Fig. 4A, B), at concentrations used in other studies (38, 39), independently inhibited PGE2-induced apoptosis. These findings suggest that PGE2 requires activation of both caspase 8 and caspase 9 to induce fibroblast apoptosis.
Figure 4.
PGE2 promotes fibroblast apoptosis via both caspases 8 and 9. A) IMR-90 fibroblasts were treated with PGE2 (0.5 μM) in the presence or absence of the caspase 8 inhibitor Z-IETD-FMK (25 μM) or the caspase 9 inhibitor Z-LEHD-FMK (25 μM) for 24 h. Caspase 3 activity was measured as described in Materials and Methods; data are expressed as a percentage of activity relative to the no-PGE2 control (n=4). *P < 0.05. B) IMR-90 fibroblasts were treated with PGE2 (0.5 μM), Z-IETD-FMK (25 μM), and/or Z-LEHD-FMK (25 μM) for 24 h. Lysates were immunoblotted for cleaved PARP. Top panel: representative immunoblot. Bottom panel: densitometry of cleaved PARP normalized to α-tubulin (n=3).
PTEN is required for PGE2-induced apoptosis
PTEN is a lipid phosphatase that antagonizes the phosphatidylinositol-3-kinase (PI3K) pathway by dephosphorylating phosphatidylinositol 3,4,5-trisphosphate (PIP3) (40). PIP3 is a lipid mediator that recruits and activates proteins with pleckstrin homology domains such as Akt, which has been shown to be an important prosurvival mediator (41) that can inhibit both the intrinsic (42) and extrinsic (43) apoptotic pathways. Previous work has shown that PGE2 activation of PTEN, with subsequent loss of Akt activity, is responsible for PGE2 inhibition of fibroblast migration (21). We sought to determine whether this same pathway is responsible for PGE2-mediated apoptosis of fibroblasts.
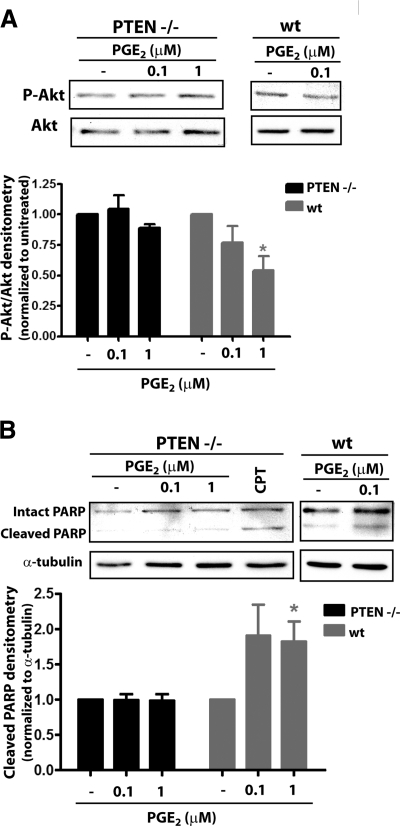
We first sought to examine the apoptotic effects of PGE2 in murine fibroblasts from embryonic PTEN−/− mice (PTEN−/− mice do not survive to gestation). As expected, PGE2 was unable to decrease Akt phosphorylation in PTEN−/− fibroblasts, in contrast to its ability to do so in wild-type embryonic fibroblasts (Fig. 5A). Unlike control wild-type embryonic fibroblasts, fibroblasts from PTEN−/− mice were likewise not susceptible to apoptosis by PGE2 (Fig. 5B). However, these cells were still susceptible to the alternative inducer of apoptosis, the topoisomerase inhibitor CPT. These findings suggest that activation of PTEN and subsequent inhibition of Akt is necessary for PGE2 to induce apoptosis.
Figure 5.
Diminished capacity for PGE2-induced apoptosis in PTEN−/− fibroblasts. PTEN−/− and wild-type (wt) murine embryonic fibroblasts were treated for 24 h with PGE2 at the indicated concentrations. Phosphorylated Akt (A) and cleaved PARP (B) were assayed by immunoblot analysis. Top panels: representative immunoblots. Bottom panels: relative densitometry normalized to untreated controls (wt, n=3; PTEN−/−, n=4). *P < 0.05.
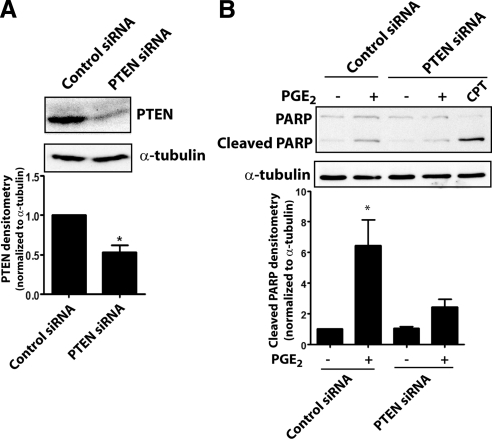
To confirm the importance of PTEN in PGE2-induced apoptosis, we also silenced PTEN expression in IMR-90 cells using siRNA (Fig. 6A). As with cells from PTEN−/− mice, fibroblasts treated with PTEN siRNA similarly failed to undergo apoptosis in response to PGE2 but were susceptible to cell death evoked by CPT (Fig. 6B). These findings show that PTEN is critical to PGE2-induced apoptosis in fibroblasts.
Figure 6.
PTEN silencing diminishes PGE2-induced apoptosis in fibroblasts. A) PTEN expression was assayed by immunoblot in cells treated with control or PTEN siRNA for 48 h. Top panel: representative blot. Bottom panel: mean densitometric data (n=4). *P < 0.05. B) PTEN siRNA- and control siRNA-treated cells were exposed to PGE2 (0.1 μM) for 24 h, and cleaved PARP was assayed by immunoblot. Top panel: representative blot. Bottom panel: densitometry of cleaved PARP/α-tubulin (n=3). CPT (2 μg/ml) was used as a positive control. *P < 0.05 vs. no-PGE2 treatment.
PGE2 decreases survivin expression and increases Fas receptor expression
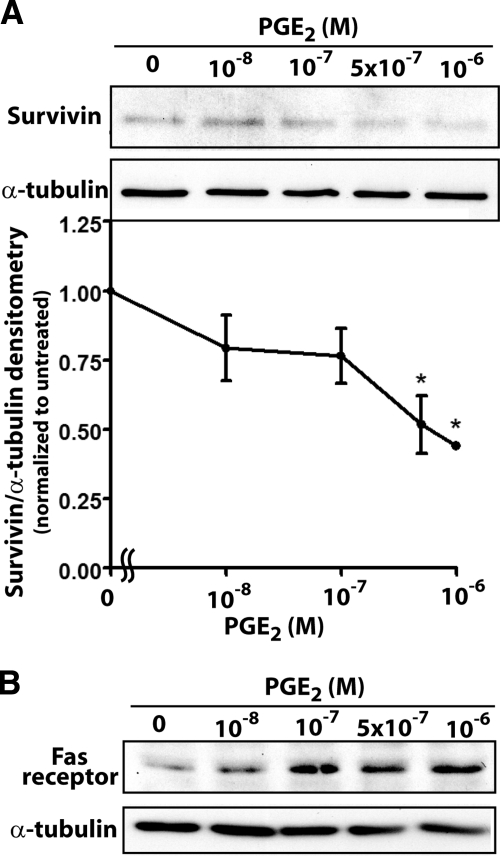
Akt activates multiple downstream pathways that are responsible for its prosurvival effects, including the induction of multiple antiapoptotic genes (41). These include the inhibitors of apoptosis proteins (IAPs) which directly inhibit caspases. Among the IAPs, survivin has been shown to be important in lung cancer (44) and colorectal cancer cells (45). Cancer cells exhibit increased survivin expression, which directly inhibits caspases (46). It is down-regulated by Akt signaling (47) and has been shown to be up-regulated by PGE2 in lung cancer cells (48). Because survivin is a target of both Akt and PGE2 signaling, we examined its expression in fibroblasts treated with PGE2. Survivin expression was dose-dependently inhibited by PGE2 (Fig. 7A), which is in contrast to the action of this prostanoid observed in cancer cells (48, 49).
Figure 7.
PGE2 modulates fibroblast expression of survivin and Fas receptor. IMR-90 fibroblasts were treated for 24 h with the indicated concentrations of PGE2, and survivin expression (n=3) (A) and Fas receptor expression (n=3) (B) were determined by immunoblot analysis. *P < 0.05 vs. no-PGE2 treatment.
Because Akt has also been shown to protect cells from the extrinsic apoptotic pathway (50) and because we found that PGE2 potentiates FasL-induced apoptosis, we also examined the effects of PGE2 on expression of the Fas receptor. PGE2 dose-dependently increased Fas receptor expression (Fig. 7B). Together, these data show that PGE2 can affect expression of multiple targets that all enhance fibroblast apoptosis.
Variable resistance to PGE2-induced apoptosis is observed in fibroblasts from patients with UIP
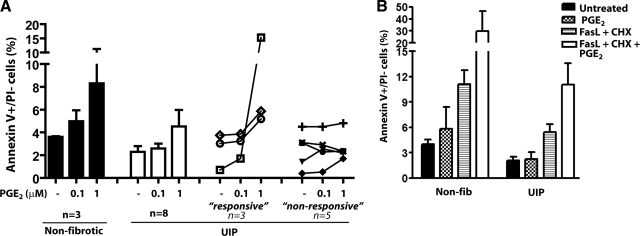
We examined the apoptotic response to PGE2 of fibroblasts from patients with UIP/IPF. Previous work in our laboratory has shown that in many of these patient-derived cell lines, fibroblasts are resistant to the suppressive effects of PGE2 on collagen expression and cell proliferation (16). Each of the three nonfibrotic fibroblast lines exhibited dose-dependent apoptosis in response to PGE2. By comparison, the group of 8 lines of UIP fibroblasts exhibited a relative degree of resistance to PGE2-elicited apoptosis (Fig. 8A). However, when the responses of individual UIP lines were examined, 3 lines exhibited dose-related apoptosis to PGE2, while 5 lines were mainly resistant. This variable response among different UIP fibroblast lines is consistent with the variable inhibitory effects of PGE2 on collagen expression and cell proliferation, which we observed previously (16).
Figure 8.
PGE2-induced apoptosis in adult control and UIP fibroblasts. A) Fibroblasts from the lungs of patients with UIP (n=8, white bars) and nonfibrotic controls (n=3, black bars) were treated with PGE2 for 24 h, and apoptosis was measured by annexin V/PI staining, with results expressed as the percentage of annexin V+/PI− (early apoptosis) cells. Responses of individual UIP lines are displayed at right, segregated within responsive and unresponsive groups. B) UIP (n=3) and nonfibrotic control (n=4) fibroblasts were treated with PGE2 (0.1 μM) ± FasL (100 ng/ml) + CHX (0.5 μg/ml) for 24 h. Cells were stained for annexin V and PI; percentage of annexin V+/PI− cells is shown.
Previous studies have also shown that UIP fibroblasts are resistant to FasL-induced apoptosis (10, 11), and data in Fig. 8B support this conclusion. In control cells, PGE2 potentiated the proapoptotic effects of FasL/cycloheximide; although this appeared to be the case in the UIP cell lines as well, the degree of potentiation was less than observed in normal cell lines (Fig. 8B). A summary of PGE2-mediated apoptotic signaling is shown in Fig. 9.
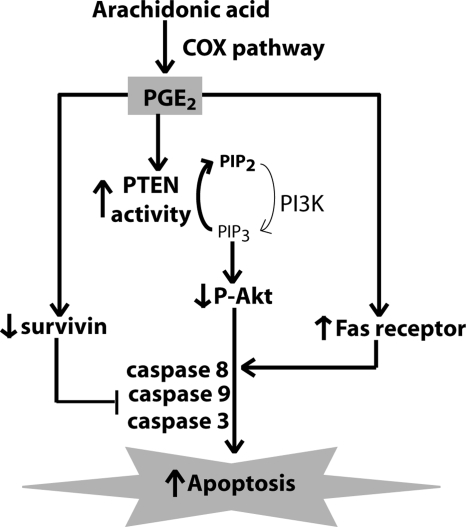
Figure 9.
Schema of PGE2-induced apoptosis in lung fibroblasts. PGE2, synthesized by the cyclooxygenase (COX) pathway, induces apoptosis by increasing PTEN activity and decreasing Akt activity. PGE2 also decreases survivin and increases Fas expression in lung fibroblasts. PGE2-induced apoptosis is mediated by ligation of the EP2 and EP4 receptors; relative contributions of EP2 and EP4 signaling to the 3 apoptosis pathways remain to be determined.
DISCUSSION
PGE2 has been shown to be antiapoptotic in multiple cell types, including normal epithelial cells (24, 25), cancer cells (26, 27), T cells (28, 29), and adipocytes (30). Despite the well-known contribution of fibroblast survival to the accumulation of this key mesenchymal cell in tissue fibrosis, there are few studies that have addressed the effects of this prostanoid on fibroblast survival. Our studies demonstrate for the first time that in contrast to its predominant antiapoptotic effect in other cell types, PGE2 promotes apoptosis of lung fibroblasts. These findings provide a novel mechanism by which PGE2 may mediate antifibrotic effects.
The pathobiology of pulmonary fibrosis is characterized by an increase in epithelial cell apoptosis (2,3,4) and a decrease in fibroblast apoptosis (5,6,7,8). The importance of TGF-β as a profibrotic mediator derives in part from its ability to promote each of these opposing effects—a phenomenon that has been termed the “apoptosis paradox” (7, 51). That PGE2 acts as an antiapoptotic signal in epithelial cells (24, 25) but as a proapoptotic signal in fibroblasts therefore signifies another such apoptosis paradox and emphasizes the pleiotropic antifibrotic potential of PGE2. The fact that impaired synthesis of PGE2 in pulmonary fibrosis (12,13,14) would be expected to promote fibroblast survival may explain the persistent fibrogenesis observed in this disease.
Our studies employing both RNA silencing and gene deletion indicate that PGE2-induced fibroblast apoptosis is dependent on PTEN activation; this results in down-regulation of Akt, a molecule that has been shown to be important in prosurvival signaling (41). The contrasting actions of PGE2 in fibroblasts vs. epithelial cells may be explained by differences in PTEN/Akt signaling, since we have shown that PGE2 activates PTEN and inhibits Akt (21) in the former, while it activates Akt in epithelial (24) and cancer cells (52). Although the measured increases in caspase 8 and caspase 9 activities were modest, both appear to participate in PGE2-induced apoptosis on the basis of data using selective inhibitors. The importance of both the intrinsic (as reflected by the role for caspase 9) and extrinsic (as reflected by the role for caspase 8) apoptosis pathways is not surprising, given their recognized capacity for crosstalk (53).
We furthermore show for the first time that PGE2 inhibits expression of survivin. This once again contrasts with findings in other cell types, in which PGE2 was shown to increase survivin expression in cancer cells (48, 49), thereby contributing to its role in promoting oncogenesis. We also found that PGE2 increases expression of Fas. These findings contrast with reports of PGE2 decreasing FasL expression in T cells, thereby preventing T-cell activation-induced cell death (29). This contrasting action on the Fas/FasL pathway further illustrates that actions of PGE2 are cell-type dependent.
Previous studies have shown that a chemical inhibitor of Akt, LY294002, does not increase baseline fibroblast apoptosis (32), suggesting that increased PTEN activity/decreased Akt activity by PGE2 may be necessary, but not sufficient, for PGE2-induced apoptosis. Rather, this may require the contributions of additional proapoptotic pathways, including increased Fas expression and decreased survivin expression. Indeed, increased Fas expression could explain our finding that PGE2 potentiates apoptosis elicited by FasL plus cycloheximide. The possible interrelationship among increased Fas expression, decreased survivin expression, and PTEN/Akt signaling requires further investigation, and the relative contributions of EP2 and EP4 receptor signaling to these three mechanisms remain to be determined. However, the observation that PGE2 modulates multiple determinants of apoptosis in lung fibroblasts may explain its ability to activate both intrinsic and extrinsic pathways, as well as its potential to influence apoptosis in diverse biological contexts.
PGE2 synthesized by neighboring epithelial cells or fibroblasts themselves may act as a paracrine or autocrine brake to limit excessive fibroblast accumulation under normal homeostatic conditions. Since PGE2 enhanced FasL-induced apoptosis even in UIP fibroblasts, which were previously shown to be resistant to FasL signaling (10, 11), it may play an important role in sensitizing cells to apoptosis. Thus, the modest magnitude of PGE2-induced apoptosis does not diminish its biological importance, and the actions of PGE2 may help potentiate other apoptosis-targeted therapies.
A previous study has shown that PGE2 protects against fibroblast apoptosis in the presence of cigarette smoke extract (54). These experiments were performed in cells treated for only 6 h, and the effects of PGE2 alone were not tested. Our findings instead examined the effects of PGE2 both alone and with the addition of FasL, employing later time points that may be more relevant to the kinetics of apoptosis. Nevertheless, the seemingly disparate findings between the two experimental models may also point to the pleiotropic actions of PGE2 in the presence of differing apoptotic signals.
Studies in UIP fibroblasts demonstrated that many, but not all, cell lines exhibited resistance to the proapoptotic effects of PGE2. This mirrored our previous findings in which some but not all UIP fibroblast lines exhibited resistance to PGE2-mediated inhibition of collagen synthesis and cell proliferation (14). Multiple mechanisms (diminished EP2 expression, diminished protein kinase A expression, and activity) accounted for resistance to PGE2 inhibition of collagen synthesis and cell proliferation (14). Given that PGE2-induced apoptosis proceeds via EP2/EP4 receptor activation, it is likely that apoptosis resistance in UIP cells reflects these same mechanisms responsible for resistance to PGE2 inhibition of collagen synthesis and cell proliferation. Interestingly, decreased PTEN expression has also been described in fibroblasts from some UIP patients (55), possibly also contributing to their resistance to apoptosis.
Although our studies address PGE2-mediated fibroblast apoptosis in vitro, the in vivo relevance of this phenomenon remains to be determined. However, it is of interest that the cyclooxygenase inhibitor indomethacin was shown to worsen bleomycin-induced fibrosis in mice (56). As PGE2 is the major cyclooxygenase-derived prostanoid produced by both alveolar epithelial cells (57) and fibroblasts (57), it has the capability to elicit fibroblast apoptosis in both a paracrine and autocrine fashion. Although we found that PGD2 likewise possesses this capability, its relevance in the setting of parenchymal fibrogenesis is less apparent.
CONCLUSIONS
As opposed to normal and malignant epithelial cells, for which PGE2 is antiapoptotic, we show that PGE2 promotes apoptosis of lung fibroblasts, and that this involves enhanced PTEN signaling and decreased Akt activation. PGE2 also decreased survivin and increased Fas receptor expression. A resistance to PGE2-induced apoptosis was observed in some UIP cells, which could be overcome with the combination of FasL and cycloheximide. Although PGE2 has previously been demonstrated to inhibit the activation of diverse fibrogenic functions in lung fibroblasts, its ability to promote apoptosis offers a potential opportunity not merely to prevent progression of pulmonary fibrosis but to intervene in and potentially reverse established disease.
Supplementary Material
Acknowledgments
We thank Dr. Rommel Sagana for providing assistance with the PTEN siRNA experiments. This work was supported by U.S. National Institutes of Health grant P50 HL56402 from the National Heart, Lung, and Blood Institute. S.K.H. was supported by a Parker B. Francis Fellowship and an American Thoracic Society Fellows Career Development Award.
References
- Danial N N, Korsmeyer S J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Barbas-Filho J V, Ferreira M A, Sesso A, Kairalla R A, Carvalho C R, Capelozzi V L. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP) J Clin Pathol. 2001;54:132–138. doi: 10.1136/jcp.54.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal B D, Joshi I, Hughes W F, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275:L1192–L1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- Plataki M, Koutsopoulos A V, Darivianaki K, Delides G, Siafakas N M, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–274. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- Selman M, King T E, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Horowitz J C, Cui Z, Moore T A, Meier T R, Reddy R C, Toews G B, Standiford T J, Thannickal V J. Constitutive activation of prosurvival signaling in alveolar mesenchymal cells isolated from patients with nonresolving acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2006;290:L415–L425. doi: 10.1152/ajplung.00276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal V J, Horowitz J C. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G J, McAnulty R J, Hill M, Chambers R. Escape from the matrix: multiple mechanisms for fibroblast activation in pulmonary fibrosis. Proc Am Thorac Soc. 2008;5:311–315. doi: 10.1513/pats.200710-159DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Moodley Y P, Caterina P, Scaffidi A K, Misso N L, Papadimitriou J M, McAnulty R J, Laurent G J, Thompson P J, Knight D A. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202:486–495. doi: 10.1002/path.1531. [DOI] [PubMed] [Google Scholar]
- Buhling F, Wille A, Rocken C, Wiesner O, Baier A, Meinecke I, Welte T, Pap T. Altered expression of membrane-bound and soluble CD95/Fas contributes to the resistance of fibrotic lung fibroblasts to FasL induced apoptosis. Respir Res. 2005;6:37. doi: 10.1186/1465-9921-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisingam C B, Jenkins R G, Harrison N K, Hernandez-Rodriguez N A, Booth H, Laurent G J, Hart S L, Foster M L, McAnulty R J. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R J, Jenkins R G, Wheeler-Jones C P, Copeman D M, Bottoms S E, Bellingan G J, Nanthakumar C B, Laurent G J, Hart S L, Foster M L, McAnulty R J. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol. 2004;165:1663–1676. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilborn J, Crofford L J, Burdick M D, Kunkel S L, Strieter R M, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B B, Ballinger M N, White E S, Green M E, Herrygers A B, Wilke C A, Toews G B, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174:5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- Huang S K, Wettlaufer S H, Hogaboam C M, Flaherty K R, Martinez F J, Myers J L, Colby T V, Travis W D, Toews G B, Peters-Golden M. Variable prostaglandin E2 resistance in fibroblasts from patients with usual interstitial pneumonia. Am J Respir Crit Care Med. 2008;177:66–74. doi: 10.1164/rccm.200706-963OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauncey J B, Peters-Golden M, Simon R H. Arachidonic acid metabolism by rat alveolar epithelial cells. Lab Invest. 1988;58:133–140. [PubMed] [Google Scholar]
- Elias J A, Rossman M D, Zurier R B, Daniele R P. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985;131:94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- Bitterman P B, Wewers M D, Rennard S I, Adelberg S, Crystal R G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986;77:700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine A, Poliks C F, Donahue L P, Smith B D, Goldstein R H. The differential effect of prostaglandin E2 on transforming growth factor-beta and insulin-induced collagen formation in lung fibroblasts. J Biol Chem. 1989;264:16988–16991. [PubMed] [Google Scholar]
- White E S, Atrasz R G, Dickie E G, Aronoff D M, Stambolic V, Mak T W, Moore B B, Peters-Golden M. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama T, Ertl R F, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard S I. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- Kolodsick J E, Peters-Golden M, Larios J, Toews G B, Thannickal V J, Moore B B. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- Tessner T G, Muhale F, Riehl T E, Anant S, Stenson W F. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H, Kizaka-Kondoh S, Insel P A, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci U S A. 2003;100:8921–8926. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Shao J, Morrow J D, Beauchamp R D, DuBois R N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- Hawcroft G, Ko C W, Hull M A. Prostaglandin E2-EP4 receptor signalling promotes tumorigenic behaviour of HT-29 human colorectal cancer cells. Oncogene. 2007;26:3006–3019. doi: 10.1038/sj.onc.1210113. [DOI] [PubMed] [Google Scholar]
- Weinlich R, Bortoluci K R, Chehab C F, Serezani C H, Ulbrich A G, Peters-Golden M, Russo M, Amarante-Mendes G P. TLR4/MYD88-dependent, LPS-induced synthesis of PGE2 by macrophages or dendritic cells prevents anti-CD3-mediated CD95L upregulation in T cells. Cell Death Differ. 2008;15:1901–1909. doi: 10.1038/cdd.2008.128. [DOI] [PubMed] [Google Scholar]
- Porter B O, Malek T R. Prostaglandin E2 inhibits T cell activation-induced apoptosis and Fas-mediated cellular cytotoxicity by blockade of Fas-ligand induction. Eur J Immunol. 1999;29:2360–2365. doi: 10.1002/(SICI)1521-4141(199907)29:07<2360::AID-IMMU2360>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Setoyama T, Tsumagari H, Miyata N, Hatano Y, Xu L, Jisaka M, Nagaya T, Yokota K. Endogenous prostaglandins E2 and F 2alpha serve as an anti-apoptotic factor against apoptosis induced by tumor necrosis factor-alpha in mouse 3T3–L1 preadipocytes. Biosci Biotechnol Biochem. 2006;70:2145–2153. doi: 10.1271/bbb.60106. [DOI] [PubMed] [Google Scholar]
- Hogaboam C M, Carpenter K J, Evanoff H, Kunkel S L. Approaches to evaluation of fibrogenic pathways in surgical lung biopsy specimens. Methods Mol Med. 2005;117:209–221. doi: 10.1385/1-59259-940-0:209. [DOI] [PubMed] [Google Scholar]
- Horowitz J C, Lee D Y, Waghray M, Keshamouni V G, Thomas P E, Zhang H, Cui Z, Thannickal V J. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-β1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Wettlaufer S H, Hogaboam C, Aronoff D M, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L405–L413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed J C. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Jin Z, El-Deiry W S. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- LeBlanc H N, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- Sauerwald T M, Oyler G A, Betenbaugh M J. Study of caspase inhibitors for limiting death in mammalian cell culture. Biotechnol Bioeng. 2003;81:329–340. doi: 10.1002/bit.10473. [DOI] [PubMed] [Google Scholar]
- Sweeney E A, Inokuchi J, Igarashi Y. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 1998;425:61–65. doi: 10.1016/s0014-5793(98)00198-7. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Tintignac L A, Zhuravleva E, Hemmings B A. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Falleni M, Pellegrini C, Marchetti A, Oprandi B, Buttitta F, Barassi F, Santambrogio L, Coggi G, Bosari S. Survivin gene expression in early-stage non-small cell lung cancer. J Pathol. 2003;200:620–626. doi: 10.1002/path.1388. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Altieri D C, Lu C D, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–5074. [PubMed] [Google Scholar]
- LaCasse E C, Baird S, Korneluk R G, MacKenzie A E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- Jeong J C, Kim M S, Kim T H, Kim Y K. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochem Res. 2008;34:991–1001. doi: 10.1007/s11064-008-9868-5. [DOI] [PubMed] [Google Scholar]
- Krysan K, Merchant F H, Zhu L, Dohadwala M, Luo J, Lin Y, Heuze-Vourc'h N, Pold M, Seligson D, Chia D, Goodglick L, Wang H, Strieter R, Sharma S, Dubinett S. COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- Lin J, Hsiao P W, Chiu T H, Chao J I. Combination of cyclooxygenase-2 inhibitors and oxaliplatin increases the growth inhibition and death in human colon cancer cells. Biochem Pharmacol. 2005;70:658–667. doi: 10.1016/j.bcp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Rosner D, Stoneman V, Littlewood T, McCarthy N, Figg N, Wang Y, Tellides G, Bennett M. Interferon-gamma induces Fas trafficking and sensitization to apoptosis in vascular smooth muscle cells via a PI3K- and Akt-dependent mechanism. Am J Pathol. 2006;168:2054–2063. doi: 10.2353/ajpath.2006.050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R A, Leof E B. TGF-β signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- Castellone M D, Teramoto H, Williams B O, Druey K M, Gutkind J S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Yin X M. Signal transduction mediated by Bid, a prodeath Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Liu X, Togo S, Kobayashi T, Shen L, Kawasaki S, Kamio K, Wang X Q, Mao L J, Rennard S I. Prostaglandin E(2) protects human lung fibroblasts from cigarette smoke extract-induced apoptosis via EP(2) receptor activation. J Cell Physiol. 2007;210:99–110. doi: 10.1002/jcp.20825. [DOI] [PubMed] [Google Scholar]
- White E S, Atrasz R G, Hu B, Phan S H, Stambolic V, Mak T W, Hogaboam C M, Flaherty K R, Martinez F J, Kontos C D, Toews G B. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B B, Coffey M J, Christensen P, Sitterding S, Ngan R, Wilke C A, McDonald R, Phare S M, Peters-Golden M, Paine R, 3rd, Toews G B. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- Lipchik R J, Chauncey J B, Paine R, Simon R H, Peters-Golden M. Arachidonate metabolism increases as rat alveolar type II cells differentiate in vitro. Am J Physiol. 1990;259:L73–80. doi: 10.1152/ajplung.1990.259.2.L73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.