Abstract
Protease-activated receptor 1 (PAR-1) mediates thrombin signaling in human endothelial cells. As a G-protein-coupled receptor, PAR-1 transmits thrombin signal through activation of the heterotrimeric G proteins, Gi, Gq, and G12/13. In this study, we demonstrated that zyxin, a LIM-domain-containing protein, is involved in thrombin-mediated actin cytoskeleton remodeling and serum response element (SRE)-dependent gene transcription. We determined that zyxin binds to the C-terminal domain of PAR-1, providing a possible mechanism of involvement of zyxin as a signal transducer in PAR-1 signaling. Data showing that disruption of PAR-1-zyxin interaction inhibited thrombin-induced stress fiber formation and SRE activation supports this hypothesis. Similarly, depletion of zyxin using siRNA inhibited thrombin-induced actin stress fiber formation and SRE-dependent gene transcription. In addition, depletion of zyxin resulted in delay of endothelial barrier restoration after thrombin treatment. Notably, down-regulation of zyxin did not affect thrombin-induced activation of RhoA or Gi, Gq, and G12/13 heterotrimeric G proteins, implicating a novel signaling pathway regulated by PAR-1 that is not mediated by G-proteins. The observation that zyxin targets VASP, a partner of zyxin in regulation of actin assembly and dynamics, to focal adhesions and along stress fibers on thrombin stimulation suggests that zyxin may participate in thrombin-induced cytoskeletal remodeling through recruitment of VASP. In summary, this study establishes a crucial role of zyxin in thrombin signaling in endothelial cells and provides evidence for a novel PAR-1 signaling pathway mediated by zyxin.—Han, J., Liu, G., Profirovic, J., Niu, J., Voyno-Yasenetskaya, T. Zyxin is involved in thrombin signaling via interaction with PAR-1 receptor.
Keywords: endothelium, G-protein-coupled receptors, actin cytoskeleton, heterotrimeric G proteins
Thrombin is a serine protease, which is produced from prothrombin on vascular injury and inflammation (1). It mediates a number of physiological responses in endothelial cells, including changes in cell shapes, activation of gene expression, increased permeability, and disintegration of the endothelial monolayer (2). Thrombin signaling is mediated by protease-activated receptors (PARs), a family of G-protein-coupled receptors (GPCRs; ref. 3). In human endothelial cells, PAR-1 is the main receptor that mediates cellular responses of thrombin (4). PAR-1 activates heterotrimeric G proteins, Gi, Gq, and G12/13 (5,6,7). It has been established that G13 and Gq mediate thrombin-induced actin stress fiber formation via activation of small G protein RhoA (8,9,10). Recently, other proteins have been reported to interact directly with cytoplasmic domain of PAR-1 and to mediate PAR-1 signaling to cytoskeleton. For instance, heat shock protein 90 (Hsp90), a chaperone molecule, is required for PAR-1-mediated signaling to cytoskeleton (11). We recently reported that Hsp90 is required for actin remodeling mediated by G12, one of the G proteins activated by PAR-1 (12).
Zyxin was cloned from human umbilical vein endothelial cells (HUVECs) and is highly expressed in lung (13). Large fractions of zyxin are localized in the focal adhesions of cells (14). The molecular structure of zyxin implicates it in the regulation of signaling and cytoskeleton dynamics. Zyxin has proline-rich repeats at the N terminus followed by a leucine-rich nuclear export signal (NES) and 3 copies of a cysteine- and histidine-rich motif called the LIM domain at the C terminus (13). The actin regulatory proteins, Ena/vasodilator-stimulated phosphoprotein (VASP) family members, and α-actinin interact with the proline-rich region of zyxin (15, 16). Zyxin can generate new actin structures in a VASP-dependent manner but does so independently of the Arp2/3 complex that cooperates with members of the family of Wiskott-Aldrich-syndrome protein (WASP) to nucleate actin filaments. This finding suggests that zyxin-VASP is a distinct actin polymerization machine in cells (17). The proline-rich domain of zyxin can bind Src homology 3 (SH3)-domain-containing proteins, most of which participate in signal transduction (18). The LIM domain is known to mediate specific protein–protein interaction (19). A number of zyxin-binding partners interacting with LIM domains have been identified, including cysteine-rich protein (CRP), large-tumor suppressor (h-wart/LATS1), and Crk-associated substrate (Cas) family members (20,21,22). Subcellular localization of zyxin is important for its functions (23). The NES allows zyxin to commute between cytosol and nucleus (14). On different stimuli, zyxin translocates to different subcellular compartments to assemble multimeric protein complexes, functioning as a scaffold for signaling proteins (24, 25).
Recently, zyxin and thyroid receptor interacting protein (TRIP6), another zyxin family member, have been reported to interact with lysophosphatidic acid receptor 2 (LPA-2 receptor), a GPCR that regulates cell migration (26). Here, we show that zyxin interacts with the C-terminal domain of PAR-1. This interaction is required for PAR-1-depenent actin stress fiber formation and serum response element (SRE)-dependent gene transcription. Notably, zyxin is not involved in PAR-1-dependent activation of heterotrimeric G proteins. Therefore, the present study defines a novel mechanism for PAR-1 signaling that is independent of heterotrimeric G proteins.
MATERIALS AND METHODS
Materials
Plasmids for FLAG-tagged zyxin and glutathione S-transferase (GST)-zyxin deletion mutants were kindly provided by Dr. Hideyuki Saya (Kumamoto University, Kumamoto, Japan). cDNA for GFP-tagged zyxin was a gift from Dr. Mary C. Beckerle (University of Utah, Salt Lake City, UT, USA). N-terminal FLAG-tagged PAR-1 cDNA was obtained from Dr. JoAnn Trejo (University of North Carolina, Chapel Hill, NC, USA). N terminus (residues 1-378) and C terminus (residues 375-572) of zyxin cDNA were subcloned into pCMV-2B1 (FLAG-tagged; Stratagene, La Jolla, CA, USA) using EcoRI/XhoI sites. C terminus (residues 375-425) of PAR-1 cDNA was inserted into pcDNA3.0-GFP construct at EcoRI/XhoI cloning sites. The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA): polyclonal anti-zyxin, monoclonal anti-FAK, anti-Myc, anti-HA, and anti-RhoA. Alexa Fluor 488 (green fluorescence), and Alexa Fluor 594 (red fluorescence) antibodies were from Molecular Probes (Eugene, OR, USA). Anti-FLAG monoclonal antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-VASP polyclonal antibody was from Calbiochem (San Diego, CA, USA). Thrombin was purchased from Enzyme Research Laboratories Inc. (South Bend, IN, USA) Protein A/G agarose beads were from Santa Cruz Biotechnology. Glutathione agarose beads, isobutylmethylxanthine (IBMX), and forskolin were purchased from Sigma-Aldrich. Dithio-bis-[succinimidyl propionate] (DSP) was from Thermo Scientific (Waltham, MA, USA).
RNA interference
To generate zyxin-specific short-hairpin RNA (shRNA) duplexes, we used the RNAi-Ready system and the shRNA Sequence Selector (BD Biosciences, San Jose, CA, USA) to design 21-nt sense and antisense sequences from the open reading frame of human zyxin. Four selected shRNA sequences were submitted to Basic Local Alignment Search Tool (BLAST; National Center for Biotechnology Information, Bethesda, MD, USA) search against the human genome sequence to ensure that only zyxin was targeted. Each part of synthesized oligonucleotides (Sigma-Aldrich) was annealed and ligated into pSIREN-RetroQ-ZsGreen vector, and the constructs were confirmed both by enzyme digestion and DNA sequencing. Of 4 sequences, zyxin 1599 was most effective in zyxin down-regulation in human umbilical vein endothelial cells (HUVECs). Sequence of zyxin 1599 is forward GATCCGTGTTACAAGTGTGAGGACTTCAAGAGAGTCCTCACACTTGTAACACTTTTTTACGCGTG, reverse AATTCACGCGTAAAAAAGTGTTACAAGTGTGAGGACTCTCTTGAAGTCCTCACACTTGTAACACG. Control sequence for zyxin 1599 forward (sense only) is GATCCGTGTTACAAGTGTGAGGACTTCAAGAGATTTTTTACGCGTG. Zyxin 1599 and its control shRNA plasmids were used in the immunofluorescence and SRE experiments. In all other experiments involving down-regulation of zyxin, small interference RNA (siRNA) oligonucleotides were transfected into HUVECs using siRNA transfection reagent and siRNA transfection medium (Santa Cruz Biotechnology) according to the manufacturer’s instructions.
Cell culture and transfection
Primary HUVECs were purchased from Lonza (Basel, Switzerland) and used at passage 3–6. Cells were cultured in endothelial growth medium EGM-2 Bullet Kit (Lonza) supplemented with 10% (v/v) of fetal bovine serum (FBS). HUVECs, at 90% confluency, were transiently transfected with cDNA constructs as indicated, using Amaxa nucleofactor device and transfection kit (Lonza), according to the manufacturer’s protocols. CHO-K1 and COS-7 cells were maintained in DMEM supplemented with 10% FBS. Transient transfections were performed using LipofectAmine 2000 reagent (Invitrogen, Carlsbad, CA, USA), according to manufacturer’s instructions. The transfection efficiency of HUVECs with cDNAs and shRNA was 20–25%; with siRNA, ∼90%. COS cells transfection efficiency was ∼90%.
Immunoprecipitation and GST-pulldown assays
COS-7 cells were transfected with cDNA constructs and lysed in Nonidet P-40 buffer [20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM EGTA; 1 mM EDTA; 1% Nonidet P-40; protease inhibitor cocktail (Sigma); 50 μM NaF; and 0.2 mM Na3VO4 unless specified]. Supernatants were precleared with protein A/G agarose 4B and immunoprecipitated with primary antibody for 3 h at 4°C, followed by incubation with protein A/G agarose 4B for 1 h at 4°C. Immunoprecipitates were washed and boiled in Laemmli buffer for 5 min. Protein samples were separated by SDS-polyacrylamide gel eletrophoresis (SDS-PAGE), transferred onto nitrocellulose membrane, and probed with appropriate antibodies. Western blots were developed using ECL plus reagents (Amersham Biosciences Inc., Piscataway, NJ, USA). GST-fusion proteins were expressed in BL21 Escherichia coli and purified onto glutathione-sepharose 4B beads (Amersham Biotechnology). The GST beads or GST-fusion protein beads were incubated with equal amounts of COS-7 cell lysates expressing GFP-cytoplasmic tail of PAR-1 for 2 h at 4°C, followed by washing 3 times with lysis buffer. The bound proteins were eluted with 2× SDS-PAGE sample buffer. Western blotting analysis was performed as described above.
Chemical crosslinking of zyxin-PAR-1 interaction in vivo
To determine the association of endogenous zyxin with PAR-1 under physiological conditions, CHO-K1 cells stably expressing PAR-1 tagged at the N terminus with FLAG epitope were exposed to PAR-1 peptide (100 nM) for 0, 2, or 30 min, immediately followed by incubation with 2 mM DSP, a cell-permeable cross-linker, for 30 min at 37°C. After cross-linking, the cells were lysed, sonicated 3 times for 10 s, immunoprecipitated, and Western blotted with anti-zyxin and anti-FLAG antibodies.
Immunofluorescent staining and confocal microscopy
HUVECs, grown on gelatin-coated coverslips, were transiently transfected with cDNA constructs as indicated, and maintained in EGM-2 supplemented with 0.2% FBS for 3 h prior to the experiments. Thereafter, cells were washed with Hank’s balanced salt solution (HBSS) and fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 1% bovine serum albumin (BSA) in HBSS for 1 h. Cells were then incubated with appropriate primary and secondary antibodies, and the coverslips were mounted with ProLong Antifade Kit (Molecular Probes). Images were taken by laser-scanning confocal microscopy on a Zeiss LSM 510 microscope equipped with an ×63 water-immersion objective and laser excitations at 488 and 594 nm (Carl Zeiss, Oberkochen, Germany).
Calculations of the total intensities of protein staining
Images of Alexa Fluor 594-stained HUVEC monolayers stimulated with thrombin were captured and analyzed using Zeiss enhanced colocalization tool software as we described before (27). Images were segmented differentially between cytosol (black) and F-actin (highest gray value) based on image grayscale levels. The actin stress fiber formation was expressed as a ratio of the cytoskeletal polymer area to the area of the whole image and normalized against controls. Data were collected from 20 cells for each experiment. Three independent experiments were performed. The values were analyzed using Student’s t test and statistically processed using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA). Differences were considered statistically significant when P < 0.05.
Real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR)
The total cellular RNA was isolated from the HUVECs transfected with control or zyxin siRNA for 24 or 48 h using the RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. We generated cDNA from 100 ng total RNA using Superscript II reverse transcriptase (Invitrogen). The primers specific for zyxin gene were purchased from Santa Cruz Biotechnology. The primer sequences for GAPDH were forward TTGCCATCAATGACCCCTTCA and reverse CGCCCCACTTGATTTTGGA. Real-time RT-PCR was performed using a kit with SYBR green (Applied Biosystems) on a real-time PCR machine (Prism 7000; Applied Biosystems). For comparative analysis of gene expression, we used relative standard curve method. mRNA levels were normalized against GADPH, and the mRNA level in zyxin siRNA-treated cells was expressed as the percentage of that in control siRNA-treated cells. Semiquantitative RT-PCR was also performed to determine zyxin and GADPH mRNA expression levels using the same RT-PCR conditions as real-time RT-PCR. The RT-PCR products were visualized on ethidium bromide gels.
Measurment of transendothelial electric resistance (TER)
Endothelial barrier function was assessed by measurement of TER using electric cell-substrate impedance sensor (Applied Biophysics, Troy, NY, USA), as described previously (28). Briefly, HUVECs were seeded onto electric cell-substrate impedance sensing (ECIS) 8W10+ E culture-ware containing 40 gold microelectrodes per well (Applied Biophysics) and transfected with control or zyxin siRNA oligonucleotides as described above. At 48 h after transfection, the cells were maintained in EGM-2 supplemented with 0.2% FBS for 3 h, and the resistance across the resting monolayer was monitored for 1 h to ensure a stable baseline recording. Thereafter, the cells were stimulated with thrombin (5 nM), and subsequent change in TER was recorded in real time. Resistance data were normalized to the initial voltage and plotted as normalized resistance. Results are expressed as means ± sd. The experiments were performed in triplicates.
Luciferase cis-reporter assay
HUVECs at 90% confluency, grown on 12-well plates, were transiently transfected with the following plasmids: luciferase cDNA under control of SRE from c-fos promoter (SRE.L), phRenilla (transfection efficiency control plasmid), and empty vector or constructs to be tested. Before the experiment, cells were serum starved for 3 h in EGM-2 containing 0.2% FBS and treated with thrombin for 5 h. Firefly and Renilla luciferase activity of each sample was measured using the Dual-Luciferase Reporter (DLR) Assay System (Promega, Madison, WI, USA). Luciferase activity of each sample was normalized to Renilla activity to correct for differences in the transfection efficiency. The data represent means ± sd of triplicate determinations.
Measurement of GTPase activity
RhoA activity was measured using GST-rhotekin Rho binding domain that specifically binds activated RhoA (29). Samples were subjected to electrophoresis on 12% SDS-polyacrylamide gel, transferred to nitrocellulose, and analyzed by Western blotting using monoclonal anti-RhoA antibody.
Inositol phosphate (IP3) accumulation
IP3 accumulation was determined as described previously (30). Briefly, HUVECs were seeded onto 12-well plates and transfected with zyxin and control siRNA oligonucleotides. At 24 h after transfection, cells were labeled with myo[3H]inositol (5 μCi/ml) for 24 h. Thereafter, the cells were washed once with HEPES-buffered Dulbecco’s modified medium containing 5 mM LiCl. Total accumulated IP3 was assayed using Dowex columns. The radioactivity was measured using scintillation counter (Perkin-Elmer, Wellesley, MA, USA), and results were expressed as folds of basal.
Cyclic AMP assay
cAMP production was determined using the cAMP Biotrack enzyme immunoassay system according to the manufacturer’s protocol (Cayman Chemical, Ann Arbor, MI, USA). Briefly, HUVECs seeded onto 12-well plates were transfected with zyxin and control siRNA oligonucleotides for 48 h and preincubated in serum-free medium supplemented with 0.5 mM IBMX for 10 min. Thereafter, the cells were treated with 25 nM thrombin for 10 min in the presence of 1 μM forskolin and IBMX. Intracellular cAMP was extracted by incubation with 0.1 M HCl for 20 min and determined using EIA kit.
RESULTS
Thrombin induces redistribution of zyxin to focal adhesions and along stress fibers in endothelial cells
Thrombin induces profound cytoskeletal and focal adhesion plaque rearrangement, leading to endothelial barrier dysfunction (31). A substantial body of evidence points to the ability of zyxin, as an important focal adhesion component, to regulate actin filament assembly. As the function of zyxin is dependent on its subcellular localization, we examined the distribution of zyxin in HUVECs after thrombin stimulation. To characterize the localization and dynamics of zyxin, we performed immunofluorescence study using two approaches: expression of green fluorescence protein (GFP)-tagged zyxin, and detection of endogenous zyxin with specific antibody (Fig. 1). Under basal conditions, only a small portion of zyxin is located to focal adhesions, as determined by colocalization with the focal adhesion kinase, FAK (Fig. 1A). Thrombin-treated HUVECs showed profound reorganization of FAK and accumulation of zyxin in focal adhesion plaques, as evident by remarkable colocalization of zyxin and FAK (Fig. 1A). In addition, the immunofluorescence analysis also revealed that zyxin colocalized with thrombin-induced actin stress fibers (Fig. 1B). This set of results indicated that thrombin stimulation could induce redistribution of zyxin to focal adhesions and along stress fibers. These observations led us to hypothesize that zyxin might be involved in thrombin signaling.
Figure 1.
Thrombin induces translocation of zyxin to the focal adhesions and stress fibers in HUVECs. A) Thrombin induces accumulation of zyxin at the focal adhesions. Confluent HUVECs, grown on gelatin-coated coverslips, were transiently transfected with GFP-tagged zyxin as indicated, stimulated with 25 nM thrombin for 5 min, fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 5 min, and blocked with blocking solution for 1 h. Thereafter, FAK was detected using anti-FAK antibody (red fluorescence), endogenous zyxin was detected using anti-zyxin antibody (green fluorescence), and localization of transfected GFP-zyxin was directly visualized by GFP. B) Thrombin induces redistribution of zyxin along stress. Cells were treated as described above. Actin fibers were detected with phalloidin Alexa Fluor 594 (red fluorescence). Images were taken by laser-scanning confocal microscopy on a Zeiss LSM 510 microscope equipped with ×63 water-immersion objective.
Zyxin is involved in thrombin-induced cytoskeletal rearrangement and SRE-dependent gene transcription
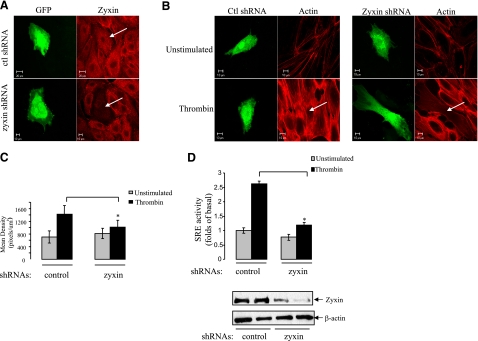
To explore the role of zyxin in thrombin signaling, we employed RNA interference technology to down-regulate the expression of endogenous zyxin. In this series of experiments (Fig. 2), we generated plasmid cDNA containing shRNA against zyxin, and GFP for detection of shRNA transfected cells, and transfected into HUVECs for immunofluorescence studies and SRE-dependent gene transcription assays. The immunostaining of endogenous zyxin was reduced markedly in zyxin shRNA-transfected HUVECs compared with control shRNA transfected and nontransfected cells, showing that zyxin shRNA effectively induced silencing of zyxin in HUVECs (Fig. 2A).
Figure 2.
Down-regulation of zyxin inhibits thrombin-induced stress fiber formation and SRE activity in HUVECs. A) Expression of GFP-tagged zyxin-specific shRNA plasmid down-regulates endogenous zyxin. HUVECs were transiently transfected with control or zyxin-specific shRNA plasmids as indicated for 48 h. Cells were then fixed and stained for endogenous zyxin with anti-zyxin antibody. B) Down-regulation of zyxin inhibits thrombin-induced stress fiber formation. HUVECs were transfected with control or zyxin shRNA plasmids for 48 h and maintained in 0.2% FBS medium for 3 h, followed by stimulation with 25 nM thrombin for 15 min. Cells were then fixed, and actin fibers were detected with phalloidin Alexa Fluor 594 (red fluorescence). Images were acquired by confocal microscopy as described in Fig. 1B. C) Stress fibers were quantified using Zeiss enhanced colocalization software. Data were collected from 20 transfected cells for each experiment; values are expressed as means ± sd. *P < 0.05. D) Down-regulation of zyxin inhibits thrombin-induced SRE activity. HUVECs, grown on 12-well plates, were transfected with shRNA plasmids together with plasmids expressing SRE-driven luciferase reporter and Renilla. At 48 h after transfection, HUVECs were maintained in medium containing 0.2% FBS for 3 h, followed by the stimulation with 25 nM thrombin for 5 h. SRE activity (top panel) was measured as described in Materials and Methods. Expression levels of endogenous zyxin in HUVECs samples were determined using Western blotting (bottom panel).
After confirming the effectiveness of zyxin shRNA, we tested how down-regulation of zyxin would affect thrombin-induced actin stress fiber formation and SRE-dependent gene transcription in endothelial cells (32, 33). HUVECs transfected with shRNA plasmids were treated with 25 nM thrombin for 15 min. Control shRNA did not affect F-actin appearance in HUVECs (Fig. 2B). Similarly, zyxin shRNA did not affect F-actin in nonstimulated cells. However, in zyxin shRNA-transfected cells, thrombin-induced actin stress fiber formation was dramatically inhibited, and the formed actin fibers were thinner and less organized (Fig. 2B). Quantification of the total intensity of F-actin staining using Zeiss enhanced colocalization tool software confirmed that thrombin-induced actin fiber formation in the cells expressing zyxin shRNA was reduced significantly (Fig. 2C). These data indicated that depletion of zyxin could inhibit thrombin-induced actin stress fiber formation and reorganization, thus suggesting the involvement of zyxin in thrombin signaling to cytoskeleton in endothelial cells.
Using the same shRNA plasmid, we next examined the effect of zyxin on thrombin-induced SRE-dependent gene transcription. HUVECs were cotransfected with plasmids expressing control or zyxin shRNAs together with SRE-driven luciferase reporter and Renilla (transfection efficiency control plasmid). Thrombin treatment increased SRE-dependent gene transcription in control shRNA-transfected HUVECs, Notably, the thrombin-induced SRE activation was inhibited significantly in cells transfected with zyxin shRNA plasmid, which indicated that depletion of zyxin could inhibit thrombin-induced SRE-dependent gene transcription (Fig. 2D).
Zyxin is involved in thrombin-induced endothelial cell barrier function
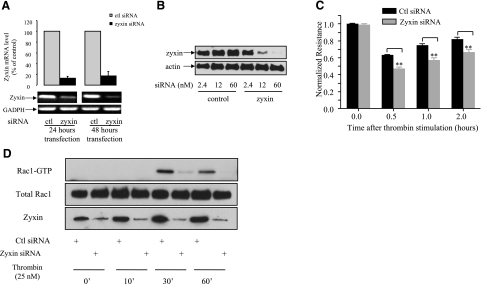
Thrombin-induced actin rearrangement is tightly correlated with the endothelial cell barrier function (34). Because our data showed a significant effect of zyxin on thrombin-induced rearrangement of the cellular F-actin network, we next tested whether zyxin is involved in thrombin-mediated increase in endothelial permeability, using ECIS to measure TER (28). In this set of experiments, we used commercially available siRNA oligonucleotides and tested their effectiveness using real-time RT-PCR (Fig. 3A) and Western blot analysis (Fig. 3B) prior to the TER measurement. Real-time RT-PCR data showed that zyxin mRNA was down-regulated by ∼80% 24 and 48 h after siRNA transfection (Fig. 3A). Western blot analysis showed that zyxin-specific siRNA down-regulated the level of zyxin expression in a dose-dependent manner (Fig. 3B). In the presented TER study, permeability of confluent monolayers of control or zyxin siRNA oligonucleotides-transfected cells was monitored in real time. The data, expressed as normalized resistance, showed that TER decreased immediately after addition of thrombin to 0.6–0.7 followed by recovery phase in control siRNA-transfected HUVEC monolayers. Transfection of zyxin siRNA significantly potentiated thrombin-induced TER decline to 0.4–0.5 and delayed the recovery time at least by 1 h (Fig. 3C), suggesting an important role of zyxin in thrombin-mediated regulation of endothelial barrier function. Depletion of zyxin using siRNA oligonucleotides did not significantly change basal endothelial barrier function over a period of 30 h after siRNA transfection (Supplemental Fig. S1).
Figure 3.
Down-regulation of zyxin enhances thrombin-induced endothelial cell barrier dysfunction. A) Transfection of zyxin-specific siRNA oligonucleotide decreases mRNA level of zyxin in HUVECs. Total mRNA of cells transfected with zyxin-specific siRNA for 24 or 48 h was isolated, and real-time RT-PCR was performed as described in Materials and Methods. Data were collected from 3 independent experiments performed in triplicates; values are means ± sd. B) Knockdown of zyxin protein by zyxin-specific siRNA transfection. HUVECs were transfected with indicated concentrations of control or zyxin-specific siRNA. At 48 h after transfection, cells were lysed, and cleared cell lysates were subjected to SDS-PAGE. C) Effect of zyxin siRNA on thrombin-induced changes in TER. Confluent HUVECs grown on gold microelectrodes were transfected with ccontrol or zyxin siRNA oligonucleotides for 48 h and used for TER measurements. Cells were stimulated with 5 nM thrombin for 30 min after initiation of recording. Results are means ± sd of 3 independent experiments performed in triplicates. Histogram shows results at different time points as indicated. **P < 0.01. D) Depletion of zyxin inhibited thrombin-induced Rac-1 activation in HUVECs. HUVECs transfected with control or zyxin siRNA oligonucleotides were exposed to 25 nM thrombin for indicated period of time, and Rac1 activity was measured. Bottom panel shows expression level of endogenous zyxin in HUVECs.
Although the basis of restoration of endothelial barrier subsequent to barrier dysfunction remains unclear, several lines of evidence indicate that activity of Rac-1, a RhoGTPase, is tightly associated with the reannealing of the endothelial barrier (35,36,37,38). Interestingly, we found that down-regulation of zyxin resulted in inhibition of thrombin-induced Rac1 activation 30 min and 1 h after thrombin stimulation (Fig. 3D), providing a possible explanation of a delayed restoration of the endothelial barrier function in the absence of zyxin.
Zyxin interacts with cytoplasmic tail of PAR-1 via LIM domains
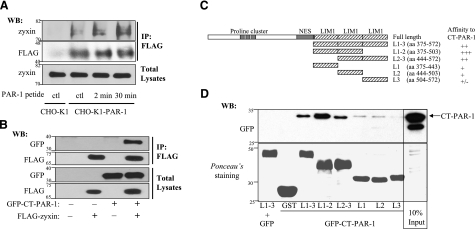
Increasing information indicates that zyxin plays a central role in the regulation of actin dynamics through recruitment of actin regulatory proteins to specific sites on stimulation from different stimuli (25, 39, 40). However, the mechanism underlying the ability of zyxin to sense outside signals, such as thrombin, is poorly understood. Recent reports provided evidence that zyxin family members can directly interact with transmembrane receptors (26, 41). These findings prompted us to investigate whether zyxin is involved in thrombin signaling through interaction with thrombin receptor PAR-1 in endothelial cells. We assessed the interaction of PAR-1 with endogenous zyxin using a cross-linking assay. We used DSP, a thiol-cleavable and membrane-permeable cross-linking agent, to capture intracellular protein–protein interactions by covalently binding the interacting proteins before lysing cells for immunoprecipitation assay. In this experiment, we used CHO-K1 cell line stably expressing FLAG-tagged PAR-1 (CHO-K1-PAR-1). Immunostaining of expressed PAR-1 is shown in Supplemental Fig. S2. To avoid cleavage of N-terminal FLAG epitope, we stimulated PAR-1 with PAR-1 peptide agonist. After stimulation with PAR-1 peptide, the cells were treated with DSP. The interaction of endogenous zyxin with PAR-1 was detected from CHO-K1-PAR-1 cells but not from CHO-K1 cells. In addition, the relative amount of protein coprecipitated with PAR-1 from PAR-1 peptide-treated cells was higher than that from nontreated cells (Fig. 4A).
Figure 4.
Zyxin associates with cytoplasmic tail of PAR-1 via its LIM domains. A) Zyxin was coimmunoprecipitated with PAR-1 stably expressed in CHO-K1 cells. CHO-K1 cells stably expressing FLAG-tagged PAR-1 were exposed to PAR-1 peptide (100 nM) for 2 or 30 min, followed by incubation with DSP for 30 min at 37°C. Cells were lysed and sonicated, and cleared lysates were then immunoprecipitated with anti-FLAG antibody and immunoblotted with anti-zyxin antibody. Nontransfected CHO-K1 cells were subjected to the same procedure, serving as a negative control. B) Zyxin coimmunoprecipitated with cytoplasmic tail of PAR-1. COS-7 cells were transiently cotransfected with FLAG-tagged zyxin together with GFP-tagged cytoplasmic tail of PAR-1 (GFP-CT-PAR-1). At 48 h after transfection, cells were lysed, and the lysates were incubated with anti-FLAG antibody, followed by incubation with A/G-agarose beads to immunoprecipitate zyxin. FLAG-tagged zyxin was detected with anti-FLAG antibody (row 2). Coimmunoprecipitated GFP-CT-PAR-1 was detected by Western blotting with anti-GFP antibody (row 1). C) Schematic diagram of GST-zyxin deletion mutants used in GST pull-down assay. The GST-zyxin constructs encode for LIM domain 1-3 (residues 375-572), LIM domain 1-2 (residues 375-503), LIM domain 2-3 (residues 444-572), LIM domain 1 (residues 375-443), LIM domain 2 (residues 444-503), and LIM domain 3 (residues 504-572). D) Zyxin interacts with PAR-1 through its LIM domains. Equal amounts of COS-7 cell lysates expressing GFP-CT-PAR-1 were incubated with GST beads or GST-zyxin-deletion mutant beads for 3 h. Thereafter, GFP-CT-PAR-1 associated with GST-zyxin-deletion mutants was pulled down and analyzed by Western blotting using anti-GFP antibody. Ponceau S staining of membrane showed expression of GST- zyxin-deletion mutants.
As the C-terminal tail of GPCRs is involved in signal transduction, we determined whether zyxin interacts with the last 50 amino acid residues of PAR-1 (residues 375-425) using coimmunoprecipitation assay. We cotransfected GFP-tagged cytoplasmic tail of PAR-1 (GFP-CT-PAR-1) and FLAG-tagged zyxin (FLAG-zyxin) into COS-7 cells. At 48 h after transfection, cell lysates were incubated with anti-FLAG antibody, followed by incubation with protein A/G beads. Transfected FLAG-zyxin and GFP-CT-PAR-1 were detected with anti-FLAG and anti-GFP antibodies, respectively, (Fig. 4B). These data showed that the C-terminal domain of PAR-1 forms a complex with full-length zyxin.
To map the domain of zyxin that interacts with the C-terminal domain of PAR-1, we performed a GST pull-down assay. The following GST-fusion domains of zyxin were generated: L1–3 (residues 375-572), containing LIM domains 1, 2, and 3; L1–2 (residues 375-503), containing LIM domains 1 and 2; L2–3 (residues 444-572), containing LIM domains 2 and 3; L1 (residues 375-443), containing LIM domain 1; L2 (residues 444-503), containing LIM domain 2; and L3 (residues 504-572), containing LIM domain 3 (Fig. 4C). Equal amounts of COS-7 cell lysates expressing GFP-CT-PAR-1 were incubated with GST beads alone or GST-fusion protein beads for 3 h, and GFP-CT-PAR-1 associated with GST-zyxin deletion mutants was pulled down and analyzed by Western blot with anti-GFP antibody. The Ponceau S staining of membrane showed the expression of GST-zyxin deletion mutants (Fig. 4D). The N-terminal domain of zyxin did not interact with GFP-CT-PAR-1 (data not shown), suggesting that the proline-rich region of zyxin is not required for the interaction with PAR-1. The data showed that LIM motifs 1 and 2 are the primary domains responsible for the interaction of zyxin with PAR-1 (Fig. 4D, top panel). In this experiment, COS-7 cell lysate expressing GFP alone was incubated with GST-LIM 1–3 beads, serving as a negative control to rule out the possibility that GST-zyxin deletion mutants interact with GFP alone.
The PAR-1-zyxin interaction is involved in thrombin-induced cytoskeletal rearrangement and SRE-dependent gene transcription in HUVECs
We investigated whether interaction of zyxin with PAR-1 is required in thrombin-induced actin stress fiber formation and SRE-dependent gene transcription. To disrupt the interaction between endogenous PAR-1 and zyxin, we used cDNA construct L1–3 (residues 375-572) containing LIM domains of zyxin that interact with PAR-1. COS-7 cells were transfected with different amounts of L1–3 together with GFP-CT-PAR-1 and FLAG-zyxin (Fig. 5A). Full-length zyxin was immunoprecipitated with FLAG antibody, and the presence of GFP-CT-PAR-1 was detected with GFP antibody. Data showed that increased amount of L1–3 resulted in decreased association of GFP-CT-PAR-1 with full-length zyxin (Fig. 5A), suggesting that overexpression of LIM domains of zyxin can disrupt the interaction between zyxin and PAR-1.
Figure 5.
Zyxin contributes to thrombin signaling via interaction with cytoplasmic tail of PAR-1. A) Expression of LIM domains of zyxin disrupted the interaction between full-length zyxin and cytoplasmic tail of PAR-1. HUVECs were cotransfected with different amount (0, 1.5, 3 μg) of plasmid encoding for Myc-tagged C-terminal LIM domain of zyxin (Myc-L1–3) together with FLAG-tagged full-length zyxin (3 μg) and GFP-CT-PAR-1 (3 μg). Coexpression of FLAG-tagged full-length zyxin, and GFP served as a negative control for this experiment, whereas coexpression of FLAG-CT-zxyin (FLAG-L1–3) and GFP-CT-PAR-1 served as a positive control for this experiment. At 48 h after transfection, cells were lysed, and the cleared cell extracts were subjected to immunoprecipitation with anti-FLAG antibody and A/G-agarose beads. Samples were analyzed by Western blotting and probed with GFP antibody to detect GFP-CT-PAR-1 coimmunoprecipitated with zyxin, probed with anti-FLAG antibody to detect immunoprecipitated FLAG-zyxin and FLAG-L1–3. B) Expression of L1–3 inhibited thrombin-induced stress fiber formation in HUVECs. HUVECs were grown on gelatin-coated coverslips, transiently transfected with plasmid encoding for Myc-tagged L1–3, and stimulated with 25 nM thrombin for 15 min. Thereafter, cells were fixed and stained with anti-Myc antibody, followed by anti-mouse Alexa Fluor 488 antibody to detect transfected Myc-L1–3 (green channel) and phalloidin Alexa Fluor 594 to detect actin fibers (red channel). C) Expression of L1–3 inhibited thrombin-induced-SRE activation in HUVECs. HUVECs were cotransfected with the plasmids encoding for Myc-L1–3, SRE-driven luciferase reporter, and Renilla were cotransfected for 48 h before the experiments. SRE assay was performed as described above. Results are expressed as means ± sd of 3 independent experiments performed in triplicates. **P < 0.01. D) Expression of L1–3 did not inhibit SRE activation induced by constitutively active RhoA (RhoA V14) in HUVECs. HUVECs were transiently transfected with plasmids encoding for Myc-L1–3 and RhoA-V14 for 48 h. SRE activity was measured as described above.
To study the role of interaction between zyxin and PAR-1 in thrombin signaling, we transfected HUVECs with L1–3, stimulated with 25 nM thrombin for 5 min, and analyzed actin stress fiber formation (Fig. 5B). The data showed that in the cells expressing L1–3, thrombin treatment did not induce actin stress fiber formation and that the F-actin network was completely disorganized, which led to a loss of cell shape (Fig. 5B).
Thrombin-induced SRE-dependent gene transcription was examined in HUVECs transfected with L1–3 (Fig. 5C). Data showed that in the cells expressing L1–3, thrombin-induced SRE-dependent gene transcription was inhibited (Fig. 5C).
As RhoA mediates thrombin-induced actin stress fiber formation and SRE-dependent gene transcription (35, 42, 43), we analyzed whether disruption of the interaction between zyxin and PAR-1 can affect RhoA-induced SRE-dependent gene transcription (Fig. 5D). HUVECs were transfected with activated RhoA (RhoAV14) with or without L1–3, and SRE activity was measured as described earlier. Data showed that RhoAV14 induced a 4-fold increase of SRE activity, which was not affected by overexpression of L1–3 (Fig. 5D). Similarly, L1–3 did not affect actin stress fiber formation induced by RhoAV14 (data not shown). These results indicate that zyxin interaction with PAR-1 is required for thrombin-induced actin stress fiber formation and SRE-dependent gene transcription but does not affect similar responses induced by RhoA, suggesting the involvement of a novel signaling pathway.
Heterotrimeric G protein-independent signaling: depletion of zyxin did not affect G-protein-mediated PAR-1 signaling in HUVECs
PAR-1 mediates thrombin signaling via activation of heterotrimeric G-proteins G12/13, Gq, and Gi 5–7. In particular, G12/13 and Gq were shown to mediate thrombin-induced cytoskeleton rearrangement and SRE-dependent gene transcription (33, 44, 45). Therefore, we determined whether zyxin itself or its interaction with cytoplasmic tail of PAR-1 would affect PAR-1-mediated activation of heterotrimeric G proteins.
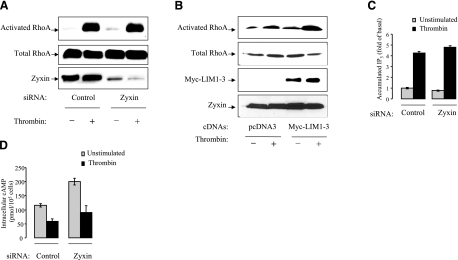
As RhoA is an established effector of Gα13 protein (46), we tested how zyxin down-regulation would affect thrombin-induced RhoA activation. HUVECs transfected with control and zyxin siRNA oligonucleotides were incubated with 25 nM thrombin for 15 min (Fig. 6A). Thereafter, the cells were lysed, and RhoA activity was determined using affinity-precipitation of activated RhoA with Rhotekin-GST beads. The result showed that depletion of zyxin did not affect thrombin-induced RhoA activation. Similarly, expression of L1–3 domain of zyxin did not affect thrombin-induced RhoA activation (Fig. 6B).
Figure 6.
Down-regulation of zyxin did not inhibit G-protein-mediated thrombin signaling in HUVECs. A) Knockdown of zyxin did not inhibit thrombin-induced RhoA activation. HUVECs transfected with control or zyxin siRNA were stimulated with 25 nM thrombin for 15 min. Cells were lysed, and the cleared cell extracts were immediately incubated with GST-RBD-conjugated beads for 90 min. Activated RhoA that precipitated with RBD-bound beads was detected by immunoblotting with anti-RhoA antibody. Total cell lysates were subjected to immunoblotting to determine the expression level of total RhoA. B) Expression of L1–3 did not inhibit thrombin-induced RhoA activation. HUVECs were transfected with L1–3 for 48 h before the experiments. RhoA activation assay was performed as described above. C) Knockdown of zyxin did not inhibit thrombin-induced generation of IP3 in HUVECs. HUVECs, grown in 12-well plates, were transfected with control or zyxin siRNA oligonucleotides. Cells were incubated with 5 μCi (1 Ci=37 GBq) [3H] myo-inositol per well for 48 h before stimulation with thrombin (25 nM) for 30 min. Accumulated intracellular IP3 was measured as described in Materials and Methods. Experiments were performed in triplicates. Data are expressed as means ± sd. D) Down-regulation of zyxin did not inhibit thrombin-induced reduction of cAMP level in HUVECs. HUVECs were transfected with control or zyxin siRNA oligonucleotides for 48 h. Cells were pretreated with 0.5 mM IMBX for 10 min and then treated with 25 nM thrombin in the presence of IMBX and 1 μM forskolin for 30 min before experiment. Intracellular cAMP levels were measured as described in Materials and Methods. Experiments were performed in triplicates. Data are expressed as means ± sd.
We tested whether down-regulation of zyxin can affect activation of other Gα subunits that are coupled to PAR-1. As Gαq protein mediates thrombin-induced IP3 accumulation (47), we assessed the effect of depletion of zyxin on thrombin-induced IP3 accumulation. HUVECs transfected with control or zyxin siRNA oligonucleotides were stimulated with thrombin. The accumulation of radioactively labeled IP3 was determined. Thrombin treatment induced ∼4 fold increase in IP3 accumulation in both control and zyxin siRNA-transfected cells, indicating that down-regulation of zyxin did not affect thrombin-induced IP3 accumulation (Fig. 6C).
Gαi proteins mediate thrombin-induced inhibition of adenylyl cyclase (48). To stimulate cAMP accumulation, HUVECs were treated with 1 μM forskolin for 30 min at 37°C. To see the inhibitory effect of thrombin, cells were pretreated with thrombin for 15 min prior to the addition of forskolin. Thrombin pretreatment inhibited forskolin-induced cAMP accumulation, which was consistent with activation of Gαi protein. However, down-regulation of zyxin did not affect thrombin-induced inhibition of adenylyl cyclase (Fig. 6D). Together, these data suggest that zyxin transduces PAR-1 signaling in a G-protein-independent manner.
Thrombin-induced redistribution of VASP is zyxin dependent in HUVECs
Zyxin is thought to regulate cytoskeleton dynamics in a VASP-dependent manner by binding and targeting VASP to the sites of dynamic actin assembly, where VASP can bind to F-actin and stimulate actin polymerization (17, 49). Zyxin and VASP are colocalized in nonstimulated HUVECs (Fig. 7A). Stimulation with thrombin resulted in apparent relocalization of both zyxin and VASP to focal adhesions and stress fibers (Fig. 7B).
Figure 7.
Translocation of VASP after thrombin stimulation is zyxin dependent in HUVECs. A) VASP translocated on thrombin stimulation and colocalizes with zyxin. HUVECs were stimulated with 25 nM thrombin for 5 min, fixed, and double-stained for zyxin and VASP with anti-zyxin and anti-VASP antibody followed by anti-goat Alexa Fluor 488 and anti-rabbit Alexa Fluor 594 antibody, respectively. B) Down-regulation of zyxin prevents VASP from translocation after thrombin stimulation. HUVECs were transfected with control or zyxin-specific shRNA plasmid for 48 h prior to stimulation with 25 nM thrombin for 5 min. Distribution of VASP was visualized by immunostaining with anti-VASP antibody, followed by anti-rabbit Alexa Fluor 594 antibody. Images were acquired by confocal microscopy as described in Fig. 1B.
To test whether zyxin is required for thrombin-induced VASP translocation, we transfected HUVECs with control or zyxin shRNA plasmids that express both shRNAs and GFP. Notably, the thrombin-induced redistribution of VASP is abolished by depletion of zyxin (Fig. 7B). As localization of VASP is critical for its function (50), our data suggested that zyxin is involved in thrombin-mediated cytoskeleton changes via recruitment of VASP in endothelial cells.
Our data suggest that zyxin is a component of a novel PAR-1 signaling pathway involved in thrombin-induced cytoskeletal rearrangement and SRE-dependent gene transcription in endothelial cells (Fig. 8). Notably, interaction of zyxin with PAR-1 does not influence PAR-1-mediated activation of heterotrimeric G-proteins, indicating that zyxin transduces PAR-1 signaling in a G-protein-independent manner. Furthermore, zyxin may mediate PAR-1 signaling to cytoskeleton in endothelial cells via recruitment of VASP.
Figure 8.
Proposed model for the role of zyxin in thrombin signaling in endothelial cells. In endothelial cells, zyxin transduces thrombin signals to cytoskeleton via interaction with cytoplasmic tail of PAR-1 in G-protein independent manner. Zyxin participates in thrombin-induced cytoskeletal alteration through recruitment of VASP.
DISCUSSION
In this study, we demonstrated that zyxin is involved in thrombin-mediated actin cytoskeleton remodeling in endothelial cells. Down-regulation of zyxin significantly potentiated thrombin-induced endothelial barrier dysfunction, indicating an important role of zyxin in the regulation of endothelial barrier function. We determined that zyxin binds to the C-terminal domain of PAR-1 and that this binding is required for thrombin-induced cytoskeletal rearrangement and SRE-dependent gene transcription. Moreover, we observed that VASP is targeted to focal adhesions and stress fibers in a zyxin-dependent manner after thrombin stimulation. These data suggested that zyxin might recruit VASP and participate in actin polymerization and reorganization after sensing extracellular signal of thrombin through direct interaction with PAR-1. Notably, we found that down-regulation of zyxin did not affect thrombin-induced activation of Gi, Gq, and G12/13 heterotrimeric G proteins, which are coupled to PAR-1 in endothelial cells. This study provides evidence that zyxin transduces PAR-1 signaling in endothelial cells independently of G-proteins.
Zyxin is involved in thrombin-mediated cytoskeletal rearrangement in endothelial cells
Thrombin generated under such pathological conditions as acute inflammation and atherosclerosis stimulates the endothelial paracellular gap formation and barrier dysfunction through regulation of cytoskeleton organization (32, 51). Focal adhesion plaques, which anchor actin filaments and distribute tension throughout the cells, are the critical regulatory sites involved in thrombin-induced endothelial barrier function (31). As a focal adhesion protein, zyxin is shown to regulate actin assembly and dynamics through targeting actin regulatory proteins to actin assembly sites. Our data showed that in HUVECs, zyxin redistributed to newly formed focal adhesions and stress fibers after thrombin stimulation. Therefore, we set out to investigate the role of zyxin in thrombin signaling to cytoskeleton in endothelial cells. Loss of function assays using zyxin RNA interference technology provided evidence for the involvement of zyxin in thrombin-induced actin remodeling and SRE-dependent gene transcription in endothelial cells. F-actin staining in zyxin shRNA transfected HUVECs showed that the thrombin-induced stress fibers were diminished, and actin filaments were also disorganized. Consistent with cytoskeletal alterations determined by immunofluorescence studies, depletion of zyxin potentiated thrombin-induced endothelial permeability, as evident by further decreased electrical resistance during the thrombin treatment. The result of TER measurement in real time also showed that depletion of zyxin delayed the recovery of endothelial barrier function, suggesting that zyxin might regulate recovery of endothelial barrier function after thrombin stimulation. Although the basis of restoration of endothelial barrier subsequent to barrier dysfunction remains unclear, several lines of evidence indicate that activity of Rac-1, a RhoGTPase, is tightly associated with the reannealing of the endothelial barrier (35,36,37,38). Interestingly, we found that down-regulation of zyxin resulted in inhibition of thrombin-induced Rac1 activation 30 min and 1 h after thrombin stimulation (Fig. 3D), providing a possible explanation of a delayed restoration of the endothelial barrier function in the absence of zyxin. It was reported that zyxin interacts with Vav, a guanine nucleotide exchange factor specific for Rac family of proteins (18). It is tempting to investigate the functional relationship between PAR-1, zyxin, and Vav in endothelial cell signaling in future studies.
Zyxin is believed to regulate actin assembly and dynamics through recruitment of VASP, a member of the Ena/VASP family of proteins (17). Particularly, recent work provided evidence that zyxin acts as a molecular sensor for mechanical force, and promotes actin polymerization and cytoskeletal reinforcement in a VASP-dependent manner (25, 39, 52). Consistent with these findings, we found that both zyxin and VASP redistributed to focal adhesions and along stress fibers after thrombin stimulation in HUVECs. Thus, we postulated that after sensing thrombin stimulation through interaction with PAR-1, zyxin recruits VASP to the sites of actin assembly and stimulates actin polymerization and reorganization.
In addition to thrombin effect on cytoskeletal rearrangement, which is the acute cellular response to thrombin stimulation, thrombin signaling in endothelial cells also results in activation of serum response factor (SRF), a transcription factor for SRE found in growth-factor-regulated gene promoters. In our study, both down-regulation of endogenous zyxin by zyxin shRNA plasmid and disruption of zyxin interaction with PAR-1 by overexpression of LIM domains of zyxin inhibited thrombin-induced SRE activation, which suggests that zyxin is critical in this cellular response to thrombin. Several lines of evidence suggest that SRF activity is regulated by alterations in actin dynamics, as actin polymerization leads to the change in the ratio of polymerized actin (F actin) to monomeric actin (G-actin) and SRF may “sense” this change and become activated (53,54,55,56,57). Because of zyxin’s capacity to promote actin polymerization, it is plausible that zyxin participates in thrombin-induced cytoskeletal alteration, resulting in activation of SRF and initiation of SRE-dependent gene transcription. In this case, the observation that zyxin contributes to thrombin-induced SRE activation provides supportive evidence for the conclusion that zyxin mediates thrombin-induced cytoskeleton rearrangement. As zyxin contains an NES, it has been implicated in regulation of gene transcription (23). Thus, we could not exclude the possibility that zyxin directly regulates SRE activity through transporting transcriptional regulators into or out of nucleus. However, as zyxin was not found shuttling between cytosol and nucleus after thrombin stimulation in HUVECs, this possibility seems unlikely.
Zyxin interacts with PAR-1 in endothelial cells
The major question addressed by our study is how zyxin contributes to thrombin signaling in endothelial cells. We identified the interaction of zyxin with cytoplasmic domain of PAR-1, providing a possible mechanism of involvement of zyxin in thrombin signaling.
PAR-1 is the predominant receptor mediating thrombin signaling in endothelial cells. The traditional paradigm for PAR-1 has been that it transduces the thrombin signal to the Gi/o, Gq, and G12/13 families of heterotrimeric G proteins, which allows the regulation of multiple signal transduction pathways (5,6,7). In endothelial cells, small GTPase RhoA is an established mediator of PAR-1 signaling to the cytoskeleton (34). Here, PAR-1 was found to interact with zyxin, and zyxin mediated PAR-1 signaling to the cytoskeleton with no effect on thrombin-induced RhoA activation. It was reported that PAR-1 interacts with creatine kinase (58) and Hsp90 (11). In contrast to zyxin, these two proteins are involved in thrombin-induced cytoskeletal rearrangement through activation of RhoA. Therefore, we believe that exact mapping of the interaction sites between PAR-1 and zyxin in combination with detailed biochemical studies in the future will allow for more specific characterization of the PAR-1-zyxin function.
We have begun the initial investigation of PAR-1-zyxin interaction in the regulation of PAR-1-dependent responses. It will be important to identify the exact interaction sites between PAR-1 and zyxin that will allow developing a peptide that could disrupt such interaction. It was reported that the N-terminal domain of zyxin is involved in actin polymerization, whereas the C-terminal domain containing LIM domain L1–3 did not affect actin dynamics (17). In addition, the L1–3 domain inhibits SRE activation induced by thrombin but not by RhoA (Fig. 5D). Similarly, data show that actin stress fibers induced by RhoA are not affected by L1–3 (data not shown).
Zyxin and G proteins are activated by thrombin
Classically, GPCRs transduce extracellular signals by coupling to heterotrimeric G proteins, although recent studies have clearly demonstrated that they can signal via G protein–independent mechanisms (59). The best-characterized and best-understood regulators of GPCR signal transduction are G-protein-coupled receptor kinases (GRKs) and β-arrestins (59). GRKs catalyze serine/threonine phosphorylation at multiple sites in the cytoplasmic tails of GPCRs, which promotes agonist-induced recruitment of β-arrestins to the cytoplasmic domain of a GPCR (59). Recently, G protein–independent signaling by the angiotensin II type 1 receptor was reported; however, the molecular mechanism of this regulation remained unknown (60). Our data uncovered a novel signaling pathway that is activated by GPCR independently of heterotrimeric G proteins. Our data uncovered a novel signaling axis, PAR-1-zyxin, that regulates actin remodeling and SRE-dependent gene transcription in a manner that is independent of heterotrimeric G proteins.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants GM56159 and P01 HL60678 and by American Heart Association predoctoral fellowship 0710113Z to J.H.
References
- Narayanan S. Multifunctional roles of thrombin. Ann Clin Lab Sci. 1999;29:275–280. [PubMed] [Google Scholar]
- Minami T, Sugiyama A, Wu S Q, Abid R, Kodama T, Aird W C. Thrombin and phenotypic modulation of the endothelium. Arterscler Thromb Vasc Biol. 2004;24:41–53. doi: 10.1161/01.ATV.0000099880.09014.7D. [DOI] [PubMed] [Google Scholar]
- Coughlin S R. How the protease thrombin talks to cells. Proc Natl Acad Sci U S A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P J, Prevost N, Molino M, Hollinger M K, Woolkalis M J, Woulfe D S, Brass L F. Thrombin responses in human endothelial cells. contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem. 2000;275:13502–13509. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- Rahman A, True A L, Anwar K N, Ye R D, Voyno-Yasenetskaya T A, Malik A B. Galpha(q) and gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ Res. 2002;91:398–405. doi: 10.1161/01.res.0000033520.95242.a2. [DOI] [PubMed] [Google Scholar]
- Vanhauwe J F, Thomas T O, Minshall R D, Tiruppathi C, Li A, Gilchrist A, Yoon E J, Malik A B, Hamm H E. Thrombin receptors activate G(o) proteins in endothelial cells to regulate intracellular calcium and cell shape changes. J Biol Chem. 2002;277:34143–34149. doi: 10.1074/jbc.M204477200. [DOI] [PubMed] [Google Scholar]
- Martin C B, Mahon G M, Klinger M B, Kay R J, Symons M, Der C J, Whitehead I P. The thrombin receptor, PAR-1, causes transformation by activation of rho-mediated signaling pathways. Oncogene. 2001;20:1953–1963. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- McLaughlin J N, Shen L, Holinstat M, Brooks J D, Dibenedetto E, Hamm H E. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- Chikumi H, Fukuhara S, Gutkind J S. Regulation of G protein-linked guanine nucleotide exchange factors for rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- Buhl A M, Johnson N L, Dhanasekaran N, Johnson G L. G alpha 12 and G alpha 13 stimulate rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- Pai K S, Mahajan V B, Lau A, Cunningham D D. Thrombin receptor signaling to cytoskeleton requires Hsp90. J Biol Chem. 2001;276:32642–32647. doi: 10.1074/jbc.M104212200. [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, Kozasa T, Voyno-Yasenetskaya T A. Interaction between the G alpha subunit of heterotrimeric G (12) protein and Hsp90 is required for G alpha(12) signaling. J Biol Chem. 2001;276:46088–46093. doi: 10.1074/jbc.M108711200. [DOI] [PubMed] [Google Scholar]
- Macalma T, Otte J, Hensler M E, Bockholt S M, Louis H A, Kalff-Suske M, Grzeschik K H, von der Ahe D, Beckerle M C. Molecular characterization of human zyxin. J Biol Chem. 1996;271:31470–31478. doi: 10.1074/jbc.271.49.31470. [DOI] [PubMed] [Google Scholar]
- Beckerle M C. Zyxin: zinc fingers at sites of cell adhesion. BioEssays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle M C, Golsteyn R M. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000;275:22503–22511. doi: 10.1074/jbc.M001698200. [DOI] [PubMed] [Google Scholar]
- Li B, Trueb B. Analysis of the alpha-actinin/zyxin interaction. J Biol Chem. 2001;276:33328–33335. doi: 10.1074/jbc.M100789200. [DOI] [PubMed] [Google Scholar]
- Fradelizi J, Noireaux V, Plastino J, Menichi B, Louvard D, Sykes C, Golsteyn R M, Friederich E. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat Cell Biol. 2001;3:699–707. doi: 10.1038/35087009. [DOI] [PubMed] [Google Scholar]
- Hobert O, Schilling J W, Beckerle M C, Ullrich A, Jallal B. SH3 domain-dependent interaction of the proto-oncogene product vav with the focal contact protein zyxin. Oncogene. 1996;12:1577–1581. [PubMed] [Google Scholar]
- Dawid I B, Toyama R, Taira M. LIM domain proteins. Compt Rendus Acad Sci III Sci Vie. 1995;318:295–306. [PubMed] [Google Scholar]
- Schmeichel K L, Beckerle M C. LIM domains of cysteine-rich protein 1 (CRP1) are essential for its zyxin-binding function. Biochem J. 1998;331:885–892. doi: 10.1042/bj3310885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Kloeker S, Jensen C C, Bockholt S, Honda H, Hirai H, Beckerle M C. Members of the zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol. 2000;149:1073–1086. doi: 10.1083/jcb.149.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D A, Fradelizi J, Bockholt S, Menichi B, Louvard D, Friederich E, Beckerle M C. Targeting of zyxin to sites of actin membrane interaction and to the nucleus. J Biol Chem. 2001;276:34759–34767. doi: 10.1074/jbc.M102820200. [DOI] [PubMed] [Google Scholar]
- Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski C C, Schaefer E, Beckerle M, Sussman M A. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshigi M, Hoffman L M, Jensen C C, Yost H J, Beckerle M C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lai Y J, Lin W C, Lin F T. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J Biol Chem. 2004;279:10459–10468. doi: 10.1074/jbc.M311891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovoy M, Niu J, Bernard O, Profirovic J, Minshall R, Neamu R, Voyno-Yasenetskaya T. LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem. 2005;280:26533–26542. doi: 10.1074/jbc.M502921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva A V, Kutuzov M A, Vaiskunaite R, Profirovic J, Meigs T E, Predescu S, Malik A B, Voyno-Yasenetskaya T. G alpha12 interaction with alphaSNAP induces VE-cadherin localization at endothelial junctions and regulates barrier function. J Biol Chem. 2005;280:30376–30383. doi: 10.1074/jbc.M502844200. [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, Adarichev V, Furthmayr H, Kozasa T, Gudkov A, Voyno-Yasenetskaya T A. Conformational activation of radixin by G13 protein alpha subunit. J Biol Chem. 2000;275:26206–26212. doi: 10.1074/jbc.M001863200. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya T, Conklin B R, Gilbert R L, Hooley R, Bourne H R, Barber D L. G alpha 13 stimulates na-H exchange. J Biol Chem. 1994;269:4721–4724. [PubMed] [Google Scholar]
- Schaphorst K L, Pavalko F M, Patterson C E, Garcia J G. Thrombin-mediated focal adhesion plaque reorganization in endothelium: Role of protein phosphorylation. Am J Resp Cell Mol Biol. 1997;17:443–455. doi: 10.1165/ajrcmb.17.4.2502. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik A B. Mechanisms of increased endothelial permeability. Canad J Physiol Pharmacol. 1996;74:787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- Mao J, Yuan H, Xie W, Simon M I, Wu D. Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J Biol Chem. 1998;273:27118–27123. doi: 10.1074/jbc.273.42.27118. [DOI] [PubMed] [Google Scholar]
- Dudek S M, Garcia J G. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley A J. Rho and rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Baumer Y, Drenckhahn D, Waschke J. cAMP induced rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol. 2008;129:765–778. doi: 10.1007/s00418-008-0422-y. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik A B. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Waschke J, Baumgartner W, Adamson R H, Zeng M, Aktories K, Barth H, Wilde C, Curry F E, Drenckhahn D. Requirement of rac activity for maintenance of capillary endothelial barrier properties. Am J Physiol Heart Circ Physiol. 2004;286:H394–401. doi: 10.1152/ajpheart.00221.2003. [DOI] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- Lele T P, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber D E. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- Yi J, Beckerle M C. The human TRIP6 gene encodes a LIM domain protein and maps to chromosome 7q22, a region associated with tumorigenesis. Genomics. 1998;49:314–316. doi: 10.1006/geno.1998.5248. [DOI] [PubMed] [Google Scholar]
- Sahai E, Alberts A S, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C S, Wynne J, Treisman R. The rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Gohla A, Offermanns S, Wilkie T M, Schultz G. Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J Biol Chem. 1999;274:17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Mehta D, Kozasa T, Minshall R D, Malik A B. Protein kinase calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart M J, Sternweis P M, Singer W D, Gilman A G, Bollag G, Sternweis P C. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Conklin B R, Chabre O, Wong Y H, Federman A D, Bourne H R. Recombinant gq alpha. mutational activation and coupling to receptors and phospholipase C. J Biol Chem. 1992;267:31–34. [PubMed] [Google Scholar]
- Katada T, Northup J K, Bokoch G M, Ui M, Gilman A G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984;259:3578–3585. [PubMed] [Google Scholar]
- Laurent V, Loisel T P, Harbeck B, Wehman A, Grobe L, Jockusch B M, Wehland J, Gertler F B, Carlier M F. Role of proteins of the Ena/VASP family in actin-based motility of listeria monocytogenes. J Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y H, Chung C Y, Wessels D, Stephens S, Titus M A, Soll D R, Firtel R A. Requirement of a vasodilator-stimulated phosphoprotein family member for cell adhesion, the formation of filopodia, and chemotaxis in dictyostelium. J Biol Chem. 2002;277:49877–49887. doi: 10.1074/jbc.M209107200. [DOI] [PubMed] [Google Scholar]
- Garcia J G, Schaphorst K L. Regulation of endothelial cell gap formation and paracellular permeability. J Invest Med. 1995;43:117–126. [PubMed] [Google Scholar]
- Hoffman L M, Jensen C C, Kloeker S, Wang C L, Yoshigi M, Beckerle M C. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J Cell Biol. 2006;172:771–782. doi: 10.1083/jcb.200512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J W, Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol Biol Cell. 2002;13:4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneste O, Copeland J W, Treisman R. LIM kinase and diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R, Copeland J W, Newsome T P, Way M, Treisman R. A role for VASP in RhoA-diaphanous signalling to actin dynamics and SRF activity. EMBO J. 2003;22:3050–3061. doi: 10.1093/emboj/cdg287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posern G, Sotiropoulos A, Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol Biol Cell. 2002;13:4167–4178. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Mahajan V B, Pai K S, Lau A, Cunningham D D. Creatine kinase, an ATP-generating enzyme, is required for thrombin receptor signaling to the cytoskeleton. Proc Natl Acad Sci U S A. 2000;97:12062–12067. doi: 10.1073/pnas.97.22.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal K, Lefkowitz R J, Rockman H A. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115:2971–2974. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, Roden D M, Wagner T, Yatani A, Vatner D E, Vatner S F, Sadoshima J. Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.