Abstract
Growing evidence shows that trafficking of the μ-opioid receptor (MOR) is a critical process in functional recovery from desensitization following activation and plays important roles in morphine tolerance and dependence largely because of the failure of morphine to promote such trafficking. However, morphine tolerance and dependence are believed to be mediated by multiple mechanisms, including well-documented biochemical changes in cAMP activity, N-methyl-d-aspartate receptors (NMDARs), glucocorticoid receptors (GRs), and c-fos. Here, we assess the consequences of promoting morphine-induced endocytosis on these biochemical changes utilizing a knock-in mouse model, RMOR, in which MORs undergo morphine-induced endocytosis. Chronic morphine treatment of wild-type (WT) mice promoted superactivation of adenylyl cyclase, alterations in NMDARs, and up-regulation of GR and c-fos in distinct brain regions. Notably, none of these biochemical changes occurred in the RMOR-knock-in mice. Together, these data demonstrate that morphine tolerance and dependence are mediated by multiple biochemical mechanisms and that MOR endocytosis plays a critical role in each of these mechanisms.—He, L., Kim, J. A., Whistler, J. L. Biomarkers of morphine tolerance and dependence are prevented by morphine-induced endocytosis of a mutant μ-opioid receptor.
Keywords: adenylyl cyclase, c-fos, glucocorticoid receptor, NMDA receptor, trafficking
Morphine is among the most effective and commonly used analgesics in clinical practice. However, its utility for the treatment of chronic pain is often hampered by the need for dose escalation due to the development of analgesic tolerance. This dose escalation not only puts patients at a greater risk for severe side effects, such as respiratory depression, but also increases the liability for physical dependence or addiction to the drug.
The effects of morphine are mediated primarily through activation of the μ-opioid receptor (MOR), as the analgesic, rewarding, and withdrawal-induced aversive effects of morphine are eliminated in mice disrupted for the MOR (1, 2). However, multiple signal transduction systems are engaged once MOR is activated by morphine. Thus, the behavioral manifestation of morphine tolerance and dependence is likely caused by a complex interplay of several events or adaptations occurring in multiple neurotransmitter systems. Indeed, changes as diverse as activation of adenylyl cyclase, alterations in N-methyl-d-aspartate receptor (NMDAR) levels, and glucocorticoid receptor (GR) levels have been implicated in the development of morphine tolerance and/or dependence (3,4,5,6,7,8,9). Thus, although they are distinct phenomena, each is considered a biochemical hallmark of morphine tolerance and dependence, because manipulations of each of these systems influence these behaviors. However, whether these adaptations are related to one another and how activity at a single receptor, the MOR, can dramatically affect so many diverse systems remains unknown.
Not all drugs that activate the MOR promote tolerance and dependence to the same degree. Indeed, morphine has a much higher liability for promoting tolerance and dependence than some other opioid drugs when these drugs are administered at equianalgesic doses (10, 11). While each opioid drug in these studies has a unique constellation of activities at the receptor, morphine is unique among opioid agonists in that it fails to promote substantial endocytosis of the MOR in vivo (12,13,14,15,16). Recently, we generated a knock-in mouse expressing a mutant form of the MOR, RMOR (for recycling MOR), that does undergo morphine-induced endocytosis and recycling, and demonstrated that promoting endocytosis of the MOR in response to morphine in these mice reduced tolerance and dependence (10). For this study, this RMOR mouse provided us with a unique tool to examine which of the many changes reportedly associated with chronic morphine treatment were influenced by the trafficking of the MOR.
MATERIALS AND METHODS
Animals and drugs
WT and RMOR mice were generated as littermates by crossing heterozygous RMOR C57BL/6 × 129SvJ mice, as previously reported (10). All animal experiments were performed in accordance with the Ernest Gallo Clinic and Research Center Institutional Animal Care and Use Committee guidelines. [3H]Adenosine 3′,5′-cyclic phosphate (cAMP) (32.1 Ci/mmol) was purchased from PerkinElmer Life Science (Boston, MA, USA). Morphine sulfate and naloxone HCl were obtained from Sigma Chemical (St. Louis, MO, USA).
Antinociception assessment and tolerance induction
Antinociceptive responses were measured with the hot-plate assay, as previously reported (10). Briefly, mice were placed on a hot plate maintained at 56°C. Drugs were dissolved in physiological saline and injected in a volume of 10 ml/kg. Mice were injected subcutaneously (s.c.) 2×d with either morphine (10 mg/kg) or saline for 5 d. Data are reported as maximal possible effect (MPE), calculated as 100% × [(drug response time − basal response time)/(cutoff time − basal response time)].
Morphine withdrawal
Morphine withdrawal was precipitated with s.c. injection of naloxone (2 mg/kg) after the hot-plate test on d 5. Four withdrawal behaviors (jumping, rearing, paw tremor, and weight loss) were counted over a 20-min period of time. A global withdrawal score was calculated for each animal, as previously reported (17).
Tissue homogenate preparation
Mice were killed by cervical dislocation following the antinociception test on d 5, and brain regions were dissected out on ice. For the adenylyl cyclase activity measurement, tissues were homogenized in buffer (20 mM Tris-HCl, pH 7.4; 2 mM EGTA; 1 mM MgCl2; and 250 mM sucrose) and centrifuged at 27,000 g for 15 min at 4°C. The pellet was resuspended in the same buffer and centrifuged for 15 min. The pellet was resuspended in ice-cold buffer (2 mM Tris-HCl, pH 7.4, and 2 mM EGTA). For the NMDAR immunoblot, tissues were homogenized in ice-cold buffer 1 (10 mM Tris acetate and 5 mM EDTA, pH 7.4) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The homogenates were centrifuged at 30,000 g for 30 min at 4°C, and the pellets were resuspended in buffer 2 (50 mM Tris acetate and 5 mM EDTA, pH 7.4) with protease inhibitor as in buffer 1. For the GR immunoblot, tissues were homogenized in ice-cold buffer (50 mM Tris-HCl, 0.1% SDS, and 1 mM EDTA) containing protease inhibitor cocktail, and were used without centrifugation. The protein concentration was measured using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA, USA), and all homogenates were portioned into aliquots and stored at −80°C until use.
Measurement of adenylyl cyclase activity via cAMP accumulation
The experiments were performed based on the method of Izenwasser (18). Tissue homogenate (20 μg of protein in 10 μl) was added to tubes (final volume of 60 μl) containing (in mM): 80 Tris-HCl, pH 7.4; 10 theophylline; 1 MgSO4; 0.8 EGTA; 30 NaCl; 0.25 ATP; 0.01 GTP; and either forskolin (3 μM) or water. Samples were incubated at 30°C for 5 min. The tubes were placed in boiling water for 2 min. Next, [3H]cAMP (final concentration of 4 nM) in citrate-phosphate buffer (pH 5.0) and a binding protein prepared from bovine adrenal glands were added to assay tubes. Additional tubes containing known amounts of cAMP were prepared without tissue, and served as standards. Tubes were incubated for 90 min at 4°C, and reactions were terminated by the addition of charcoal and centrifugation (1000 g for 15 min, at 4°C) to separate the free [3H]cAMP from that bound to the binding protein. Aliquots of the supernatant containing bound cAMP were placed into scintillation vials, and scintillation cocktail was added. The radioactivity was measured using liquid scintillation spectrometry and was converted to picomoles of cAMP by comparison to the standard curve. Results were expressed as the percentage of basal activity, measured in the absence of forskolin.
Immunohistochemistry
Mice were deeply anesthetized (ketamine, 80 mg/kg, xylazine 12 mg/kg, i.p.) and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer 30 min after the injection on d 5. The brain was dissected out, postfixed overnight, and then transferred to a 30% sucrose buffer solution. Coronal sections (30 μm) were cut on a cryostat at −20°C, preincubated in PBT solution (0.1 M phosphate buffer + 0.2% BSA and 0.2% Triton X-100) for 30 min, blocked in 5% normal goat serum in PBT solution for another 30 min, and then incubated with rabbit anti-c-fos antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:1000 dilution overnight at 4°C. Sections were extensively washed with PBT and incubated in Alexa 488-conjugated goat anti-rabbit antibody (Molecular Probes, Eugene, OR, USA) at 2 μg/ml for 2 h at room temperature. The sections were then washed and mounted on slides. C-fos immunostaining was examined with a Zeiss confocal microscope using an ×10 objective (Carl Zeiss, Oberkochen, Germany) and quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunoblot
NMDAR immunoblots were performed as previously reported (14, 19). Membrane proteins (20 μg/lane) were solubilized by boiling in 2% SDS containing 50 mM dithiothreitol, subjected to 4–12% SDS-PAGE, and transferred to PVDF membranes. The membranes were blocked in blocking buffer (5% nonfat dry milk in 20 mM Tris-HCl containing 0.1% Tween-20 and 140 mM NaCl, pH 7.4) for 1 h at room temperature and then incubated overnight at 4°C in blocking buffer containing the following primary antibodies: monoclonal anti-NMDAR1 (1:5000; Chemicon, Temecula, CA, USA), or polyclonal anti-NMDAR2A or 2B affinity-purified (1:2000; Chemicon), and monoclonal anti-β-actin (1:20,000, Sigma). After washes with TBS containing 0.1% Tween-20, the membranes were incubated 2 h at room temperature with following horseradish peroxidase-conjugated secondary antibodies: goat anti-mouse at 1:10,000 dilution for NMDAR1 and 1:50,000 for β-actin; goat anti-rabbit at 1:5000 dilution for both NMDAR2A and 2B. Following washes, proteins were visualized with ECL. For GR, the following antibodies were used: polyclonal anti-GR antibody (1:5000; Santa Cruz Biotechnologies) and monoclonal anti-β-actin antibody (1:20,000; Sigma) as primary and horseradish peroxidase-conjugated goat anti-rabbit antibody at a dilution of 1:10,000 for GR and goat anti-mouse antibody at a dilution of 1:50,000 for β-actin as secondary, respectively. Following washes, proteins were visualized with ECL. Quantifications of immunoreactive bands were performed using Scion Image software (Scion Corporation, Frederick, MD, USA). The relative levels of NMDAR and GR protein levels were determined by normalization to the anti-β-actin immunoblot from the same membrane and are expressed as a percentage relative to the saline group.
Statistics
The comparison of the antinociceptive effect of morphine between wild-type and knock-in mice at different time points was analyzed by a 2-way ANOVA followed by a Bonferroni posttest. The comparison of global withdrawal scores between wild-type and RMOR-knock-in mice was analyzed with 1-way ANOVA followed by Newman-Keuls posttest. The comparisons of cAMP, c-fos, NMDAR, and GR levels between saline and morphine treatment were analyzed using Student’s t test.
RESULTS
Chronic morphine treatment induced superactivation of cAMP in WT but not RMOR mice
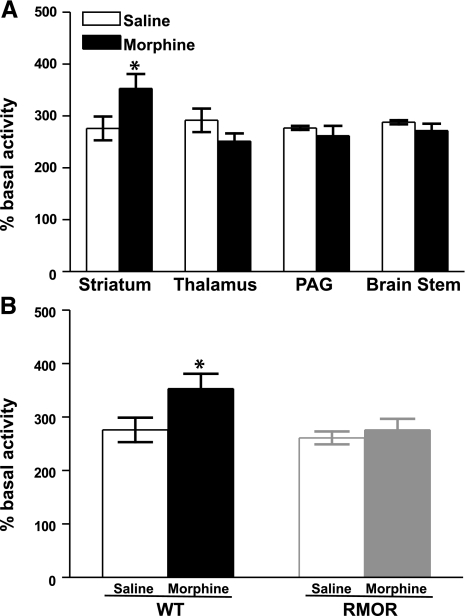
Chronic morphine treatment induced profound antinociceptive tolerance and physical dependence by d 5 in WT but not RMOR mice (Supplemental Fig. 1), consistent with previous reports (10). WT mice treated in this paradigm showed a significant superactivation of adenylyl cyclase activity in the striatum (Fig. 1A). This superactivation was brain region specific, as it was detectable in striatum but not in other brain regions examined, including the thalamus, periacqueductal gray (PAG), and brain stem (Fig. 1A). Notably, superactivation did not occur in any brain region, including striatum, in the RMOR mice (Fig. 1B).
Figure 1.
Adenylyl cyclase activity in WT and RMOR-knock-in mice after chronic morphine treatment. A) Adenylyl cyclase activity was assessed in membranes prepared from the striatum, thalamus, PAG, and brain stem of WT mice after 5 d of morphine (10 mg/kg, s.c., 2×/d) or saline treatment. B) Adenylyl cyclase activity was assessed in membranes prepared from the striatum of both WT and RMOR-knock-in mice after 5 d of morphine or saline treatment as in A. Data are means ± se from 4 independent experiments, each performed in triplicate. *P < 0.05 vs. saline group. PAG, periaqueductal gray.
Chronic morphine treatment altered NMDAR subunit levels in WT but not RMOR mice
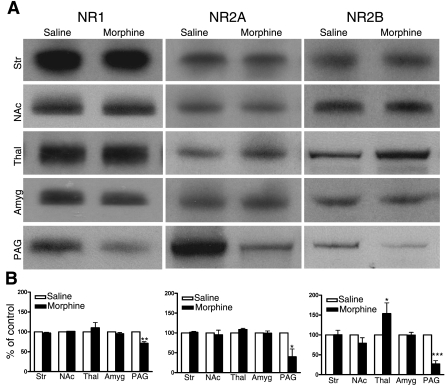
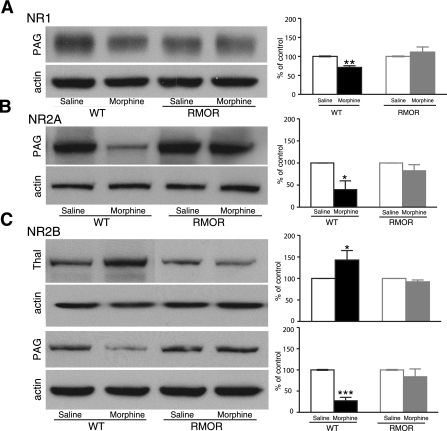
We next assessed whether this morphine treatment regimen would promote changes in NMDAR protein levels. Several brain regions were examined, including the striatum, nucleus accumbens (NAc), thalamus, amygdala, and PAG, all regions that are thought to be important for morphine-induced antinociception and/or physical dependence. In WT mice, the most predominant changes were observed in PAG, with significant reductions in NR1, NR2A, and NR2B levels after morphine treatment. We also observed a significant change in the thalamus where the NR2B subunit was up-regulated after morphine treatment (Fig. 2). We next examined whether any of these changes observed in the WT mice occurred in the RMOR mice. Notably, neither the down-regulation in PAG nor the up-regulation in thalamus occurred in the RMOR mice (Fig. 3).
Figure 2.
Region- and subunit-specific regulation of NMDAR subunit levels after chronic morphine treatment. A) Protein levels of the NMDAR subunits NR1, NR2A, and NR2B were assessed in mouse brain regions by immunoblot in WT mice treated with morphine (10 mg/kg, s.c., 2×/d) or saline. B) Results were quantified by normalization to β-actin, where saline was defined as 100%. Data are expressed as means ± se. Tissue samples were analyzed from ≥3 separate experiments. Str, striatum; NAc, nucleus accumbens; Thal, thalamus; Amyg, amygdala; PAG, periaqueductal gray. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline group.
Figure 3.
NMDAR subunit levels are not altered in RMOR-knock-in mice after chronic morphine treatment. Protein levels of the NMDAR subunits NR1 (A), NR2A (B), and NR2B (C) were assessed in several mouse brain regions by immunoblot in both WT and RMOR-knock-in mice treated with morphine or saline. Data are expressed as means ± se. Tissue samples were analyzed from ≥3 separate experiments. Thal, thalamus; PAG, periaqueductal gray. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline group.
Effects of chronic morphine treatment on GR protein levels
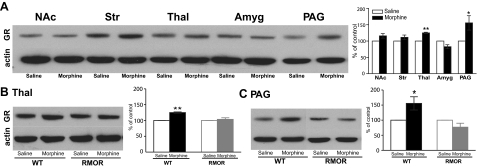
We then examined whether this morphine treatment promoted changes in GR protein levels in the same brain regions as above. In WT mice, morphine treatment caused a significant increase in GR protein level in both the thalamus and PAG (Fig. 4A). Notably, RMOR mice did not show significant up-regulation of GR in any brain region analyzed, including the thalamus and PAG (Fig. 4B, C).
Figure 4.
Effects of chronic morphine treatment on GR levels. A) Chronic morphine induced a significant increase in GR immunoreactivity in the thalamus and PAG of WT mice. B, C) Chronic morphine caused no change in RMOR mice in either thalamus (B) or PAG (C). In all cases, data are expressed as means ± se of ≥3 separate experiments. NAc, nucleus accumbens; Str, striatum; Thal, thalamus; Amyg, amygdala; PAG, periaqueductal gray. *P < 0.05, **P < 0.01 vs. saline group.
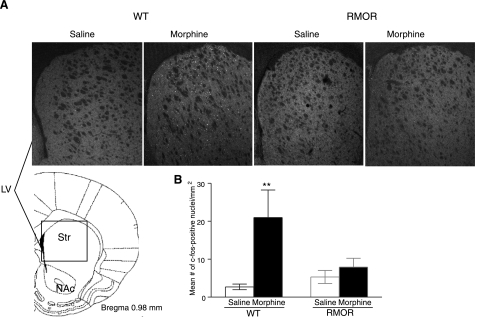
The c-fos response to chronic morphine treatment
The c-fos levels in the striatum of mice were measured with an immunohistochemical assay, and a significant increase of c-fos protein level was observed in the striatum of WT mice treated with chronic morphine. Again, no significant difference was found for RMOR mice between saline and morphine treatment (Fig. 5).
Figure 5.
Immunohistochemical staining of c-fos-positive nuclei in the striatum of mice. A) Representative images of c-fos protein staining in the striatum of WT and RMOR mice treated with repeated saline or morphine. A significantly higher number of c-fos-positive nuclei was seen only for the WT mice treated with morphine. B) Quantification of c-fos-positive nuclei for A. Data are expressed as means ± se of 4 experiments. LV, lateral ventricle; NAc, nucleus accumbens; Str, striatum. **P < 0.01 vs. saline group.
DISCUSSION
Many biochemical changes occur following chronic morphine exposure, and several of these changes have been implicated in the development of morphine analgesic tolerance and physical dependence. Here, we examined four of these biochemical hallmarks, comparing WT mice that develop tolerance and dependence to morphine, and RMOR mice that do not. In WT mice, morphine treatment that produced tolerance and dependence within 5 d also caused changes in adenylyl cyclase activity, NMDAR levels, GR levels, and c-fos expression in a brain region-selective fashion. Notably, none of these changes occurred in the RMOR mice. Together, these data indicate that the failure of the WT MOR to endocytose in response to morphine plays a critical role in inducing these diverse biochemical changes. Notably, these data also provide clear evidence that these diverse changes are likely contributing to the development of tolerance and dependence and are not merely epiphenomena of chronic morphine exposure unrelated to the behavioral phenotype. Previously, although changes in these “biomarkers” were found to be associated and correlated with morphine tolerance and dependence and pharmacological modulators of some of these molecules [such as the NMDAR (6) and the GR (45)] could affect the development of tolerance and/or dependence, one could not rule out that these changes were merely a consequence of chronic morphine exposure unrelated to the behavioral phenotype. This was because one always had to compare treated and untreated animals. Here, for the first time, we could compare changes in these biomarkers in two populations of mice (wild-type MOR and knock-in RMOR), all of which received the same morphine regimen, but which display differences in tolerance and dependence.
Previous studies have implicated the endocytic properties of the MOR in opioid tolerance and dependence. For example, opioids with the ability to promote MOR endocytosis cause reduced tolerance compared to morphine (11, 20,21,22,23). Furthermore, enhancing morphine-induced MOR endocytosis has been shown to significantly reduce the development of tolerance and/or dependence in both in vitro and in vivo models (10, 13, 14, 21, 22, 24,25,26). However, how and why endocytosis decreases tolerance have not been fully elucidated.
Chronic morphine exposure results in a compensatory up-regulation of the cAMP pathway (27,28,29), a phenomenon often called cAMP superactivation. These elevated cAMP levels reflect cellular adaptive changes, including increased expression of certain types of adenylyl cyclase, protein kinase A (PKA), and cAMP response element binding protein (CREB) (reviewed in refs. 30, 31). These compensatory changes mask the effects of morphine on this signal transduction cascade. Here, we evaluated adenylyl cyclase activity in several brain regions of mice chronically treated with morphine. Consistent with previous studies (32), we observed a moderate, but significant, up-regulation of cAMP levels in the striatum of WT mice. It is not clear why the striatum appears more responsive than other brain regions to this adaptive change. However, chronic morphine also induces much higher c-fos levels in striatum than in other brain regions, such as the NAc and septum (33), indicating that the striatum might be more sensitive to the effects of chronic morphine than other regions. Notably, no cAMP superactivation was found in the striatum of RMOR-knock-in mice, strongly suggesting that reduced morphine tolerance and dependence observed in these mice is mediated, at least in part, by preventing superactivation of the cAMP pathway.
It is increasingly believed that aspects of drug addiction, including morphine tolerance and dependence, are partly a process of neural and behavioral plasticity mediated through the NMDAR system (34). Indeed, multiple studies, including pharmacological (6), cellular (35), genetic (4), and electrophysiological (36), have demonstrated an important role of altered NMDAR function in modulating morphine tolerance and/or dependence. In agreement with this notion, we found significant changes in the levels of NMDAR subunits in WT mice treated with chronic morphine. Specifically, we observed significant up-regulation of NR2B in the thalamus and down-regulation of NR1, NR2A, and NR2B in the PAG (Fig. 2A). The latter findings are in contrast to some other studies, which have shown an up-regulation of NMDAR in response to chronic morphine exposure even in the PAG (4, 7, 37, 38). The exact reasons for the discrepancy are not clear. It is possible, however, that the decreases in NMDAR protein might reflect down-regulation of receptor in the presence of an increased glutamate tone, similar to that observed after chronic exposure to exogenous NMDA receptor agonists (39, 40). Indeed, chronic morphine has been shown to reduce glutamate transporter levels, which contributes to chronic morphine-induced hyperalgesia (35). Most important, none of the changes in NMDAR level were observed in the RMOR-knock-in mice treated with morphine, implying that all of these changes depend on the inability of morphine to promote endocytosis of the MOR (Fig. 3B, C).
The GR is important in a broad range of physiological functions, including stress (41,42,43,44), which has been shown to significantly contribute to addictive-like behaviors (3, 44). In addition, locomotor responses to morphine are modulated by GR, probably through modification of dopamine release in the nucleus accumbens (9). Together, these results suggested that the GR plays a role in the development of morphine tolerance and dependence by integrating several different systems. Consistent with previous reports (7, 8, 45), we observed significant increases in GR protein levels in the thalamus and PAG regions of WT mice tolerant to morphine. Once again, notably, none of these changes in GR were observed in RMOR-knock-in mice.
In addition to a decreased analgesic effect manifesting as tolerance, repeated morphine exposure also produces some sensitized behaviors, including the progressive and persistent increase in the psychomotor response to the drug, which is considered a critical process mediating drug reinforcement and addiction. The underlying cellular mechanisms mediating drug sensitization are not fully understood; however, the activation of c-fos, one of the immediate early genes, in mesolimbic brain regions has been implicated in behavioral sensitization (33, 46,47,48,49,50). Similar to previous reports, we found a significant increase in c-fos protein levels in the striatum of WT mice treated with repeated morphine. Interestingly, the same morphine treatment did not induce a significant increase in c-fos expression in RMOR-knock-in mice. Future experiments examining morphine preference, self-administration, and the effect of repeated morphine exposure on drug sensitization in RMOR-knock-in mice will provide more information on how or whether altered MOR trafficking could be affecting the reinforcing properties of morphine.
In summary, our findings clearly indicate that several biochemical adaptations that occur during the development of morphine tolerance and dependence are caused, at least in part, by the inability of morphine to induce substantial endocytosis of the MOR. Our data also suggest that all of these adaptations are important for tolerance and dependence to morphine, since they are all reversed in the RMOR mice that do not develop morphine tolerance and dependence. Taken together, these results provide the first evidence that these diverse, and seemingly unrelated, biomarkers of dependence are all possibly triggered by aberrant trafficking of a single receptor, the MOR.
Supplementary Material
Acknowledgments
The authors thank Drs. Anuradha Madhavan, Dawn Thompson, and Johan Enquist for critical reading of the manuscript. We thank Stacy Taylor and Madeline Ferwerda for maintenance and genotyping of the mouse colony. This work was supported by National Institute on Drug Abuse grant R01 DA015232 and DA19958 to J.L.W., and funds were provided by the state of California for medical research on alcohol and substance abuse through the University of California–San Francisco to J.L.W.
References
- Matthes H W, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques B P, Kieffer B L. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay R S, Donovan D M, Miner L L, Uhl G R. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zheng X, Wang Y, Cao J, Dong Z, Cai J, Sui N, Xu L. Stress enables synaptic depression in CA1 synapses by acute and chronic morphine: possible mechanisms for corticosterone on opiate addiction. J Neurosci. 2004;24:2412–2420. doi: 10.1523/JNEUROSCI.5544-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci. 2003;23:6529–6536. doi: 10.1523/JNEUROSCI.23-16-06529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak G W. When it comes to opiates, just say NO. J Clin Invest. 2007;117:3185–3187. doi: 10.1172/JCI34035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo K A, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Zeng Q, Sung B, Yang L, Mao J. Expression of spinal NMDA receptor and PKCγ after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–11154. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Wang S, Zeng Q, Sung B, Mao J. Spinal glucocorticoid receptors contribute to the development of morphine tolerance in rats. Anesthesiology. 2005;102:832–837. doi: 10.1097/00000542-200504000-00020. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza P V. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci U S A. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J A, Bartlett S, He L, Nielsen C K, Chang A M, Kharazia V, Waldhoer M, Ou C J, Taylor S, Ferwerda M, Cado D, Whistler J L. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn B C. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith D E, Jr, Bunnett N W, von Zastrow M, Evans C, Brecha N C. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler J L. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- He L, Whistler J L. An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol. 2005;15:1028–1033. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Trafton J A, Abbadie C, Marek K, Basbaum A I. Postsynaptic signaling via the μ-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. 2000;20:8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D E, Anton B, Murray S R, Zaki P A, Chu P C, Lissin D V, Monteillet-Agius G, Stewart P L, Evans C J, von Zastrow M. μ-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- Koob G F, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Buzas B, Cox B M. Differential regulation of adenylyl cyclase activity by μ and δ opioids in rat caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 1993;267:145–152. [PubMed] [Google Scholar]
- Wang Y H, Bosy T Z, Yasuda R P, Grayson D R, Vicini S, Pizzorusso T, Wolfe B B. Characterization of NMDA receptor subunit-specific antibodies: distribution of NR2A and NR2B receptor subunits in rat brain and ontogenic profile in the cerebellum. J Neurochem. 1995;65:176–183. doi: 10.1046/j.1471-4159.1995.65010176.x. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bartzsch K, Widera A, Becker A, Hollt V, Koch T. Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology. 2006;186:177–184. doi: 10.1007/s00213-006-0365-8. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, Hollt V. C-terminal splice variants of the mouse μ-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem. 2001;276:31408–31414. doi: 10.1074/jbc.M100305200. [DOI] [PubMed] [Google Scholar]
- Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker E A, Yoburn B C. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol. 2007;563:92–101. doi: 10.1016/j.ejphar.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg L O, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Whistler J L, Chuang H H, Chu P, Jan L Y, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Zollner C, Mousa S A, Fischer O, Rittner H L, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A K, Whistler J L. Endocytosis of the μ-opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Sharma S K, Klee W A, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson D B, Krystal J H, Aghajanian G K, Nestler E J. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z. Chronic opioid treatment induces adenylyl cyclase V superactivation. Involvement of Gβγ. J Biol Chem. 1996;271:21309–21315. doi: 10.1074/jbc.271.35.21309. [DOI] [PubMed] [Google Scholar]
- Williams J T, Christie M J, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Nestler E J. Under siege: The brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Bohn L M, Gainetdinov R R, Lin F T, Lefkowitz R J, Caron M G. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Liu J, Nickolenko J, Sharp F R. Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-d-aspartate receptors. Proc Natl Acad Sci U S A. 1994;91:8537–8541. doi: 10.1073/pnas.91.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo K A. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology. 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji R R, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Thomson L M, Aicher S A, Terman G W. Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci. 2006;26:12033–12042. doi: 10.1523/JNEUROSCI.2530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Crawford E F, Roberto M, Madamba S G, Siggins G R. Chronic morphine treatment alters expression of N-methyl-d-aspartate receptor subunits in the extended amygdala. J Neurosci Res. 2006;83:532–537. doi: 10.1002/jnr.20756. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L W, Ortiz J, Hamedani A G, Nestler E J. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink A, Villa M, Benke D, Hidaka H, Mohler H, Balazs R. Characterization of agonist-induced down-regulation of NMDA receptors in cerebellar granule cell cultures. J Neurochem. 1996;66:369–377. doi: 10.1046/j.1471-4159.1996.66010369.x. [DOI] [PubMed] [Google Scholar]
- Resink A, Villa M, Boer G J, Mohler H, Balazs R. Agonist-induced down-regulation of NMDA receptors in cerebellar granule cells in culture. Eur J Neurosci. 1995;7:1700–1706. doi: 10.1111/j.1460-9568.1995.tb00691.x. [DOI] [PubMed] [Google Scholar]
- Thomson F, Craighead M. Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem Res. 2008;33:691–707. doi: 10.1007/s11064-007-9518-3. [DOI] [PubMed] [Google Scholar]
- Kolber B J, Wieczorek L, Muglia L J. Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress. 2008;11:321–338. doi: 10.1080/10253890701821081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E R, de Jong I E, Oitzl M S. Neuropharmacology of glucocorticoids: focus on emotion, cognition and cocaine. Eur J Pharmacol. 2008;585:473–482. doi: 10.1016/j.ejphar.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Koob G F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Wang S, Zeng Q, Sung B, Mao J. Evidence for a long-term influence on morphine tolerance after previous morphine exposure: role of neuronal glucocorticoid receptors. Pain. 2005;114:81–92. doi: 10.1016/j.pain.2004.11.019. [DOI] [PubMed] [Google Scholar]
- David V, Matifas A, Gavello-Baudy S, Decorte L, Kieffer B L, Cazala P. Brain regional Fos expression elicited by the activation of μ- but not δ-opioid receptors of the ventral tegmental area: evidence for an implication of the ventral thalamus in opiate reward. Neuropsychopharmacology. 2008;33:1746–1759. doi: 10.1038/sj.npp.1301529. [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Linke R, Riechert U, Hollt V. Long-lasting sensitization towards morphine in motoric and limbic areas as determined by c-fos expression in rat brain. Brain Res Mol Brain Res. 1999;72:1–16. doi: 10.1016/s0169-328x(99)00184-9. [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Riechert U, Grecksch G, Hollt V. Identification of brain regions that are markedly activated by morphine in tolerant but not in naive rats. Brain Res Mol Brain Res. 1998;61:51–61. doi: 10.1016/s0169-328x(98)00197-1. [DOI] [PubMed] [Google Scholar]
- Nye H E, Nestler E J. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- Ferguson S M, Thomas M J, Robinson T E. Morphine-induced c-fos mRNA expression in striatofugal circuits: modulation by dose, environmental context, and drug history. Neuropsychopharmacology. 2004;29:1664–1674. doi: 10.1038/sj.npp.1300465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.