Abstract
The role of the actin filament-associated protein nebulin on mechanical and kinetic properties of the actomyosin motor was investigated in skeletal muscle of wild-type (wt) and nebulin-deficient (nebulin−/−) mice that were 1 d old, an age at which sarcomeric structure is still well preserved. In Ca2+-activated skinned fibers from psoas muscle, we determined the Ca2+ dependence of isometric force and stiffness, the rate of force redevelopment after unloaded shortening (kTR), the power during isotonic shortening, and the unloaded shortening velocity (V0). Our results show a 65% reduction in isometric force in nebulin−/− fibers at saturating [Ca2+], whereas neither thin-filament length nor the Ca2+ sensitivity of the contractile system is affected. Stiffness measurements indicate that the reduction in isometric force is due to a reduction in the number of actin-attached myosin motors, whereas the force of the motor is unchanged. Furthermore, in nebulin−/− fibers, kTR is decreased by 57%, V0 is increased by 63%, and the maximum power is decreased by 80%. These results indicate that, in the absence of nebulin, the attachment probability of the myosin motors to actin is decreased, revealing a direct role for nebulin in promoting strong actomyosin interactions responsible for force and power production.—Bang, M.-L., Caremani, M., Brunello, E., Littlefield, R., Lieber, R. L., Chen, J., Lombardi, V., Linari, M. Nebulin plays a direct role in promoting strong actin-myosin interactions.
Keywords: muscle performance, muscle force generation, cytoskeletal proteins
Nebulin is a giant skeletal muscle protein (500–900 kDa), which extends along the thin actin-containing filament and is constituted mainly of 35 amino acid modules (M1-185), which are further organized into super-repeats of 7. Each module binds to a single actin monomer, and each super-repeat associates with the tropomyosin-troponin regulatory complex along the thin filament (1,2,3). The N-terminal unique region of nebulin binds to the actin pointed end capping protein tropomodulin, a protein critical for maintaining thin-filament length (4, 5), whereas its C-terminal end binds to the actin barbed-end capping protein CapZ that regulates the growth of thin filaments (6, 7). Furthermore, the C-terminal modules of nebulin are connected to the intermediate filament desmin in the periphery of the Z line (8), and an Src homology 3 (SH3) domain in the extreme C-terminal end of nebulin interacts with titin and myopalladin in the Z line (9,10,11). Based on the close association of nebulin with the thin filament and the correlation between nebulin isoform size and variations in thin-filament length within different muscle types (3, 12), nebulin has long been believed to act as a molecular ruler, determining skeletal muscle thin-filament length. However, recent studies have shown that nebulin does not extend the whole way to the pointed end of the thin filament (13), and in nebulin-deficient (nebulin−/−) mice, the presence of shorter but uniform thin-filament lengths at birth reveals the existence of a nebulin-independent mechanism of thin-filament length regulation (14).
The critical role of nebulin in skeletal muscle function is further illustrated by human mutations in the nebulin gene, which are causative for nemaline myopathy, the most important nondystrophic congenital myopathy. This disease is characterized by muscle weakness and hypotonia due to sarcomeric disarray and the formation of rod-like “nemaline” bodies in the muscle fibers, composed of aggregates of Z-line and I-band proteins (15, 16). Furthermore, studies of nebulin−/− mice have demonstrated nebulin’s role in maintaining sarcomeric integrity during contraction (9, 14). Although nebulin−/− mice are normal in size at birth, their weight barely increases after birth, and most nebulin−/− mice die before 11 d of age with muscle weakness due to progressive sarcomeric disorganization, misalignment, and Z-line widening, reminiscent of nemaline myopathy (9, 14).
During the past 10 yr, the role of nebulin in muscle contractility has been studied both in vitro and in situ. In in vitro motility assays, recombinant nebulin fragments of 7–8 modules near the A-I junction have been shown to bind with high affinity to both actin and myosin and to inhibit actomyosin ATPase activity and the sliding velocity of actin over myosin (17, 18), suggesting that nebulin might optimize actomyosin interactions (17). More recently, a large reduction in isometric force (∼50%) (9, 14, 19) as well as abnormal Ca2+ sensitivity of the contractile system was reported in nebulin−/− mouse muscle (9). However, most of these studies were performed on 10-d-old nebulin−/− mice, which already exhibit Z-line misalignment and sarcomeric disarray, making the mechanical tests unreliable. Here, we studied the role of nebulin in skeletal muscle contractility in Ca2+-activated, demembranated, fibers from psoas muscle of nebulin−/− (14) and wild-type (wt) mice that were 1 d old, an age at which nebulin−/− mice have well-preserved sarcomeric structure. Our results show that the absence of nebulin does not affect the Ca2+ sensitivity of the contractile system but results in a decrease in the number of strong actomyosin interactions because of a reduced rate of attachment of myosin to actin. Consequently, the force during isometric contraction, the rate of force redevelopment after unloaded shortening, and the power during isotonic shortening at intermediate loads are decreased, whereas the force developed by each myosin motor is unaffected. These results provide a molecular basis for the impaired mechanical performance of nebulin−/− skeletal muscle.
MATERIALS AND METHODS
Generation of nebulin−/− mice
The generation of nebulin−/− mice has been described elsewhere (14). In brief, nebulin−/− mice were obtained by gene targeting, resulting in deletion and replacement of exon 1 by Cre recombinase cDNA as well as the neomycin resistance gene flanked by frt sites.
Electron microscopy and thin-filament length measurements
Transmission electron microscopy was performed on psoas muscle as described previously (14). For immunofluorescence studies, isolated myofibrils from 1-d-old mice were prepared as described previously (20). In brief, isolated psoas muscles were stretched with pins on pieces of cork and immersed in EGTA-Ringer’s relaxing buffer (100 mM NaCl, 2 mM KCl, 2 mM MgCl2, 6 mM potassium phosphate, 1 mM EDTA, and 0.1% glucose, pH 7.0) overnight at 4°C. Subsequently, myofibrils were stored in a 50:50 solution of rigor buffer (100 mM KCl, 2 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, and 10 mM potassium phosphate, pH 7.0) and glycerol at −20°C. Muscles were homogenized in rigor buffer, and the quality of isolated myofibrils was determined under the microscope during the procedure. After centrifugation at ∼1000 rpm to remove nuclei and any remaining pieces of muscle, myofibril solution was applied on slides and allowed to settle before fixation in 4% paraformaldehyde for 5 min. As described by Bang et al. (14), isolated myofibrils were stained with rhodamine-phalloidin or costained for α-actinin and tropomodulin by indirect immunofluorescence and subsequently imaged on a laser scanning confocal microscope (Radiance 2000; Bio-Rad Laboratories, Hercules, CA, USA) with an ×60 plan apochromat objective, NA 1.4 (Carl Zeiss Microimaging, Inc., Thornwood, NY, USA). Thin-filament lengths were measured by image analysis (64.7 nm/pixel) using distributed deconvolution, as described previously (14, 20).
Preparation of fibers for mechanical measurements
Fiber bundles used in these experiments were obtained from psoas muscles of 11 wt and 13 nebulin−/− mice. One-day-old mice were sacrificed by decapitation in accordance with the official regulations of the Community Council on Use of Laboratory Animals, and all procedures were approved by the institutional animal care and use committees at the Universities of Milan and Florence. Psoas muscles attached to the bones were stored in skinning solution containing 75% glycerol at −80°C for up to 3–4 wk. For mechanical experiments, the muscles were transferred to a Petri dish with the bottom covered by Sylgard (Dow Corning Ltd., Allesley, UK) and kept at 4–6°C. Bundles of ∼20 fibers (fiber diameter 10–15 μm, bundle length at ∼2.6 μm sarcomere length, l0, 0.8–1.6 mm) were dissected under a stereomicroscope (Stemi SV11; Carl Zeiss MicroImaging, Inc.) with dark field illumination and pinned down on the Sylgard surface at both ends. Extremities were clamped by aluminum T clips for attachment to transducer hooks.
Mechanical protocol
The fiber bundle was mounted in a drop of relaxing solution between the lever arms of a loudspeaker motor (21) and a capacitance force transducer (22), which are elements of the servo system that can control either fiber length (feedback signal from the position of the motor lever) or force (feedback signal from the force transducer). The drop of solution is one of the series of drops in the system that allows for rapid solution exchange (23). The average sarcomere length (sl) of several fibers and the width (w) and height (h) of the bundle were measured in 1 to 2 points along the bundle with an ×40 dry objective (NA 0.60; Carl Zeiss MicroImaging, Inc.) and an ×25 eyepiece. The sl of both wt and nebulin−/− fiber bundles was set to 2.57–2.62 μm. The cross-sectional area (CSA) of the bundle was determined assuming elliptical geometry. CSA was 4500 ± 2700 μm2 in wt bundles (mean ± sd; 11 bundles) and 5300 ± 1700 μm2 in nebulin−/− bundles (13 bundles). By means of the rapid solution-exchange system, the bundle was transferred from relaxing solution to preactivating solution at 3°C and, after 3 min, to activating solution at low temperature, where the isometric force developed is ∼0.1 the isometric force developed at the test temperature (∼13°C). The bundle was then transferred to the activating solution at the test temperature for ∼6 s and subsequently transferred to relaxing solution at the same temperature. This protocol prevents the development of most of the force during diffusion of activating solution and thus minimizes the development of sarcomere nonuniformities because of the diffusion-limited time of activation across the fiber. The baseline force in the activating solution at the test temperature was determined by superimposing on the isometric contraction a fast shortening (rise time 3 ms, amplitude 10% of the initial bundle length L0) that induces slack in the fiber. Step changes in fiber length to determine stiffness were imposed at the plateau of the isometric force. The force velocity relation was determined by imposing step reduction in force (range 0.2–0.8 T0) 100 ms after switching to force control. The test temperature was set to 12–14°C, according to most of the mechanical studies on skinned mammalian fibers (23,24,25,26,27,28,29,30,31). In fact, at higher (more physiological) temperatures, the quick force recovery after a length step would be faster and would produce a larger truncation of the elastic response, with a consequent underestimate of the stiffness.
Data collection and analysis
Force and motor-position signals were recorded with a multifunction I/O board (PCI-6110E; National Instruments, Austin, TX, USA) at both 10-μs and 0.5-ms sampling rates. A program written in LabVIEW (National Instruments) was used for signal recording and analysis. Data are expressed as means ± se except for the CSA (mean ± sd). Data points were interpolated with the SigmaPlot fitting procedure. The difference of parameters was statistically tested for significance with Student’s t test. P < 0.05 indicates a significant difference.
Solutions
Relaxing, preactivating, and activating solutions had compositions as reported previously (ref. 23 and references therein). Protease inhibitors (20 μg/ml leupeptin and 10 μM E-64; Sigma-Aldrich, St. Louis, MO, USA) were added to all solutions to preserve sarcomeric proteins.
RESULTS
Characterization of psoas muscle
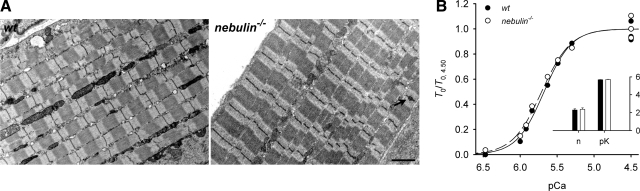
All mechanical experiments were performed on fiber bundles from psoas muscle of 1-d-old wt and nebulin−/− mice. At this age, nebulin−/− mice are still similar in size to their wt littermates, and the localization of other sarcomeric proteins (actin, α-actinin, tropomyosin, α-myosin, desmin, tropomodulin, and palladin) is unaffected (14). In addition, transmission electron microscopy studies of psoas muscle from 1-d-old nebulin−/− mice showed normal sarcomeric organization and well-aligned sarcomeres with only a small percentage of widened Z lines (Fig. 1A). This is in contrast to the result with nebulin−/− tibialis anterior muscle, in which sarcomere misalignment and fragmentation of Z lines occur already at postnatal day 1 (14). Determination of thin-filament lengths by immunofluorescence analysis of labeled actin filaments (14) revealed thin-filament lengths of ∼1.0 μm in psoas muscle both from 1-d-old nebulin−/− and wt mice (Table 1). In contrast, thin-filament lengths in gastrocnemius, tibialis anterior, vastus lateralis, and extensor digitorum longus muscles from 1-d-old nebulin−/− mice have been found to be reduced by up to 25%, from 1.16 to 1.29 μm to a uniform length of ∼1.0 μm (14). Thus, psoas muscle is a good choice for studying the effect of nebulin on the mechanical performance of skeletal muscle, not only because of its common use for fine mechanical studies of mammalian skeletal muscle (23, 25, 32) but also because confounding effects due to sarcomere misalignment and variations in thin-filament length are excluded. Furthermore, its length (∼4 mm in 1-d-old mice) facilitates the attachment of fiber segments (0.8–1.6 mm) to the force and length transducers.
Figure 1.
Comparison of sarcomere structure and force-pCa relation between wt and nebulin−/− psoas muscle fibers from 1-d-old mice. A) Transmission electron microscopy on longitudinal sections of psoas muscle from wt and nebulin−/− mice. Arrow indicates a zone of Z-line widening. Scale bar = 2 μm. B) Force-pCa relation in fiber bundles from wt and nebulin−/− mice. Solid (wt) and dashed (nebulin−/−) lines are Hill sigmoidal equations (Eq. 1) fitted to data. Inset: mean ± se values of the parameters. wt psoas: bundle length, 1.00 mm; sarcomere length, 2.57 μm; CSA, 2300 μm2; T0, 71 kPa; temperature, 13.5°C. nebulin−/− psoas: bundle length, 1.50 mm; sarcomere length, 2.60 μm; CSA, 4300 μm2; T0, 24 kPa; temperature, 13.4°C.
TABLE 1.
Thin-filament length in psoas muscle from 1-d-old wt and nebulin−/− mice
| Muscle | Probe | Length (μm)
|
P | |
|---|---|---|---|---|
| wt | nebulin−/− | |||
| Psoas | Tmod/α-actinin | 1.08 ± 0.03 (9) | 0.98 ± 0.09 (6) | >0.2 |
| Phalloidin | 1.00 ± 0.02 (33) | 1.03 ± 0.01 (28) | >0.2 | |
Data (means ± se) obtained with distributed deconvolution image analysis of immunofluorescently labeled myofibrils stained for α-actinin/tropomodulin or phalloidin. Numbers of myofibrils contributing to each value are shown in parentheses.
Force-pCa relation
Steady isometric force T0 developed at any [Ca2+] was significantly lower in nebulin−/− compared with wt fibers and was reduced by ∼65% at saturating [Ca2+] (pCa 4.50) (Table 2). The effect of nebulin on the Ca2+ sensitivity of the contractile system was tested by determining the force-pCa relation (range of pCa 6.47–4.50) (Fig. 1B). T0 at any [Ca2+] was normalized to T0 at saturating [Ca2+] (T0, 4.50) and the force-pCa relationship was fitted by the sigmoid Hill equation (33):
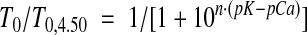 |
1 |
where n is the Hill coefficient indicating the steepness of the relation and thus the cooperativity in the Ca2+ activation process (34), and pK = pCa at T0 = 0.5 T0,4.50, a measure of the Ca2+ sensitivity of the contractile material.
TABLE 2.
Mechanical parameters during isometric contraction of fiber bundles from wt and nebulin−/− mice
| Genotype | T0, 4.50 (kPa) | n | pK | Cf + Ce (%L0/MPa) | s0 (%L0) | kTR (s−1) |
|---|---|---|---|---|---|---|
| wt | 65 ± 10 (11) | 2.29 ± 0.13 (5) | 5.68 ± 0.02 (5) | 6.3 ± 2.9 (3) | 0.72 ± 0.11 (3) | 2.56 ± 0.26 (4) |
| nebulin−/− | 23 ± 3 (13) | 2.37 ± 0.16 (4) | 5.71 ± 0.02 (4) | 9.4 ± 2.4 (3) | 0.77 ± 0.03 (3) | 1.10 ± 0.15 (5) |
| P | <0.01* | >0.6 | >0.6 | >0.4 | >0.6 | <0.01* |
T0, 4.50, isometric force at saturating pCa; n, Hill’s coefficient; pK, pCa at which isometric force is 0.5 T0, 4.50; Cf + Ce, the compliance in series to the array of myosin motors, given by the sum of equivalent myofilament compliance Cf and extra compliance due to the attachment of the fiber to the transducer ends Ce; s0, average strain in the attached myosin head; kTR, rate of force redevelopment after a period of unloaded shortening measured as the reciprocal of the half-time for maximum force redevelopment. Values are means ± se. Numbers of bundles contributing to each value are shown in parentheses.
P < 0.05.
As shown in Fig. 1B, the nebulin−/− relation essentially superimposes on the wt relation. Thus, neither the degree of cooperativity of Ca2+ activation (n) nor the Ca2+ sensitivity of the myofilaments (pK) is influenced by nebulin (Table 2).
Effects of nebulin absence on the force and number of myosin motors and the rate of force redevelopment after unloaded shortening
In the absence of sarcomeric disorganization, the reduction of isometric force in nebulin−/− fibers can be accounted for by a reduction in the number of active myosin motors and/or the force per motor. To distinguish between these two possibilities, we estimated the average strain in the myosin motors by determining the strain-force relation (Y0-T0) at different [Ca2+] (23, 27). It has previously been shown that [Ca2+] determines the isometric force exerted by the array of motors in each half-myosin filament by modulating the number of motors attached to actin, without affecting the force of each motor (23, 27). Under these conditions, the ordinate intercept of the Y0-T0 relation estimates the strain of the motors, whereas the slope of the relation estimates the compliance in series to the motors.
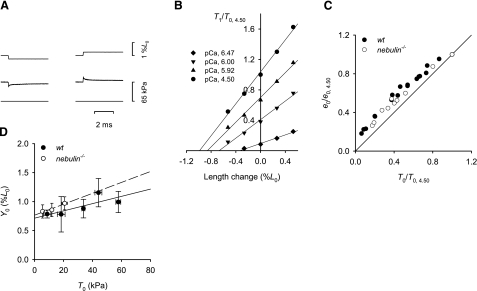
The analysis was conducted by imposing small steps in length on the isometrically contracting fibers, and for each steady force T0 obtained at different [Ca2+], the fiber stiffness (e0) was estimated as the slope of the relation between the force attained at the end of the step (T1) and the size of the step (T1 relation) (35) (Fig. 2A, B). The linear fit to the T1 points at any [Ca2+] in both wt and nebulin−/− mice shows a decrease in fiber stiffness with reduced T0 that is less than in proportion to the reduction of T0 (Fig. 2C). The abscissa intercept Y0 of the regression line to the T1 relation is a measure of the extension in the fiber elasticity during the isometric condition preceding the step (35). The relations between Y0 and the Ca2+-modulated force in nebulin−/− and wt mice are plotted in Fig. 2D. Under the conditions of the present experiments, in which the length signal is measured by the position of the motor lever at one end of the fiber bundle, the compliance, estimated by the slope of the Y0-T0 relation, is the sum of the equivalent actin and myosin filament compliance (Cf) and the extracompliance due to the attachment of the fiber ends to the transducers (Ce). A large variation in Cf + Ce was found among fibers of the same type, probably related to the large variation in Ce. The slope of the linear fit to the data is higher in nebulin−/− than in wt fibers, a finding that can be explained by an additional compliance, probably due to the observed occasional widening of Z lines in nebulin−/− psoas muscle (Fig. 1A). However, the difference is not significant (Table 2) and does not affect the value of the ordinate intercept of the Y0-T0 relation, an estimate of the average strain in the myosin motors (23, 36, 37), which is similar in wt and in nebulin−/− fibers (Table 2). These results indicate that the absence of nebulin results in a decrease in isometric force by a reduction in the number of motors without affecting the force per motor.
Figure 2.
Relation between strain and isometric force in fiber bundles from wt and nebulin−/− psoas muscles. A) Force response (middle trace) to a length step (top trace) in a fiber bundle from wt mouse activated at pCa 4.50; left, step release; right, step stretch of similar size; bottom trace, force baseline. Bundle length, 1.37 mm; average sarcomere length, 2.60 μm; CSA, 4400 μm2; temperature, 13.2°C. B) T1 relations for 4 pCa values. T1 values are plotted relative to T0 at pCa 4.50 (T0,4.50). Different symbols refer to different pCa values, as indicated in the inset. Continuous lines are linear regressions fitted to the experimental points for each pCa. Same fiber bundle as in A. C) Relation between stiffness e0 relative to that at pCa 4.50 (e0,4.50), and relative isometric force determined at different pCa values both in wt and nebulin−/− mice. Continuous line expresses direct proportionality. Data are pooled from 3 fibers for both wt and nebulin−/− mice. D) Relation between fiber strain Y0 (mean ± se) and isometric force at different pCa values, determined in 3 fiber bundles each from wt and nebulin−/− mice and grouped in classes of force. Lines are linear regressions to points from wt (solid line) and nebulin−/− (dashed line) data. Mean ± se values of the parameters for wt and nebulin−/− mice are reported in Table 2.
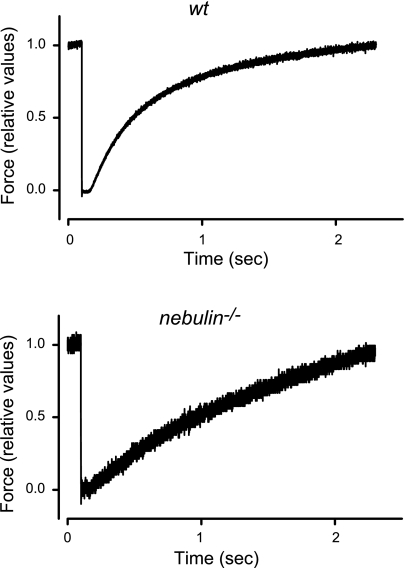
The reduction in the number of motors in nebulin−/− fibers could be due to a reduction in the rate of cross-bridge attachment, an increase in the rate of cross-bridge detachment, or a combination of both. This can be investigated by determining the rate of isometric force redevelopment at saturating Ca2+ after a period of unloaded shortening (kTR), which, based on a simple two-state kinetic model (38 (f) and the detachment rate constant (g) (see also ref. 32). kTR, as estimated by the reciprocal of the half-time for maximum force redevelopment (Fig. 3), was reduced by 57% in nebulin−/− fibers compared with wt fibers (Table 2). Thus, in the absence of nebulin, the number of motors is decreased because of a reduction in the rate constant of cross-bridge attachment.
Figure 3.
Isometric force redevelopment after a period of unloaded shortening in maximally activated fiber bundles from wt and nebulin−/− mice (pCa 4.50). Force is normalized to the value preceding the ramp release (∼10% L0). wt psoas: bundle length, 1.26 mm; average sarcomere length, 2.62 μm; CSA, 4200 μm2; isometric force, 50 kPa; temperature, 12.8°C. nebulin−/− psoas: bundle length, 0.92 mm; average sarcomere length, 2.60 μm; CSA, 4500 μm2; isometric force, 20 kPa; temperature, 13.0°C.
Effect of nebulin absence on dynamic properties
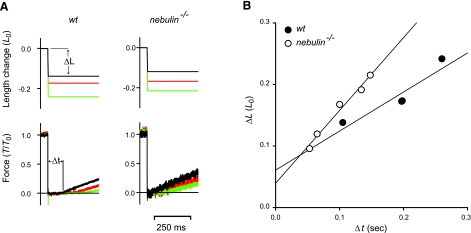
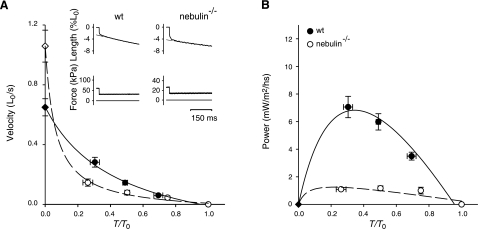
Unloaded shortening velocity (V0) was measured in wt and nebulin−/− fibers activated at saturating [Ca2+] (pCa 4.50) using the slack-test method (39) (Fig. 4A). V0 was determined as the slope of the relation between the amount of rapid shortening imposed to the fiber (ΔL) and the time necessary for force to redevelop (Δt). As shown in Fig. 4B, V0 was significantly higher (∼63%) in nebulin−/− fibers compared with wt fibers (1.06±0.11 vs. 0.65±0.06 L0/s). The force-velocity relations in fully activated nebulin−/− and wt fibers were determined by measuring the velocity of steady shortening V after a drop in force from the isometric value T0 to a preset value T < T0 (Fig. 5A) (37). On the ordinate of the T-V relation, the value of V0 (Fig. 5A, diamond), estimated with the slack-test method (see above) is also reported. All force-velocity points from both nebulin−/− and wt fibers were fitted to the Hill hyperbolic equation (40):
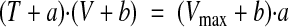 |
2 |
where a, b, and Vmax are the regression parameters.
Figure 4.
Unloaded shortening velocity V0 determined using the slack test in fiber bundles from wt and nebulin−/− mice. A) Force responses (bottom panels) to rapid shortening steps of different magnitudes (ΔL; top panels). Δt (shown here for the smaller ΔL in a wt fiber bundle) is the time necessary for the force to begin to redevelop. B) Relation between ΔL and Δt in wt and in nebulin−/− fibers. V0 (± se), measured by the slope of the relation, is 0.64 ± 0.17 L0/s in wt and 1.18 ± 0.10 L0/s in nebulin−/− mice. wt psoas: bundle length, 1.04 mm; average sarcomere length, 2.60 μm; CSA, 3500 μm2; isometric force, 82 kPa; temperature, 13.1°C. nebulin−/− psoas: same fiber bundle as in Fig. 1.
Figure 5.
Relation between shortening velocity and power output vs. force in fiber bundles from wt and nebulin−/− psoas muscles. A) Force-velocity relations from wt (4 bundles) and nebulin−/− mice (4 bundles). Inset: sample records of length (top trace) after a drop of force from T0 to 0.5 T0 (middle trace); bottom trace, force baseline. Left panel, wt; right panel, nebulin−/−. Slope of the dashed line fitted to the steady shortening phase measures shortening velocity. Diamonds on the ordinate are V0 values measured with the slack-test method (see Fig. 4). Force T is relative to isometric value T0. Points are means ± se from data grouped in classes of force; n = 4–7. Solid (wt) and dashed (nebulin−/−) lines are Hill hyperbolic equations (Eq. 2) fitted to the points. Mean ± se values of parameters of the hyperbolic equations are reported in Table 3. B) Power-force relations obtained from the same data as in A. Lines are obtained from the Hill equation fitted to the force-velocity data in A.
At intermediate loads, the shortening velocity was lower in nebulin−/− mice than in wt mice, and a/T0, the parameter that estimates the curvature of the force-velocity relation, was about 7 times smaller in nebulin−/− than in wt mice (Table 3). Consequently, power, calculated as the product T · V, was significantly reduced in the absence of nebulin (Fig. 5B). At the load for the maximum power, ∼0.3 T0, the power was ∼6 times lower in nebulin−/− than in wt mice (Table 3).
TABLE 3.
Mechanical parameters during isotonic shortening of fiber bundles from wt and nebulin−/− mice
| Genotype | Vmax (L0/s) | a/T0 | b (L0/s) | Wmax (mW/m2/h) |
|---|---|---|---|---|
| wt | 0.65 ± 0.03 | 0.44 ± 0.13 | 0.30 ± 0.11 | 7.06 ± 0.77 |
| nebulin−/− | 1.06 ± 0.05 | 0.06 ± 0.04 | 0.06 ± 0.01 | 1.10 ± 0.08 |
| P | <0.05* | <0.05* | <0.01* | <0.01* |
Vmax, a/T0, and b are regression parameters of the Hill hyperbolic equation (Eq. 2) fitted to the pooled data for wt and nebulin−/− fibers. Wmax is the maximum power, obtained with a load ∼1/3 the isometric force. Values are means ± se.
P < 0.05.
DISCUSSION
In the current study, we investigated the role of nebulin in modulating the mechanical performance of skeletal muscle by comparing the relevant mechanical parameters in demembranated psoas muscle fiber bundles from 1-d-old nebulin−/− and wt mice. Our study demonstrates that nebulin deficiency results in reduced isometric force due to a reduced number of myosin motors attached to actin; a reduced rate of force redevelopment after unloaded shortening kTR, indicating a reduced probability of cross-bridge formation; increased unloaded shortening velocity; and reduced power. On the other hand, nebulin deficiency does not affect the Ca2+ sensitivity of the regulatory system or the cooperativity of the Ca2+ activation process.
Based on immunofluorescence analyses, we previously found an up to 25% reduction in thin-filament lengths to a uniform length of ∼1.0 μm in gastrocnemius, tibialis anterior, vastus lateralis, and extensor digitorum longus muscles from 1-d-old nebulin−/− mice compared with wt mice (9, 14). In this study, using the same method, we found thin-filament lengths of ∼1.0 μm both in wt and nebulin−/− psoas muscle (Table 1). The missing effect of nebulin deficiency on thin-filament lengths in psoas muscle could be due to the relatively short thin-filament lengths in wt psoas muscle (∼1.0 μm) compared with other muscles that have thin-filament lengths of up to ∼1.3 μm (9, 14). This finding is consistent with our previous hypothesis that thin-filament lengths of ∼1.0 μm are determined by a nebulin-independent mechanism, whereas nebulin is required to guide further actin filament growth (14). In 10-d-old tibialis anterior muscle, shorter, but more variable, actin thin-filament lengths have been reported in nebulin−/− mice compared with wt mice (9). The nonuniform thin-filament lengths at this stage may be due to progressive muscle degeneration in nebulin−/− mice secondarily affecting thin-filament length and not a direct result of the genotype.
Isometric force in psoas fibers from 1-d-old mice was reduced by 65% in the absence of nebulin (65±10 vs. 23±3 kPa) (Table 2), in agreement with the previously reported reduction in isometric force in tibialis anterior and soleus muscle from nebulin−/− mice (9, 14, 19). The average strain per myosin motor during isometric contraction, as estimated by the ordinate intercept of the Y0-T0 relation, was the same in both wt and nebulin−/− fibers (Fig. 2D), indicating that the force per motor is unaffected and that the 65% reduction in isometric force in the absence of nebulin is due to an equivalent reduction in the number of motors attached to actin. The average strain per myosin motor measured here is more than twice the value of that previously estimated in single fibers from adult rabbit psoas muscle (23). This difference could be due to variations in myosin isoform expression between muscles isolated from neonatal mice and adult rabbit, as suggested by a recent study demonstrating that a single amino acid mutation in the converter domain of the myosin head doubles the stiffness of the myosin motor (41). Myosin isoform expression does not differ between 1-d-old nebulin−/− and wt mice (14), and no major ultrastructural abnormalities (Fig. 1A) or significant differences in thin-filament lengths have been found in psoas muscle from 1-d-old nebulin−/− mice compared with wt mice (Table 1). Thus, the reduced number of myosin motors during isometric contraction of nebulin−/− fibers can be explained only by a direct effect of nebulin on the actin-myosin binding process.
The shortening velocity at loads from 0.8 to 0.2 T0 was lower in nebulin−/− than in wt fibers (Fig. 5A), resulting in a corresponding reduction in power: the maximum power is ∼6 times smaller in nebulin−/− than in wt psoas bundles (Fig. 5B). The decrease in the number of myosin motors interacting during isometric contraction, the rate of isometric force development kTR, and the power during isotonic shortening can all be explained in terms of the simple 2-state kinetic model of Huxley (38) by a reduction in the rate constant for attachment f of the myosin motor to actin. In fact, according to the 2-state model, the fraction of attached motors is expressed by the ratio f/(f+g), where g is the rate constant for motor detachment, and the rate of isometric force development is expressed by the equation kTR = f + g. Thus, the observed 65% reduction in the fraction of attached motors and 57% reduction in kTR implies a decrease in f to (0.35×0.43) = 0.15. This reduction also explains the increase in the curvature of the T-V relation (proportional to the reciprocal of kTR) and thus the reduction in power (see also ref. 42). These results suggest for the first time that nebulin plays a role at the interface between the myosin motor domain and actin, assisting the formation of the strong bound complex. After the strong actin-myosin interaction has occurred, nebulin has no effect on the force generated by the myosin motor.
The increase in the unloaded shortening velocity V0 in the absence of nebulin is in agreement with the finding that nebulin fragments reduce the sliding velocity of actin over myosin in in vitro motility assays (18). Under zero load, sliding velocity does not depend on the number of motors (38) but only on the rate of detachment under negative strain that prevents motors at the end of their working stroke from exerting a resistance to sliding. Thus, the rate of detachment under negative strain is increased in nebulin−/− fibers. In this respect it should be noted that the degree of decrease in the force and rate of force development during isometric contraction indicate that the reduction in the rate constant for attachment f is not accompanied by an increase in the rate of cross-bridge detachment g but rather by a reduction. For instance, if in wt fibers the fraction of myosin motors attached during isometric contraction were 0.3, as found in rabbit psoas fibers (23), a drop in f to 0.15 in nebulin−/− fibers compared with that in wt fibers (see above) would imply a drop in g to 0.52 of the control value according to the simple 2-state model (38). It seems evident that this model may be inadequate to correctly describe the shortening velocity under zero load, considering that, under this condition the interacting myosin motors would be predominantly weakly bound to actin (ref. 31 and references therein). Because weakly bound myosin motors under negative strain should exert a reduced resistance to sliding with respect to strongly bound motors, the increase in V0 in nebulin−/− fibers could be explained by an increase in the proportion of weakly bound motors at the expense of strongly bound motors.
Using psoas fibers from 1-d-old mice, we did not find any effect of nebulin deficiency on the degree of cooperativity of Ca2+ activation (expressed by the parameter n) or Ca2+ sensitivity of the contractile system (expressed by the parameter pK) (Table 2). This contradicts previous work by Witt et al. (9), who reported a reduction in n in tibialis anterior muscle from 10-d-old nebulin−/− mice compared with wt mice. The discrepancy between the two studies may be due to the different ages of the mice. Our experiments were performed in 1-d-old mice because at that age, nebulin−/− mice still have well-preserved sarcomere structure (Fig. 1A). In contrast, 10-d-old nebulin−/− mice exhibit various structural abnormalities, such as severe sarcomere misalignment and Z-line widening that are likely to affect not only the mechanical output but also the cooperative mechanism of activation and thus the value of n. It should be noted that the value of n reported here for 1-d-old wt and nebulin−/− psoas muscle (∼2.3) (Table 2) is about one-half of that reported for adult (43) and 10-d-old wt muscle (9, 43). Thus, at an early stage, the value of n rises with age, which emphasizes the need to conduct comparative tests on muscles of the same age. The value of pCa for half-maximum isometric force (∼5.7) (Table 2), which expresses the sensitivity of the contractile system to Ca2+, was similar in wt and nebulin−/− fibers and comparable to that reported previously by Witt et al. (9) and thus apparently independent of both the age of mice and the presence of nebulin.
CONCLUSIONS
Here we provide the first experimental evidence that nebulin plays a role in determining the mechanical performance of skeletal muscle. Comparing steady force and stiffness during isometric contraction as well as rate of isometric force redevelopment and power during isotonic shortening of psoas fibers of wt and nebulin−/− mice, we demonstrate that nebulin influences the myosin-actin interface, increasing the probability of strong actomyosin interactions. The direct role of nebulin in modulating the mechanical performance of skeletal muscle may contribute to the muscle weakness that is observed in nemaline myopathy patients with nebulin gene mutations.
Acknowledgments
The authors acknowledge the Telethon Electron Microscopy core facility (directed by R. S. Polishchuk) for the preparation and analysis of electron microscopic specimens and thank Mario Dolfi for skilled technical assistance. This research was supported by the U.S. National Institutes of Health (grant R01 AR49033), Ministero dell’Università e della Ricerca (MiUR-Cofin 2006), and Ministero del Lavoro, della Salute e delle Politiche Sociali (grant RF-MUL-2007-666195). M.-L.B. is an Assistant Telethon Scientist supported by Telethon-Italy (grant TCP07006) and Fondazione Cariplo (grant 2007.5812).
References
- Jin J P, Wang K. Nebulin as a giant actin-binding template protein in skeletal muscle sarcomere. Interaction of actin and cloned human nebulin fragments. FEBS Lett. 1991;281:93–96. doi: 10.1016/0014-5793(91)80366-b. [DOI] [PubMed] [Google Scholar]
- Pfuhl M, Winder S J, Pastore A. Nebulin, a helical actin binding protein. EMBO J. 1994;13:1782–1789. doi: 10.1002/j.1460-2075.1994.tb06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Knipfer M, Huang Q Q, van Heerden A, Hsu L C, Gutierrez G, Quian X L, Stedman H. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture: sequence motifs and affinity profiles of tandem repeats and terminal SH3. J Biol Chem. 1996;271:4304–4314. doi: 10.1074/jbc.271.8.4304. [DOI] [PubMed] [Google Scholar]
- Littlefield R, Almenar-Queralt A, Fowler V M. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol. 2001;3:544–551. doi: 10.1038/35078517. [DOI] [PubMed] [Google Scholar]
- McElhinny A S, Kolmerer B, Fowler V M, Labeit S, Gregorio C C. The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J Biol Chem. 2001;276:583–592. doi: 10.1074/jbc.M005693200. [DOI] [PubMed] [Google Scholar]
- Schafer D A, Hug C, Cooper J A. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol. 1995;128:61–70. doi: 10.1083/jcb.128.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J F, Craig S W, Maack D J, Brown A E. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J Cell Biol. 1987;105:371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M L, Gregorio C, Labeit S. Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J Struct Biol. 2002;137:119–127. doi: 10.1006/jsbi.2002.4457. [DOI] [PubMed] [Google Scholar]
- Witt C C, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 2006;25:3843–3855. doi: 10.1038/sj.emboj.7601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Wang K. Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett. 2002;532:273–278. doi: 10.1016/s0014-5793(02)03655-4. [DOI] [PubMed] [Google Scholar]
- Bang M L, Mudry R E, McElhinny A S, Trombitas K, Geach A J, Yamasaki R, Sorimachi H, Granzier H, Gregorio C C, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–427. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B. The complete primary structure of human nebulin and its correlation to muscle structure. J Mol Biol. 1995;248:308–315. doi: 10.1016/s0022-2836(95)80052-2. [DOI] [PubMed] [Google Scholar]
- Castillo A, Nowak R, Littlefield K P, Fowler V M, Littlefield R S. A nebulin ruler does not dictate thin filament lengths. Biophys J. 2009;96:1856–1865. doi: 10.1016/j.bpj.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M L, Li X, Littlefield R, Bremner S, Thor A, Knowlton K U, Lieber R L, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol. 2006;173:905–916. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Donner K, Sewry C, Bijlsma E, Lammens M, Bushby K, Giovannucci Uzielli M L, Lapi E, Odent S, Akcoren Z, Topaloglu H, Pelin K. Mutations in the nebulin gene can cause severe congenital nemaline myopathy. Neuromuscul Disord. 2002;12:674–679. doi: 10.1016/s0960-8966(02)00065-2. [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Pelin K, Hilpela P, Donner K, Porfirio B, Graziano C, Swoboda K J, Fardeau M, Urtizberea J A, Muntoni F, Sewry C, Dubowitz V, Iannaccone S, Minetti C, Pedemonte M, Seri M, Cusano R, Lammens M, Castagna-Sloane A, Beggs A H, Laing N G, de la Chapelle A. Clinical and genetic heterogeneity in autosomal recessive nemaline myopathy. Neuromuscul Disord. 1999;9:564–572. doi: 10.1016/s0960-8966(99)00061-9. [DOI] [PubMed] [Google Scholar]
- Root D D, Wang K. High-affinity actin-binding nebulin fragments influence the actoS1 complex. Biochemistry. 2001;40:1171–1186. doi: 10.1021/bi0015010. [DOI] [PubMed] [Google Scholar]
- Root D D, Wang K. Calmodulin-sensitive interaction of human nebulin fragments with actin and myosin. Biochemistry. 1994;33:12581–12591. doi: 10.1021/bi00208a008. [DOI] [PubMed] [Google Scholar]
- Ottenheijm C A, Fong C, Vangheluwe P, Wuytack F, Babu G J, Periasamy M, Witt C C, Labeit S, Granzier H. Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle. FASEB J. 2008;22:2912–2919. doi: 10.1096/fj.07-104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield R, Fowler V M. Measurement of thin filament lengths by distributed deconvolution analysis of fluorescence images. Biophys J. 2002;82:2548–2564. doi: 10.1016/S0006-3495(02)75598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V, Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol. 1990;431:141–171. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A F, Lombardi V. A sensitive force transducer with resonant frequency 50 kHz. J Physiol. 1980;305:15P–16P. [Google Scholar]
- Linari M, Caremani M, Piperio C, Brandt P, Lombardi V. Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys J. 2007;92:2476–2490. doi: 10.1529/biophysj.106.099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J A, Goldman Y E, Millar N C, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y E, Hibberd M G, Trentham D R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5′-triphosphate. J Physiol. 1984;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Guth K, Winnikes K, Haist C, Ruegg J C. The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflügers Arch. 1987;408:1–9. doi: 10.1007/BF00581833. [DOI] [PubMed] [Google Scholar]
- Linari M, Bottinelli R, Pellegrino M A, Reconditi M, Reggiani C, Lombardi V. The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms. J Physiol. 2004;554:335–352. doi: 10.1113/jphysiol.2003.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N C, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers: a steady-state and transient kinetic study. J Biol Chem. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Regnier M, Morris C, Homsher E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am J Physiol. 1995;269:C1532–C1539. doi: 10.1152/ajpcell.1995.269.6.C1532. [DOI] [PubMed] [Google Scholar]
- Sleep J, Irving M, Burton K. The ATP hydrolysis and phosphate release steps control the time course of force development in rabbit skeletal muscle. J Physiol. 2005;563:671–687. doi: 10.1113/jphysiol.2004.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle R, Brenner B. Cross-bridge attachment during high-speed active shortening of skinned fibers of the rabbit psoas muscle: implications for cross-bridge action during maximum velocity of filament sliding. Biophys J. 2000;78:1458–1473. doi: 10.1016/S0006-3495(00)76699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A V. The combinations of haemoglobin with oxygen and with carbon monoxide. I. Biochem J. 1913;7:471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A M, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Huxley A F, Simmons R M. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Brunello E, Bianco P, Piazzesi G, Linari M, Reconditi M, Panine P, Narayanan T, Helsby W I, Irving M, Lombardi V. Structural changes in the myosin filament and cross-bridges during active force development in single intact frog muscle fibres: stiffness and X-ray diffraction measurements. J Physiol. 2006;577:971–984. doi: 10.1113/jphysiol.2006.115394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore D B, Irving T C, Irving M, Lombardi V. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Huxley A F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Edman K A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A V. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Kohler J, Winkler G, Schulte I, Scholz T, McKenna W, Brenner B, Kraft T. Mutation of the myosin converter domain alters cross-bridge elasticity. Proc Natl Acad Sci U S A. 2002;99:3557–3562. doi: 10.1073/pnas.062415899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R M, Jewell B R. Mechanics and models of muscular contraction. Linden R J, editor. London: Churchill Livingstone; 1974:87–147. [Google Scholar]
- Fink R H, Stephenson D G, Williams D A. Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J Physiol. 1986;373:513–525. doi: 10.1113/jphysiol.1986.sp016060. [DOI] [PMC free article] [PubMed] [Google Scholar]