Abstract
Transforming growth factor-β (TGF-β) is a ubiquitous cytokine with dual roles in tumor suppression and promotion, and these dichotomous functions have frustrated the development of therapies targeting oncogenic signaling by TGF-β. In comparison, Abl is well established as an initiator of hematopoietic cancers; however, a clear role for Abl in regulating solid tumor development remains elusive. Here, we investigated the role of Abl in TGF-β-mediated epithelial-mesenchymal transition (EMT) in normal and metastatic mammary epithelial cells (MECs). In doing so, we identified Abl as an essential regulator of MEC morphology and showed that Abl inactivation was sufficient to induce phenotypic and transcriptional EMT in normal MECs. Increasing Abl activity in metastatic MECs resulted in their complete morphological reversion, restored their cytostatic response to TGF-β, and blocked their secretion of matrix metalloproteinases induced by TGF-β. Constitutively active Abl expression blocked TGF-β-responsive mammary tumor growth in mice, while Imatinib therapy afforded no clinical benefit in mice bearing mammary tumors. Collectively, this investigation establishes Abl as a potent mediator of MEC identity, and as a suppressor of oncogenic TGF-β signaling during mammary tumorigenesis. Notably, our findings strongly caution against the use of pharmacological Abl antagonists in the treatment of developing and progressing mammary tumors.—Allington, T. M., Galliher-Beckley, A. J., Schiemann, W. P. Activated Abl kinase inhibits oncogenic transforming growth factor-β signaling and tumorigenesis in mammary tumors.
Keywords: EMT, matrix metalloproteinases, organotypic cultures, Imatinib, signal transduction
Transforming growth factor-β (TGF-β) is a ubiquitous cytokine with important roles in embryogenesis, cellular differentiation, wound healing, and immune response regulation. In normal mammary epithelial cells (MECs), TGF-β promotes differentiation and maintenance of normal tissue architecture. However, as premalignant MECs develop and become invasive, they subvert the tumor-suppressive activities of TGF-β and convert this cytokine into a potent promoter of invasion and metastasis (1,2,3,4,5). An important component of TGF-β signaling is its potential to induce epithelial-mesenchymal transition (EMT), a process in which epithelial cells lose cell-cell contacts, down-regulate genes expressed in terminally differentiated epithelial cells, and up-regulate mesenchymal genes needed for motility and invasion (3, 6,7,8). In addition, TGF-β stimulates the secretion of matrix metalloproteinases (MMPs), capable of degrading and remodeling the extracellular matrix (ECM), thus allowing invasive cells to penetrate the surrounding tissue (9,10,11,12). Understanding this paradoxical switch from tumor suppressing to tumor promoting TGF-β function is key to effectively blocking the oncogenic activities of TGF-β and, consequently, to restoring its tumor suppressive roles.
Abl is a multifunctional protein tyrosine kinase (PTK) that possesses both nuclear and cytoplasmic roles, including its ability to transduce signals from growth factor receptors, to promote growth arrest in response to DNA damage, to regulate actin cytoskeletal rearrangements, particularly in neurite outgrowth, and to interact with various adaptor proteins and scaffold complexes (13,14,15,16). Abl and its relative Arg are the only known kinases with direct actin-binding domains, and both PTKs serve to dynamically regulate alterations in the actin cytoskeleton and adherens junctions in response to cell attachment and extracellular signals (15, 17). Several groups have published studies indicating that Abl activity serves to promote breast cancer survival and motility (18,19,20,21,22); however, an equally compelling body of literature has described important genome-guarding functions for Abl in mediating growth arrest and apoptosis in response to DNA damage (23,24,25,26). Furthermore, EphB4 receptor was found to suppress breast cancer tumorigenicity through activation of an Abl-Crk pathway (27). Abl PTK inhibitors have been extremely effective in treating chronic myelogenous leukemia (CML), and several clinical trials have investigated the efficacy of these same Abl antagonists against solid tumors, including breast, pancreatic, and prostate cancer (28,29,30,31,32,33,34,35). Collectively, these clinical trials have yielded mixed therapeutic results, and as such, a definitive role for Abl in either suppressing or promoting solid tumor progression remains uncertain. Thus, it is clear that a deeper understanding of Abl and its signaling systems in mediating solid tumor development is required to facilitate the improved targeting of this PTK in human cancers.
Because of the morphological changes associated with EMT, in which adherens junctions dissolve to enable cell spreading and motility, and to the established functions of Abl in regulating actin cytoskeletal reorganization, we hypothesized that Abl was an important mediator of EMT and oncogenic signaling stimulated by TGF-β in normal and malignant MECs. The objective of this study was to test this hypothesis and to establish the potential chemotherapeutic value of targeting Abl PTK activity during oncogenic signaling by TGF-β in breast cancer cells. Contrary to our expectations, we discovered a novel and exciting role for Abl as an essential regulator of normal MEC morphology and as a strong suppressor of oncogenic TGF-β signaling during mammary tumorigenesis.
MATERIALS AND METHODS
Cell lines and retroviral vectors
Murine metastatic 4T1 and normal NMuMG cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in a humidified atmosphere at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (complete medium) and in the case of NMuMG cells, with insulin (10 μg/ml).
Retroviral vectors (pMSCV-hygromycin) encoding various murine c-Abl (type IV) derivatives were generously provided by Dr. Tony Hunter (Salk Institute, La Jolla, CA, USA) and included kinase-dead (KD) Abl and constitutively active (CST) Abl (13, 14, 36). Retroviral particles were produced by cotransfecting individual retroviral (10 μg/plate) and pCL-Eco (10 μg/plate) vectors into 70% confluent EcoPack2 cells (Clontech, Mountain View, CA, USA) using LT1 liposomes (Mirus, Madison, WI, USA). Forty-eight hours after transfection, medium containing the retroviral particles was collected, passed through a 0.45-μm syringe filter, diluted 1:1 with fresh complete medium, and supplemented with polybrene (4 μg/ml) prior to administration to target cells for 48 h (NMuMG or 4T1 cells at 60% confluency on 10-cm plates). Transduced cells were isolated by hygromycin selection (100 μg/ml; Mediatech, Manassas, VA, USA), and the resulting stable polyclonal populations of KD-Abl- or CST-Abl-expressing cells were expanded. Differences in Abl protein expression were monitored by immunoblotting whole-cell extracts with an anti-c-Abl antibody (BD Biosciences, Franklin Lakes, NJ, USA).
Lentiviral vectors encoding shRNA against murine c-Abl (cat. no. RMM4534-NM_001112703; Open Biosystems, Huntsville, AL, USA) or scrambled shRNA in pLKO.1 (plasmid 1864; Addgene, Cambridge, MA, USA) were cotransfected (12 μg/plate) into 70% confluent 293T cells (American Type Culture Collection) on 10-cm plates with the packaging vectors pMD2.G (4 μg/plate), pRRE (2 μg/plate), and pRSV (2 μg/plate) (Addgene) using LT1 liposomes. Transduced cells were selected with puromycin (5 μg/ml), and the extent of Abl-deficiency was monitored by c-Abl immunoblotting as above.
A pMSCV-puromycin-based retroviral vector (Clontech) that encodes firefly luciferase was produced by subcloning the luciferase gene from pGL3-luciferase (Promega, Madison, WI, USA). Stable luciferase-expressing control (i.e., empty vector) and CST-Abl-expressing 4T1 cells were generated by their transduction with pMSCV-luciferase-puromycin retrovirus and selection with puromycin as above.
ATP consumption assays
Parental and Abl-manipulated NMuMG or 4T1 cells were cultured on 10-cm plates, and on reaching 80% confluency, were rinsed once with PBS and immediately lysed in buffer H/0.1% Triton-X100 (37). The resulting whole-cell extracts were clarified by microcentrifugation, and 700 μg of total protein was immunoprecipitated with rotation overnight at 4°C with anti-c-Abl antibodies (BD Biosciences). Captured immunocomplexes were washed twice with PBS and once with kinase assay buffer (40 mM Tris, 20 mM MgCl2, and 0.1 mg/ml BSA, pH 7.5) and subsequently were resuspended in 250 μl kinase assay buffer, which then was divided into 100-μl aliquots that received either diluent or Imatinib myselate (10 μM; LC Laboratories, Woburn, MA, USA), a pharmacological Abl kinase inhibitor. Abl protein kinase reactions were initiated by addition of 100 μl of 2× kinase reaction cocktail, which contained the Abl peptide substrate (10 μM; New England Biolabs, Ipswich, MA, USA) and ATP (1 μM). Positive controls contained activated Abl1 kinase (50 ng/reaction; Cell Signaling, Boston, MA, USA), which represented maximal (i.e., 100%) Abl kinase activity. Abl PTK reactions were allowed to proceed at room temperature for 1 h under continuous rotation, at which point 50 μl was removed, mixed with an equal volume of Kinase Glo-Max reagent (Promega), and incubated at room temperature for 10 min. Luminescence was quantified over a 0.5-s integration window on a luminescent microplate reader (Turner Biosystems, Sunnyvale, CA, USA). Specific Abl kinase activity was determined by subtracting the luminescence, measured in relative light units (RLU), observed in Imatinib-containing samples from that measured for their corresponding diluent containing counterparts, which subsequently was normalized to the RLU observed in Abl-positive control samples.
Three-dimensional (3-D) culture system
NMuMG or 4T1 cells were diluted to 3 × 104 cells/ml in complete medium supplemented with 5% Cultrex reconstituted basement membrane (R&D Systems, Minneapolis, MN, USA), and subsequently they were plated in 48-well plates on top of a Cultrex cushion. Substrate stiffness was manipulated by adding type I collagen (0–3 mg/ml; BD Biosciences) to the Cultrex cushion. Cells were stimulated with TGF-β1 (5 ng/ml; R&D Systems) in the absence or presence of the following inhibitors: Imatinib myselate (20 μg/ml); MMP 2/3 (30 μM) or MMP 2/9 inhibitor (500 nM) (Calbiochem, San Diego, CA, USA); or DECMA-1/E-cadherin blocking antibody (0.5 mg/ml, Sigma, St. Louis, MO, USA). The medium/Cultrex mixture was replaced every 3 d, and organoids were allowed to grow for 4–7 d, at which point they were monitored by bright-field photography. The resulting organoids also were monitored by 3-D proliferation assays using the MTS reagent kit (Promega) according to the manufacturer’s instructions. Afterward, 100 μl of the medium/MTS mixture was transferred to a 96-well plate, and the absorbance at 450 nm was determined on a microplate reader (Turner Biosystems). Specific proliferation signals were calculated by subtracting reagent blanks from those containing cells.
In some experiments, the resulting parental and CST-Abl-expressing 4T1 acini were stained with FITC-phalloidin to visualize the actin cytoskeleton, and with DAPI to visualize nuclei. Briefly, 4T1 cell derivatives were cultured for 7 d on compliant Cultrex cushions in 8-well chamber slides (5000 cells/well; Thermo Fisher, Rochester, NY, USA), at which point the organoids were rinsed with PBS supplemented with CaCl2 (0.5 mM) and MgCl2 (0.9 mM) (PBS2+) and fixed in 4% paraformaldehyde/PBS2+. Afterward, the organoids were rinsed in PBS2+ and permeabilized with 0.1% Triton X-100/PBS2+ for 5 min, followed by an additional PBS2+ rinse prior to blocking in 1% BSA/PBS2+ for 45 min. Organoids were incubated with FITC-phalloidin (0.5 μM, Sigma) in BSA/PBS2+ for 30 min, followed by sequential PBS2+ and water washes. Afterward, the stained cultures were dried for 2 h at 37°C; subsequently, they were mounted with ProLong Gold Antifade that contained DAPI (Invitrogen, Carlsbad, CA, USA). Stained organoids were visualized on a Leica DM5000 microscope (×40; Leica Microsystems, Bannockburn, IL, USA) and photographed using Image-Pro Plus 5.1 software (Media Cybernetics, Inc., Bethesda, MD, USA).
MMP fluorimetric and immunoblot assays
NMuMG and 4T1 cells were plated onto 6-well tissue culture plates (3×105/well) and allowed to adhere overnight. The following morning, the cells were rinsed once with PBS and incubated in serum-free medium (SFM; 2 ml/well) in the absence or presence of TGF-β1 (5 ng/ml) for 24 h. The resulting conditioned medium and paired whole-cell extract was harvested and analyzed for MMP expression or activity using two complementary methods: fluorimetric and immunoblotting assays. For fluorimetric assays, equal volumes of conditioned medium (1.5 ml) were immunoprecipitated with anti-MMP-9 antibody (2 μg; Sigma) overnight at 4°C under continuous rotation. The resulting immunocomplexes were rinsed 3 times with PBS and resuspended in 140 μl PBS, followed by their activation with APMA (1 mM; AnaSpec, San Jose, CA, USA) for 2 h at 37°C. The activated samples were assayed for MMP-9 activity using the SensoLyte 520 MMP Sampler Kit (AnaSpec) and the MMP substrate SB14 (10 μM; AnaSpec), according to the manufacturer’s instructions. Duplicate samples were incubated at room temperature for 60 min, followed by reading of fluorescence intensity at Ex/Em = 490/520 nm. In addition, equal volumes (30 μl) of paired whole-cell extracts prepared in buffer H/0.1% Triton X-100 (37) and clarified by microcentrifugation were immunoblotted for β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as a loading control. For MMP immunoblotting assays, 50 μl of conditioned medium was loaded onto SDS-PAGE and subsequently was immunoblotted for MMP-9 or MMP-3 (1:500 dilution; Sigma). β-Actin controls were performed in tandem as described above.
Invasion assays
Invasion assays were conducted by culturing 3.5 × 105 cells/well on Matrigel-coated (diluted 1:25 in SFM; BD Biosciences) modified Boyden chambers, and the cells were induced to invade by addition of 4% serum in the absence or presence of TGF-β1 (5 ng/ml) for 48 h. The chambers were harvested as described elsewhere (37, 38), and specific cell invasion was quantified by measuring the optical density of stained chamber membranes in ImageJ 1.40g software (National Institutes of Health, Bethesda, MD, USA).
Semiquantitative real-time PCR
NMuMG cells were plated in 6-well plates (3.5×105/well) and allowed to adhere overnight before adding fresh complete medium the following morning. Afterward, the cells were treated with TGF-β1 (5 ng/ml) in the absence or presence of Imatinib myselate (10 μg/ml) for 48 h before harvesting total RNA using the RNeasy kit (Qiagen, Valencia, CA, USA). Total RNA (1 μg) was reverse-transcribed to cDNA using the cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), and the resulting cDNAs (10 μl) were diluted 40-fold before analysis. Semiquantitative RT-PCR was performed in 25-μl reaction volumes that contained 12.5 μl 2× SYBR Green (Bio-Rad), 200 nM PCR primer set, 2 μl nuclease-free water, and 10 μl cDNA sample. PCR cycling was performed on a Bio-Rad MJ Mini thermal cycler with Opticon 3.0 software using the following parameters: 1) 95°C for 10 min (1 cycle), and 2) 95°C for 15 s, followed 55°C for 1 min (40 cycles). The threshold for quantity calculations was adjusted to the linear range of amplification for each experiment, which subsequently was normalized to corresponding GAPDH mRNA expression levels. Individual primer sequences are presented in Supplemental Table 1.
Actin cytoskeletal immunofluorescence
NMuMG cells were plated at 3 × 105/well onto gelatin-coated (0.1%) glass coverslips in 24-well plates and allowed to adhere overnight. Afterward, the cells were incubated in the absence or presence of Imatinib (10 μg/ml) or TGF-β1 (5 ng/ml) as indicated. On completion of drug or agonist stimulation, the cells were fixed and stained with FITC-phalloidin (0.25 μM, Sigma), as described previously (39). Stained cells were visualized using an ×60 oil objective on a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) and photographed using SlideBook software (Intelligent Imaging Innovations, Inc., Denver, CO, USA).
Tumor studies
Luciferase-expressing parental (i.e., empty vector) or CST-Abl-expressing 4T1 cells (103/injection in sterile PBS) were engrafted onto the 4th inguinal mammary fat pad of syngeneic 6- to 8-wk-old female Balb/C mice (Jackson Laboratories, Bar Harbor, ME, USA). For the adjuvant chemotherapy trials, vehicle (i.e., sterile water) or Imatinib myselate was administered orally at 50 mg/kg/d dissolved in 100 μl sterile water beginning at d 8 after tumor cell inoculation. Tumor growth was monitored 3 times weekly by palpation and measurement using digital calipers (Thermo Fisher Scientific, Waltham, MA, USA). Tumor volume was calculated using the following equation: volume = 0.5(x)2(y), where x is the tumor length and y is the tumor width. Bioluminescent imaging of tumors was accomplished by injecting d-luciferin (150 μl at 15 mg/ml; Caliper Life Sciences, Hopkinton, MA, USA) intraperitoneally into isofluorane-anesthetized animals, which were imaged on a Xenogen IVIS200 (Caliper Life Sciences) for 30–120 s. Emitted light (photons/s/cm2/sr) was determined using Living Image 2.60.1 software (Xenogen), and the resulting min/max total flux (photons/s) was adjusted so that images collected on the same day could be displayed using the same light-intensity scale. On completion of the studies, the primary 4T1 tumors were excised and weighed between d 28 and 30, and again on d 51 postinoculation. All animal studies were performed according to animal protocol procedures approved by the Institutional Animal Care and Use Committee of the University of Colorado.
RESULTS
Abl inactivation morphologically and transcriptionally mimics EMT induced by TGF-β
EMT is a critical process in embryogenesis and wound healing and is activated aberrantly in malignant tumors that are invading their surrounding tissue (6, 8). TGF-β is a potent activator of EMT (7), and Abl has been well studied for its ability to regulate F-actin-dependent processes and cytoskeletal rearrangements (13, 15). Thus, we hypothesized that Abl was an essential player in determining MEC morphology and in regulating EMT induced by TGF-β. We tested this hypothesis by manipulating Abl expression or PTK activity using several complementary approaches: treating MECs with Imatinib, a pharmacological Abl PTK inhibitor; rendering MECs deficient in Abl expression using shRNA that specifically targeted c-Abl; and overexpression of either a KD or CST Abl mutant (Fig. 1). As shown in Supplemental Fig. 1, parental and scrambled shRNA-expressing (scram) NMuMG cells displayed a normal cuboidal morphology and strong cell-cell adhesion in 2-D tissue culture systems. However, in response to Imatinib treatment, these same NMuMG cells lost their cell-cell junctions and exhibited cell spreading reminiscent of EMT. An identical EMT phenotype was observed in NMuMG cells that expressed a shRNA that targeted c-Abl (shAbl) (Supplemental Fig. 1B). In addition, NMuMG cells that stably expressed KD-Abl acquired a more fibroblastoid appearance, while those that expressed CST-Abl maintained unusually strong cell-cell contacts and formed densely packed monolayers (Supplemental Fig. 1A). These morphological alterations were also readily detected in the actin cytoskeleton architectures of these same cells (Fig. 2A, B). Indeed, parental, scrambled shRNA-, and CST-Abl-expressing NMuMG cells exhibited highly regular cortical actin staining, while cells treated with Imatinib or those lacking Abl expression (shAbl) or activity (KD-Abl) displayed dysregulated cytoskeletons characterized by the loss of cortical actin and by the formation of stress fibers and lamellipodia (Fig. 2A, B). To determine whether these morphological changes induced by Abl deficiency were unique to NMuMG cells, we also treated normal human MCF10A MECs with Imatinib and observed similar cell spreading and loss of cell-cell contacts in 2-D tissue culture systems (Fig. 2C). Furthermore, culturing NMuMG cells in compliant (i.e., a soft and yielding microenvironment), 3-D organotypic cultures in the presence of Imatinib demonstrated that inhibition of Abl PTK activity disrupted normal acinar formation (Fig. 2D). Taken together, these observations indicate that Abl PTK activity is essential for MECs to maintain normal epithelial morphologies in 2-D and 3-D culture systems.
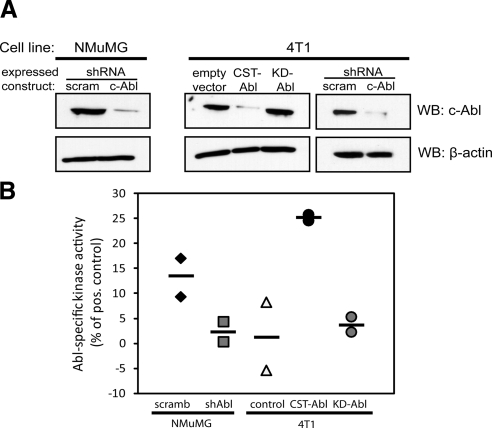
Figure 1.
Manipulation of Abl expression and activity in normal and malignant MECs. A) Immunoblotting for c-Abl expression in NMuMG and 4T1 cells engineered to express either a scrambled (scram) or an Abl-targeting shRNA (shAbl), or empty vector (pMSCV-hygromycin), CST-Abl, or KD-Abl, as indicated. Differences in protein loading were monitored by reprobing stripped membranes with anti-β-actin antibodies. B) ATP consumption assays were performed to compare Abl PTK activity in manipulated NMuMG and 4T1 cell lines. Horizontal lines show means of 2 independent experiments.
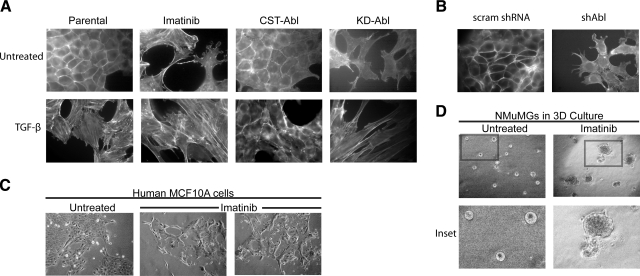
Figure 2.
Abl inactivation morphologically mimics EMT induced by TGF-β in normal MECs. A) Direct actin immunofluorescence using FITC-conjugated phalloidin on Abl-manipulated NMuMG cells before and after their stimulation with TGF-β1 (5 ng/ml) or those treated with Imatinib (20 μg/ml) for 24 h as indicated. Images are representative of 3 independent experiments. B) FITC-conjugated phalloidin immunofluorescence on control (i.e., scrambled shRNA) or Abl-deficient NMuMG cells. Images are representative of 3 or 4 independent experiments. scram, scrambled shRNA; shAbl, Abl-targeting shRNA. C) Normal human MCF10A cells were cultured in the absence or presence of Imatinib (30 μg/ml) for 56 h, at which point bright-field images (×70) were collected. Data are representative of 2 independent experiments. D) NMuMG cells were cultured in compliant 3-D organotypic cultures in the absence or presence of Imatinib (20 μg/ml) for 5 d, at which point bright-field images (×70) were collected. Insets: magnified views of boxed regions. Data are representative of 3 independent experiments.
Because Abl inactivation induced such dramatic changes in MEC morphology (Fig. 2 and Supplemental Fig. 1), we reasoned that inactivating Abl PTK activity in NMuMG cells would increase their motility and result in transcriptional changes associated with EMT. As such, we treated NMuMG cells with TGF-β and/or Imatinib for 24 h and subsequently performed semiquantitative real-time PCR and immunoblot analyses to monitor changes in epithelial (E-) cadherin expression. In accordance with our hypothesis, administration of TGF-β and Imatinib both resulted in decreased E-cadherin mRNA, a transcriptional change observed in classical EMT (Fig. 3A; refs. 6, 8). Moreover, combining both treatments tended to further reduce E-cadherin mRNA expression (Fig. 3A), while Imatinib treatment was observed to rapidly (i.e., within 3 h) induce the loss of E-cadherin protein expression in NMuMG cells (Fig. 3B). Consistent with these changes in E-cadherin expression, we also found that the expression of CST-Abl in NMuMG cells inhibited TGF-β-induced invasion in vitro, while KD-Abl expressing cells invaded readily without added TGF-β (Fig. 3C). Furthermore, real-time PCR analysis on parental and Abl-deficient NMuMG cells revealed that Abl deficiency elevated the expression of the mesenchymal markers, N-cadherin and vimentin, as well as the EMT-associated transcription factor Twist, constituting an EMT transcriptional signature in resting Abl-deficient NMuMG cells (Fig. 3D). Note that Abl deficiency failed to replicate the effects of Imatinib treatment on E-cadherin mRNA levels in NMuMG cells (Fig. 3A, D). Although the reasons underlying this disparate finding remain to be elucidated fully, we suspect that signaling imbalances between the loss of Abl PTK activity vs. those associated with its adaptor and scaffolding functions may account for these differences. Taken together, Abl inactivation leads to morphological, transcriptional, and migrational changes associated with EMT in normal MECs.
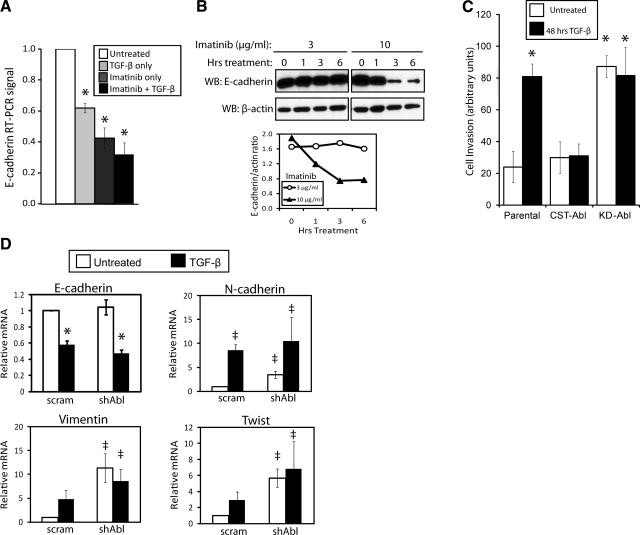
Figure 3.
Abl inactivation transcriptionally mimics EMT and cell invasion induced by TGF-β in normal MECs. A) Quiescent NMuMG cells were treated with Imatinib (10 μg/ml), TGF-β1 (5 ng/ml), or both agents for 24 h as indicated. Afterward, relative levels of E-cadherin mRNA were detected by semiquantitative real-time PCR and normalized to corresponding GAPDH signals. Data are expressed as means ± se; n = 3. B) Quiescent NMuMG cells were incubated with increasing concentrations of Imatinib (3–10 μg/ml) for 0–6 h as indicated. Afterward, altered E-cadherin expression was monitored by immunoblotting with anti-E-cadherin antibodies, followed by immunoblotting for β-actin to monitor differences in protein loading. Accompanying graph depicts ratio of E-cadherin/β-actin signals. Data are from a representative experiment that was performed 3 times with similar results. C) Parental, CST-Abl-, or KD-Abl-expressing NMuMG cells were induced to invade synthetic basement membranes by a 4% serum stimulus, or by 4% serum plus TGF-β1 (5 ng/ml) for 48 h. Data are means ± se; n = 2. D) Quiescent NMuMG cells expressing either a scrambled (scram) or Abl-targeting shRNA (shAbl) were stimulated with TGF-β1 (5 ng/ml) for 48 h. Afterward, the relative mRNA expression levels of E- and N-cadherins, vimentin, and Twist were determined by semiquantitative real-time PCR. Data are means ± se; n = 3. *P < 0.01, ‡P < 0.05 vs. corresponding control; Student’s t test).
Abl activation restores normal MEC morphology and TGF-β-mediated cytostasis in metastatic MECs grown in compliant microenvironments
Our findings in NMuMG cells, in which Abl inactivation induced an EMT-like phenotype and transcriptional signature, prompted us to ask whether expression of a CST-Abl mutant to mimic Abl activation in metastatic MECs could normalize and revert their morphologies. Thus, we stably expressed CST-Abl in metastatic murine 4T1 breast cancer cells, which resulted in low-level CST-Abl protein expression (Fig. 1A) that likely reflects the significantly shortened half-life previously ascribed to constitutively activated Abl kinases (40). However, despite the relatively low level of expression, CST-Abl cells did indeed possess significantly higher levels of Abl PTK activity as compared to their parental counterparts (Fig. 1B). In contrast, expression of the KD-Abl construct in 4T1 cells resulted in elevated Abl protein levels (Fig. 1A) but no measurable increase in the levels of Abl PTK activity (Fig. 1B). Similar to NMuMG cells, we found that expression of CST-Abl promoted stronger cell-cell junctions and reduced the spreading of 4T1 cells in 2-D cultures (Fig. 4A). However, culturing these same cells on compliant 3-D organotypic reconstituted basement membranes greatly magnified the subtle morphological differences observed in 2-D culture (Fig. 4B). Indeed, although both parental (i.e., empty vector) and KD-Abl-expressing 4T1 cells formed large, irregularly shaped organoids characteristic of malignant MECs, 4T1 cells that expressed CST-Abl formed smaller acinar structures that were perfectly spherical (Fig. 4B). To confirm that the overall reduction in Abl protein expression observed in CST-Abl cells was not responsible for the phenotypic reversion of 4T1 cell morphology, we also cultured Abl-deficient 4T1 cells in 3-D organotypic cultures and observed a phenotype that resembled that of their parental counterparts (Fig. 4B). Most strikingly, we found that parental 4T1 cells failed to form hollow acinar structures when grown in compliant 3-D-organotypic cultures, an expected outcome that was wholly consistent with the aggressive tumorigenic phenotype of 4T1 cells (Fig. 4C). In stark contrast, culturing CST-Abl-expressing 4T1 cells under identical conditions resulted in their formation of small, spherical acini that appeared to be partially hollow (Fig. 4C). This dramatic finding suggests that Abl activation was capable of inducing the morphological and phenotypic reversion of 4T1 cells. Moreover, although expression of CST-Abl tended to inhibit the proliferation of 4T1 cells, both parental and CST-Abl-expressing 4T1 cells failed to undergo growth arrest in response to TGF-β when cultured on tissue culture plastic (i.e., a stiff microenvironment; Fig. 4D). Quite surprisingly, significant proliferative differences between these cell lines did manifest on their growth in compliant 3-D organotypic cultures, which was wholly sufficient in restoring the cytostatic function of TGF-β (Fig. 4D). Note that while we failed to observe any effect of manipulating Abl expression or activity on the ability of TGF-β to activate Smad2/3 and p38 MAPK (Supplemental Fig. 2), we did find that expressing CST-Abl greatly potentiated the coupling of TGF-β to p21Cip1 mRNA expression in 4T1 cells (Fig. 4E). Thus, the heightened induction of p21Cip1 expression by TGF-β in CST-Abl-expressing cells may account for the significant suppression in their proliferation relative to that of their parental counterparts (Fig. 4D). In addition, we found that rendering normal MECs deficient in Abl had no effect on their cytostatic response to TGF-β, as parental and Abl-deficient NMuMG cells, both exhibited similar extents of growth arrest when stimulated with TGF-β (data not shown). Taken together, these findings show for the first time that exposing aggressive, metastatic mouse mammary carcinoma cells to compliant microenvironmental signals reinstates their cytostatic response to TGF-β, and in effect, partially reestablishes the tumor-suppressing functions of TGF-β in malignant MECs. Equally important, our findings show that Abl activation is sufficient to phenotypically and morphologically normalize the architecture of metastatic mouse mammary carcinoma cells.
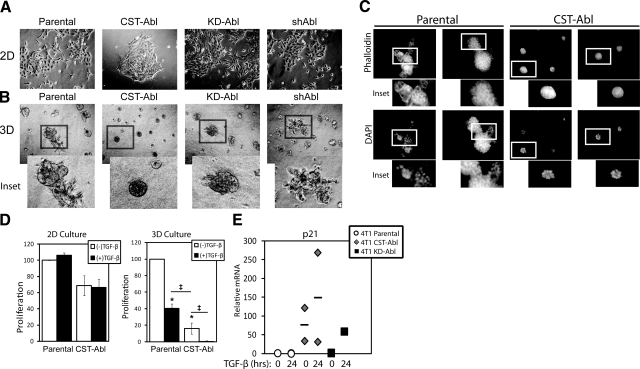
Figure 4.
Abl activation promotes normal MEC morphology and TGF-β-mediated cytostasis in metastatic MECs grown in compliant microenvironments. A, B) Bright-field images (×70) of parental, CST-Abl-, KD-Abl, or shAbl-expressing 4T1 cells grown in 2-D (A) or compliant 3-D organotypic (B) culture systems as indicated. Insets: magnified views of boxed regions. Images are representative of 3 independent experiments. C) Parental or CST-Abl-expressing 4T1 cells were grown in compliant 3-D organotypic cultures for 7 d as indicated. Afterward, resulting acinar structures were stained with FITC-phalloidin and DAPI. Insets: magnified views of boxed regions. Images are representative of 2 independent experiments. D) Parental or CST-Abl-expressing 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for 48 h in 2-D (left panel) or compliant 3-D organotypic (right panel) cultures as indicated. Differences in proliferation were determined by employment of MTS proliferation assays. Data are means ± se; n = 2–5. *P < 0.01, ‡P < 0.05 vs. untreated parental 4T1 cells; Student’s t test. E) 4T1 cells expressing empty vector, CST-Abl, or KD-Abl were stimulated with TGF-β1 (5 ng/ml) for 24 h. Afterward, relative mRNA expression of p21Cip1 transcripts was determined by semiquantitative real-time PCR. Data are combined from 2 independent experiments.
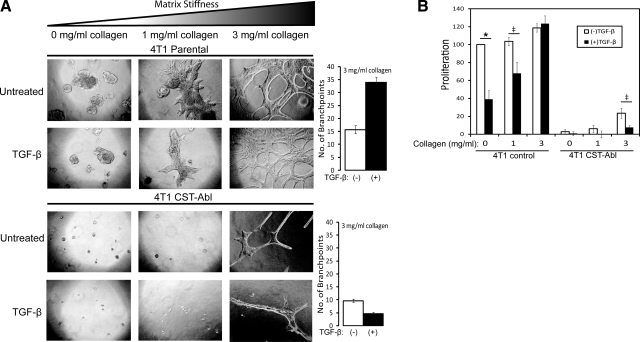
Our findings above clearly show the ability of compliant organotypic cultures to restore the cytostatic activities of TGF-β in metastatic breast cancer cells, a physiological reaction that is independent of altered genotypes. Along these lines, several studies have found that increasing ECM stiffness and rigidity can promote the development of malignant phenotypes in 3-D organotypic cultures and that the nearly infinite stiffness of 2-D culture systems can mask important morphological and functional differences that exist between normal and metastatic MECs (41,42,43). We, therefore, investigated the effect of ECM stiffness on the morphological and proliferative responses of parental (i.e., empty vector) and CST-Abl-expressing 4T1 cells to TGF-β. To do so, we added increasing amounts of type I collagen to the Cultrex basement membrane cushions, which typically are compliant and possess an elastic modulus approximating that of normal breast tissue, while supplementation with type I collagen (3 mg/ml) approximates the stiffness of tumors and their accompanying stroma (44). Figure 5A shows that parental 4T1 cells became increasingly branched and dysmorphic in response to increasing ECM stiffness, while their CST-Abl-expressing counterparts were highly resistant to rigidity-induced morphological alterations. Indeed, CST-Abl-expressing 4T1 cells formed considerably smaller organoids as compared to parental 4T1 cells and failed to exhibit any overt morphological alterations until the highest concentrations of type I collagen were reached (3 mg/ml) (Fig. 5A). Most important, ECM rigidity was observed to dose dependently override the cytostatic activities of TGF-β. Indeed, parental 4T1 cells, which do not arrest growth in response to TGF-β in 2-D cultures (Fig. 4D), recovered a cytostatic response to TGF-β when grown in compliant 3-D organotypic cultures (Fig. 4D). However, increasing 3-D organotypic culture rigidity dose dependently reduced and ultimately ablated the cytostatic response of parental 4T1 cells to TGF-β (Fig. 5B), a finding consistent with a more malignant phenotype elicited by increasing microenvironmental stiffness (41,42,43). In stark contrast, 4T1 cells expressing CST-Abl retained their ability to undergo cytostasis in response to TGF-β even in rigid microenvironments (Fig. 5B). Collectively, these findings show for the first time that Abl activation can override microenviromental signals that promote tumorigenesis, and in doing so, can dictate normal MEC morphologies that restore the tumor-suppressing activities of TGF-β in metastatic breast cancer cells.
Figure 5.
Abl activation abrogates rigidity-induced proliferation and maintains TGF-β cytostatic response in metastatic MECs. Parental and CST-Abl-expressing 4T1 cells were grown in the absence or presence of TGF-β1 (5 ng/ml) for 7 d in compliant or rigid (i.e., 1–3 mg/ml type I collagen) 3-D organotypic cultures as indicated. A) Left panels: differences in cell morphology and proliferation were monitored by bright-field microscopy (×70). Right panels: differences in organoid branching at 3 mg/ml collagen. B) Differences in organoid proliferation were monitored by MTS assays. Data are expressed as means ± se (n=3). *P < 0.01, ‡P < 0.05 vs. untreated parental 4T1 cells; Student’s t test.
Abl activation alleviates basal and TGF-β-induced MMP expression and secretion
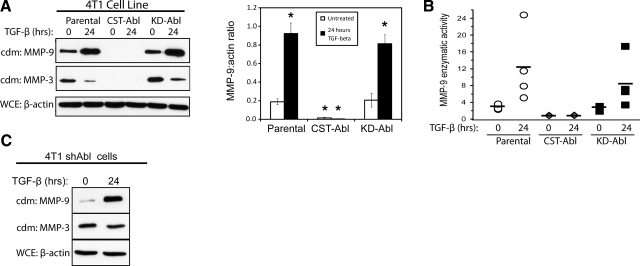
MMPs are enzymes capable of remodeling the ECM through the cleavage of fibronectins, laminins, collagens, and other components of the microenvironment, and through the release of a variety of second messengers that modify the ECM and cell behavior (45). A number of MMPs have been shown to be crucial for cancer cell motility, most notably MMPs 1-3 and -9 (46,47,48,49). In addition, in some contexts, TGF-β promotes cancer cell invasiveness by stimulating MMP expression or activity (9,10,11,12). The striking role of Abl in determining the morphological output of MECs in response to altered microenvironmental tension (Figs. 4 and 5) led us to investigate a function for Abl in regulating MMP expression and secretion. First, zymography of parental and Abl-expressing 4T1 cells showed that TGF-β readily induced MMP secretion in parental and KD-Abl-expressing cells; however, medium conditioned by CST-Abl-expressing cells contained markedly less gelatinase activity (Supplemental Fig. 3A). We also sought to identify which specific MMPs were being secreted by Abl-manipulated 4T1 cells through the employment of immunoblots and fluorimetric assays to detect MMP expression and activity. We found that TGF-β stimulated significant up-regulation of MMP-9 (Fig. 6) and MMP-13 (Supplemental Fig. 3B), but it downregulated that of MMP-3 (Fig. 6) in parental and KD-Abl-expressing 4T1 cells. Significantly, both basal and TGF-β-regulated secretion of MMPs 3, 9, and 13 was abrogated by CST-Abl expression in 4T1 cells (Fig. 6 and Supplemental Fig. 3B). Again, to rule out the possibility that Abl-deficiency (Fig. 1A) was responsible for these effects on MMP secretion, we also performed immunoblot analyses to monitor MMP-3 and MMP-9 secretion by Abl-deficient 4T1 cells, which expressed both MMPs in a manner identical to that of their parental counterparts (Fig. 6C). In addition, we found that Abl deficiency was not sufficient to induce MMP secretion in normal cells, as parental and Abl-deficient NMuMG cells secreted similar levels of MMP-3 and MMP-9 both basally and in response to TGF-β (data not shown). Taken together, these results show that CST-Abl is a potent and broad spectrum inhibitor of both basal and TGF-β-stimulated production of MMPs in metastatic breast cancer cells.
Figure 6.
Abl activation alleviates basal and TGF-β-induced MMP expression and secretion. A) Quiescent parental, CST-Abl-, and KD-Abl-expressing 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for 24 h, at which point the resulting conditioned medium (cdm) was collected and immunoblotted for MMPs 9 and 3 as indicated. Differences in cell numbers produced were monitored by immunoblotting for β-actin in corresponding whole-cell extracts (WCE). Graph depicts mean ± se ratios of MMP-9/β-actin signals; n = 5. *P < 0.01; Student’s t test. B) Conditioned medium was prepared as in A and was immunoprecipitated with anti-MMP-9 antibodies for use in fluorescent MMP-9 enzymatic assays. Symbols represent data obtained in 4 independent experiments; horizontal bars depict mean MMP-9 activity. C) Abl-deficient 4T1 cells (shAbl) were stimulated with TGF-β1 (5 ng/ml) for 24 h, at which point conditioned medium was collected and immunoblotted for MMPs 9 and 3 as indicated. Differences in cell numbers were monitored by immunoblotting corresponding whole-cell extracts with anti-β-actin antibodies. Images are from a representative experiment that was performed 2 times with identical results.
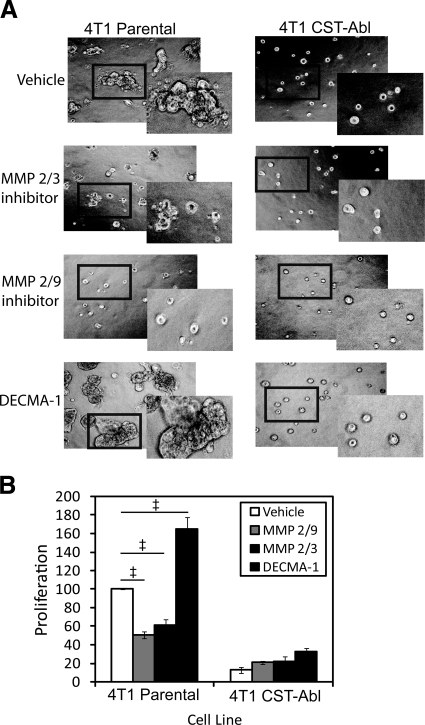
Considering the ability of CST-Abl expression to inhibit secretion of several MMPs (Fig. 6) and to dictate normal MEC morphology in 3-D organotypic cultures (Fig. 4), we endeavored to block MMP function pharmacologically in parental 4T1 cells to attempt to recapitulate the effects of CST-Abl expression. To do so, we cultured parental and CST-Abl-expressing 4T1 cells in compliant 3-D organotypic cultures and found that treatment with inhibitors against MMP2/3 and MMP2/9 both markedly reduced abnormal organoid branching and proliferation in parental 4T1 cells (Fig. 7). Predictably, these same experimental conditions had no measurable impact on organoid formation in CST-Abl-expressing cells. In view of the results involving NMuMG cells in which maintenance of E-cadherin expression correlated with normal MEC morphology (Fig. 3), we also treated these same 4T1 cell lines with a neutralizing E-cadherin antibody (DECMA-1) and observed a dramatic increase in organoid size and dysmorphic features only in parental 4T1 cells on E-cadherin inactivation (Fig. 7). Treatment with a nonspecific rat IgG (control) had no impact on organoid size (data not shown). Collectively, these findings coalesce to emphasize the importance of matrix remodeling and cell-cell contacts in determining MEC morphology and behavior, as well as their response to TGF-β.
Figure 7.
MMP and E-cadherin inhibitors alter the morphology and proliferation of metastatic MECs in 3-D organotypic cultures. Parental and CST-Abl-expressing 4T1 cells were cultured in the absence or presence of an MMP 2/3 inhibitor (30 μM), an MMP 2/9 inhibitor (500 nM), or a neutralizing E-cadherin antibody (DECMA-1; 0.5 mg/ml) for 5 d in compliant 3-D organotypic cultures as indicated. Differences in cell morphology and proliferation were monitored by bright field microscopy (×70; A), or by MTS proliferation assays (B). Insets: magnified views of boxed regions. Data are means ± se; n = 3. ‡P < 0.05 vs. untreated parental 4T1 cells; Student’s t test).
Abl activation prevents 4T1 tumor growth in mice
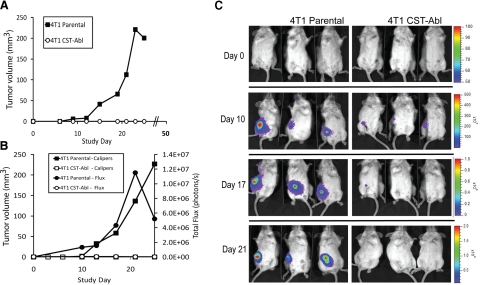
Our findings thus far have shown Abl activation to promote normal MEC morphology, especially in compliant 3-D organotypic cultures and to restore the cytostatic activities of TGF-β in malignant MECs, even when faced with tumor-promoting signals elicited by rigid microenvironments (Figs. 4 and 5). We, therefore, asked whether these findings had any bearing on mammary tumor formation in mice by comparing the growth of parental (i.e., empty vector) and CST-Abl-expressing 4T1 cells following their orthotopic injection into syngeneic Balb/C mice (12 animals/cell line). In doing so, all mice injected with parental 4T1 cells developed palpable tumors by d 10, which then proceeded to grow exponentially over the next 2–3 wk (Fig. 8A), ultimately resulting in excessive tumor burden and euthanization of the mice by d 28. Remarkably, not a single animal injected with CST-Abl-expressing 4T1 cells developed palpable tumors (Fig. 8A). At d 28, eight of the mice injected with CST-Abl-expressing 4T1 cells were euthanized and autopsied for signs of tumor nodules, which again failed to provide evidence of disease. The remaining 4 mice were allowed to survive until d 51, at which point they too were sacrificed and examined for evidence of disease. Quite surprisingly, these mice also remained free from signs of disease.
Figure 8.
Abl activation prevents 4T1 tumor growth in mice. A) Parental and CST-Abl-expressing 4T1 cells were injected orthotopically (103 cells/injection) into female Balb/C mice (12 animals/cell line), and 4T1 tumor growth was measured using digital calipers. B) Luciferase-expressing parental and CST-Abl-expressing 4T1 cells were engrafted onto the mammary fat pads of Balb/C mice (6 mice/cell line) as in A. Primary tumor growth was monitored using digital calipers (squares) or bioluminescent imaging (circles) measured as total flux of light emitted from imaged animals. C) Representative images of parental and CST-Abl-expressing 4T1 tumor-bearing mice imaged on d 0, 10, 17, and 21 postengraftment.
In an effort to exclude injection error or other anomalous events for the failure of CST-Abl-expressing 4T1 tumors to grow in Balb/C mice, we repeated these analyses using 4T1 cells engineered to stably express luciferase, which enabled us to track the tumors through bioluminescent imaging. A total of 6 animals were injected with parental or CST-Abl-luciferase-expressing 4T1 cells, as described above. By d 10 postinjection, the parental 4T1 tumors began to expand rapidly and form palpable tumors that were readily traceable by bioluminescent imaging (Fig. 8B, C). In contrast, the CST-Abl-expressing 4T1 cells were only detected transiently via bioluminescent imaging, and as above, these cells once again failed to form palpable tumors (Fig. 8B, C). Indeed, during the ensuing 25 d, the parental 4T1 cells again formed large tumors similar to those noted above, while their CST-Abl-expressing counterparts regressed to undetectable levels (Fig. 8C). Consistently, the limited expansion of CST-Abl-expressing cells in vivo mirrored the stunted proliferation of these cells in compliant 3-D organotypic culture (Figs. 4 and 5). Thus, Abl kinase activation affords protection against tumor progression in a late-stage model of TGF-β-responsive breast cancer (50,51,52). Moreover, these findings suggest that chemotherapies that target Abl inactivation are likely to offer little clinical benefit in the treatment of breast cancer.
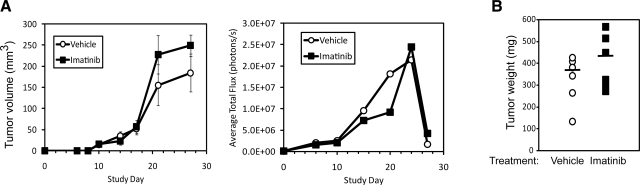
We tested the above hypothesis by monitoring the efficacy of pharmacological Abl inhibition to reduce primary tumor growth and metastasis in mice bearing 4T1 tumors. As above, 4T1 cells tagged with luciferase were injected orthotopically into 12 Balb/C mice, which uniformly developed tumors by d 8, at which point, they were administered either diluent or Imatinib (50 mg/kg/d, orally). By d 24, one Imatinib-treated mouse showed signs of lethargy and weight loss, and as a result, 3 control and 3 Imatinib-treated mice were immediately euthanized. The remaining mice were euthanized on d 27. Figure 9 clearly shows that Imatinib provided no therapeutic activity against the growth of 4T1 tumors in mice. In fact, Imatinib administration tended toward the production of larger tumors compared to diluent-treated controls (Fig. 9). Posteuthanasia imaging following removal of the primary tumors enabled us to sensitively visualize 4T1 metastases in these animals, and in doing so, we failed to detect any significant differences in the metastatic burden in these two experimental cohorts (data not shown). Taken together, these findings clearly show that Imatinib administration fails to effectively inhibit the growth and metastasis of mammary tumors.
Figure 9.
Imatinib administration fails to inhibit 4T1 tumor growth in mice. A) Luciferase-expressing 4T1 cells were injected orthotopically (103 cells/injection) into female Balb/C mice. Palpable tumors were readily detected in all mice at d 8 postinjection, at which point 6 mice received vehicle (i.e., sterile water) and 6 mice received Imatinib (50 mg/kg/d; oral administration). Primary tumor growth was monitored using digital calipers (left panel; means ± se) or bioluminescent imaging (right panel) measured as average total flux of light emitted from imaged animals. B) Primary 4T1 tumors were surgically removed at d 24 and 27 postengraftment and weighed. Bars indicate mean tumor weight for each group (6 mice/group).
DISCUSSION
Abl has been studied intensely for its role in CML; consequently, the mechanisms underlying oncogenic signaling by the BCR-Abl fusion protein in hematopoietic cancers is well established and understood (53). Although dysregulated Abl PTK activity clearly promotes tumorigenesis in hematopoietic cells, the role of Abl in regulating tumorigenesis in epithelial tumors remains controversial. Indeed, the difficulty in assigning specific functions to c-Abl likely reflects the diversity of intrinsic (e.g., DNA damage and reactive oxygen species) and extrinsic (e.g., growth factors, cytokines, and matrix molecules) signals that activate this PTK in highly spatiotemporal manner within epithelial cells (18, 54). Thus, the pathophysiological output of Abl activation ultimately reflects a conglomeration of the initiating signal, the cellular context, and the cellular locale, wherein Abl is stimulated. With respect to cancers of the breast, Abl activation has been associated with enhanced breast cancer cell proliferation, invasion, and survival, and with their transformation by Src (18,19,20,21,22). Unfortunately, the overall significance of these findings is limited by their failure to adequately exclude off-target effects of Imatinib (i.e., inhibition of PDGFR, c-Kit, or DDR1), and by their inability to translate these in vitro findings to in vivo models of breast cancer development and progression. Moreover, Abl PTK inhibitors have been included in clinical trials for treatment of prostate, pancreatic, and other cancers with limited success (28, 32,33,34,35). Along these lines, three independent Imatinib trials recently were performed in women with invasive breast cancer, and in all cases, no clinical benefit was attributed to Imatinib administration (29,30,31). In fact, one of these studies found Imatinib to cause significant toxicity and to elicit disease progression in breast cancer patients (29). Thus, at face value, Imatinib administration appears to be contraindicated for patients with developing breast and other carcinomas, a suggestion bolstered significantly by our findings showing that Imatinib was powerless to slow the growth and metastasis of 4T1 tumors in mice (Fig. 9). Moreover, this notion is consistent with recent findings identifying Abl as being essential for the ability of EphrinB2/EphB4 to suppress mammary tumorigenesis (27). In addition, Abl-mediated phosphorylation of Crk inhibits fibroblast migration (55), while Imatinib administration initiates thyroid cell migration via a HGF-dependent mechanism (56). Clearly, the functions of endogenous c-Abl are many and are subject to a variety of sensitive and diverse contextual regulatory events that may, in fact, preferentially suppress tumorigenesis in epithelial-derived tumors (13, 15, 36).
Equally complex are the dichotomous roles played by TGF-β in mediating stage-specific tumor suppression and promotion, events that have frustrated the development of chemotherapies capable of specifically targeting the oncogenic activities of TGF-β in developing and progressing human malignancies, including those of the breast (57, 58). Interestingly, despite the pathophysiological parallels that exist between TGF-β and Abl in epithelial cells, a definitive role for Abl in mediating either the tumor suppressing or promoting activities of TGF-β in normal and malignant MECs remains unexplored. Our study establishes a novel and potent tumor suppressing function for Abl in determining not only the phenotypes and morphologies of normal and malignant MECs, but also their response to TGF-β. For instance, rendering normal MECs deficient in either Abl expression or activity elicited morphological alterations and transcriptional signatures reminiscent of EMT stimulated by TGF-β (Figs. 2 and 3). Conversely, constitutive Abl activation induced a “hyperepithelial” morphology that wholly normalized and reverted the phenotypes of metastatic breast cancer cells both in vitro and in vivo (Figs. 45678), and in part via suppression of the MMP pathway (Figs. 6 and 7). Collectively, our findings clearly implicate Abl as a suppressor, not a promoter, of mammary tumorigenesis; they also show that Abl activation overrides and circumvents the oncogenic activities of TGF-β in malignant MECs, in part, by inducing a mesenchymal-epithelial transition that phenotypically normalizes and reverts MEC architectures. It is important to note that our findings in epithelial cells are in stark contrast from those obtained in fibroblasts, wherein TGF-β activation of c-Abl appears essential for fibrogenesis by renal and lung fibroblasts. Indeed, in these systems, Imatinib treatment is effective in blocking the profibrogenic activities of TGF-β (59,60,61,62). The disparate actions of Imatinib in fibroblasts (e.g., suppression of fibrogenesis) and MECs (e.g., promotion of tumorigenesis) does not preclude a tumor-suppressing function for Abl in MECs and likely is explained by differences in cell lineage and genotype and by the sum of various off-target activities of Imatinib in these respective cell types.
Along these lines, our finding that Abl deficiency induced EMT in normal MECs has important bearings on the use of Abl kinase inhibitors in the treatment of breast cancer, especially for localized tumors. Indeed, EMT has become increasing recognized as an important early step in local invasion and metastasis, and more recently, it was found to confer stem cell-like properties on human MECs to promote tumorigenesis (63). Thus, EMT induced by administration of Abl PTK inhibitors could enable or encourage the spread of otherwise premalignant tumors. This finding alone contends that the use of Imatinib or other Abl PTK antagonists is inadvisable for the treatment of well-differentiated epithelial-derived tumors, findings supported by our own preclinical Imatinib adjuvant studies.
Equally important is our conclusion that Abl activation promoted the phenotypic and morphological reversion of metastatic MECs in both 2-D and 3-D culture systems, results that clearly identify Abl as being a potent tumor suppressor in MECs. Perhaps even more remarkable was our novel finding that culturing metastatic MECs that are fully resistant to the antiproliferative activities of TGF-β in compliant organotypic cultures was sufficient in restoring their cytostatic response to TGF-β. Moreover, we show that simply reintroducing ECM rigidity to these compliant cultures dose dependently inactivates the antiproliferative activities of TGF-β, an untoward response that is abrogated by Abl activation. It has long been argued that “phenotypes dominate genotypes,” a statement referring to the ability of the ECM and cell microenvironments to either suppress or promote tumorigenesis in a manner independent of genotypic alternations in MECs (64). Dramatic evidence supporting this idea was provided originally by the pioneering work of Beatrice Mintz, who demonstrated that normal mice could develop from blastocysts injected with stable teratoma cells (65). In addition, injecting nuclei isolated from melanoma cells into enucleated oocytes led to the generation of ES cell lines capable of producing viable, healthy chimeric mice (66). Finally, infecting chicken embryos with RSV in ovo only elicited cellular transformation and tumor formation in response to tissue wounding, a response mediated by TGF-β (64). Thus, subjecting genotypically abnormal cells to normal microenvironmental signals not only normalizes and reverts their malignant behaviors, but also imparts normal cell architectures and morphologies in these previously dysmorphic cells. Our findings clearly identify Abl as an essential mediator of this morphological and phenotypical reversion, and as a principle mediator that oversees the cytostatic activities of TGF-β. Indeed, a natural extension of our findings suggests that the identification and characterization of novel small molecules capable of activating Abl allosterically will greatly improve the therapeutic response of patients with metastatic breast cancers.
Finally, our findings demonstrating that Abl activation abrogated basal and TGF-β-induced MMP expression and secretion in metastatic MECs presents a potentially novel alternative for targeting MMPs therapeutically. In general, administration of MMP antagonists have yielded disappointing therapeutic efficacy in clinical trials (67, 68), suggesting perhaps that alternative carcinoma-specific strategies for targeting MMP production in cancer cells may prove to be more effective than pan-MMP antagonism. As such, our findings indicate that measures capable of eliciting Abl activation may achieve this goal by preventing the secretion and activation of the MMP signaling cascade. Experiments designed to test this clinically relevant hypothesis are currently ongoing.
Supplementary Material
Acknowledgments
We thank Dr. Tony Hunter (Salk Institute, La Jolla, CA, USA) for providing wild-type, kinase-dead, and constitutively-active murine c-Abl (type IV) constructs. We also thank members of the W.P.S. laboratory for critical comments and reading of the manuscript. Research support was provided in part by the National Institutes of Health (CA129359) and the Komen Foundation (BCTR0706967) to W.P.S., and by the Department of Defense Predoctoral Training Fellowship (BC083323) to T.M.A.
References
- Benson J R. Role of TGF-β in breast carcinogenesis. Lancet Oncol. 2004;5:229–239. doi: 10.1016/S1470-2045(04)01426-3. [DOI] [PubMed] [Google Scholar]
- Dumont N, Arteaga C L. TGF-β and breast cancer: Tumor promoting effects of TGF-β. Breast Cancer Res. 2000;2:125–132. doi: 10.1186/bcr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali K, Moustakas A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Wakefield L M, Piek E, Bottinger E P. TGF-β signaling in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:67–82. doi: 10.1023/a:1009568532177. [DOI] [PubMed] [Google Scholar]
- Wakefield L M, Roberts A B. TGF-β signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Thiery J P. EMT in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-β-induced EMT. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin C H. Signaling networks guiding EMT during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominsky S L, Doucet M, Thorpe M, Weber K L. MMP-13 is over-expressed in renal cell carcinoma bone metastasis and is induced by TGF-β1. Clin Exp Metastasis. 2008;25:865–870. doi: 10.1007/s10585-008-9202-2. [DOI] [PubMed] [Google Scholar]
- Sinpitaksakul S N, Pimkhaokham A, Sanchavanakit N, Pavasant P. TGF-β1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. Biochem Biophys Res Commun. 2008;371:713–718. doi: 10.1016/j.bbrc.2008.04.128. [DOI] [PubMed] [Google Scholar]
- Yan C, Boyd D D. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- Mott J D, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodring P J, Litwack E D, O'Leary D D, Lucero G R, Wang J Y, Hunter T. Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J Cell Biol. 2002;156:879–892. doi: 10.1083/jcb.200110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodring P J, Meisenhelder J, Johnson S A, Zhou G L, Field J, Shah K, Bladt F, Pawson T, Niki M, Pandolfi P P, Wang J Y, Hunter T. c-Abl phosphorylates Dok1 to promote filopodia during cell spreading. J Cell Biol. 2004;165:493–503. doi: 10.1083/jcb.200312171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast A M. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- Pendergast A M. Nuclear tyrosine kinases: from Abl to WEE1. Curr Opin Cell Biol. 1996;8:174–181. doi: 10.1016/s0955-0674(96)80063-9. [DOI] [PubMed] [Google Scholar]
- Zandy N L, Pendergast A M. Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases. Cell Cycle. 2008;7:444–448. doi: 10.4161/cc.7.4.5452. [DOI] [PubMed] [Google Scholar]
- Lin J, Arlinghaus R. Activated c-Abl tyrosine kinase in malignant solid tumors. Oncogene. 2008;27:4385–4391. doi: 10.1038/onc.2008.86. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Sims J T, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- Plattner R, Kadlec L, DeMali K A, Kazlauskas A, Pendergast A M. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirvent A, Boureux A, Simon V, Leroy C, Roche S. The tyrosine kinase Abl is required for Src-transforming activity in mouse fibroblasts and human breast cancer cells. Oncogene. 2007;26:7313–7323. doi: 10.1038/sj.onc.1210543. [DOI] [PubMed] [Google Scholar]
- Li L S, Morales J C, Hwang A, Wagner M W, Boothman D A. DNA mismatch repair-dependent activation of c-Abl/p73α/GADD45α-mediated apoptosis. J Biol Chem. 2008;283:21394–21403. doi: 10.1074/jbc.M709954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M W, Li L S, Morales J C, Galindo C L, Garner H R, Bornmann W G, Boothman D A. Role of c-Abl kinase in DNA mismatch repair-dependent G2 cell cycle checkpoint arrest responses. J Biol Chem. 2008;283:21382–21393. doi: 10.1074/jbc.M709953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer G, Gademan I S, Kal H B, de Rooij D G. Role for c-Abl and p73 in the radiation response of male germ cells. Oncogene. 2001;20:4298–4304. doi: 10.1038/sj.onc.1204568. [DOI] [PubMed] [Google Scholar]
- Ongkeko W M, An Y, Chu T S, Aguilera J, Dang C L, Wang-Rodriguez J. Gleevec suppresses p63 expression in head and neck squamous cell carcinoma despite p63 activation by DNA-damaging agents. Laryngoscope. 2006;116:1390–1396. doi: 10.1097/01.mlg.0000225941.60901.0f. [DOI] [PubMed] [Google Scholar]
- Noren N K, Foos G, Hauser C A, Pasquale E B. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Gharibo M, Patrick-Miller L, Zheng L, Guensch L, Juvidian P, Poplin E. A phase II trial of imatinib mesylate in patients with metastatic pancreatic cancer. Pancreas. 2008;36:341–345. doi: 10.1097/MPA.0b013e31815d50f9. [DOI] [PubMed] [Google Scholar]
- Modi S, Seidman A D, Dickler M, Moasser M, D'Andrea G, Moynahan M E, Menell J, Panageas K S, Tan L K, Norton L, Hudis C A. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2005;90:157–163. doi: 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]
- Chew H K, Barlow W E, Albain K, Lew D, Gown A, Hayes D F, Gralow J, Hortobagyi G N, Livingston R. A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: Southwest Oncology Group Study 0338. Clin Breast Cancer. 2008;8:511–515. doi: 10.3816/CBC.2008.n.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Morandi P, Krishnamurthy S, Reuben J M, Lee B N, Francis D, Booser D J, Green M C, Arun B K, Pusztai L, Lopez A, Islam R, Valero V, Hortobagyi G N. Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-Kit or PDGFR-β: clinical activity and biological correlations. Ann Oncol. 2008;19:1713–1719. doi: 10.1093/annonc/mdn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A M, Rini B I, Derynck M K, Weinberg V, Park M, Ryan C J, Rosenberg J E, Bubley G, Small E J. A phase I trial of docetaxel/estramustine/imatinib in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. 2007;5:323–328. doi: 10.3816/CGC.2007.n.011. [DOI] [PubMed] [Google Scholar]
- Lin A M, Rini B I, Weinberg V, Fong K, Ryan C J, Rosenberg J E, Fong L, Small E J. A phase II trial of imatinib mesylate in patients with biochemical relapse of prostate cancer after definitive local therapy. BJU Int. 2006;98:763–769. doi: 10.1111/j.1464-410X.2006.06396.x. [DOI] [PubMed] [Google Scholar]
- Bajaj G K, Zhang Z, Garrett-Mayer E, Drew R, Sinibaldi V, Pili R, Denmeade S R, Carducci M A, Eisenberger M A, DeWeese T L. Phase II study of imatinib mesylate in patients with prostate cancer with evidence of biochemical relapse after definitive radical retropubic prostatectomy or radiotherapy. Urology. 2007;69:526–531. doi: 10.1016/j.urology.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Chen J, Rocken C, Nitsche B, Hosius C, Gschaidmeier H, Kahl S, Malfertheiner P, Ebert M P. The tyrosine kinase inhibitor imatinib fails to inhibit pancreatic cancer progression. Cancer Lett. 2006;233:328–337. doi: 10.1016/j.canlet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Woodring P J, Hunter T, Wang J Y. Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J Biol Chem. 2001;276:27104–27110. doi: 10.1074/jbc.M100559200. [DOI] [PubMed] [Google Scholar]
- Schiemann W P, Blobe G C, Kalume D E, Pandey A, Lodish H F. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by TGF-β and affects protein kinase cascades. J Biol Chem. 2002;277:27367–27377. doi: 10.1074/jbc.M200148200. [DOI] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman H K, Martin G R, Aaronson S A, Kozlowski J M, McEwan R N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Schiemann B J, Neil J R, Schiemann W P. SPARC inhibits epithelial cell proliferation in part through stimulation of the TGF-β-signaling system. Mol Biol Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Pendergast A M. Activated c-Abl is degraded by the ubiquitin-dependent proteasome pathway. Curr Biol. 2001;11:1759–1765. doi: 10.1016/s0960-9822(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Butcher D T, Alliston T, Weaver V M. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Weaver V M. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler J T, Weaver V M. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M J, Zahir N, Johnson K R, Lakins J N, Rozenberg G I, Gefen A, Reinhart-King C A, Margulies S S, Dembo M, Boettiger D, Hammer D A, Weaver V M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78:1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- Radisky D C, Przybylo J A. Matrix metalloproteinase-induced fibrosis and malignancy in breast and lung. Proc Am Thorac Soc. 2008;5:316–322. doi: 10.1513/pats.200711-166DR. [DOI] [PubMed] [Google Scholar]
- Przybylo J A, Radisky D C. Matrix metalloproteinase-induced EMT: tumor progression at Snail’s pace. Int J Biochem Cell Biol. 2007;39:1082–1088. doi: 10.1016/j.biocel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bissell M J, Kenny P A, Radisky D C. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky D C, Levy D D, Littlepage L E, Liu H, Nelson C M, Fata J E, Leake D, Godden E L, Albertson D G, Nieto M A, Werb Z, Bissell M J. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher-Beckley A J, Schiemann W P. Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J R, Schiemann W P. Altered TAB1:IκB kinase interaction promotes TGF-β-mediated NF-κB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y H, Albig A R, Regner M, Schiemann B J, Schiemann W P. Fibulin-5 initiates EMT and enhances EMT induced by TGF-β in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29:2243–2251. doi: 10.1093/carcin/bgn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soverini S, Martinelli G, Iacobucci I, Baccarani M. Imatinib mesylate for the treatment of chronic myeloid leukemia. Expert Rev Anticancer Ther. 2008;8:853–864. doi: 10.1586/14737140.8.6.853. [DOI] [PubMed] [Google Scholar]
- Wang J Y. Eph tumour suppression: the dark side of Gleevec. Nat Cell Biol. 2006;8:785–786. doi: 10.1038/ncb0806-785. [DOI] [PubMed] [Google Scholar]
- Kain K H, Klemke R L. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J Biol Chem. 2001;276:16185–16192. doi: 10.1074/jbc.M100095200. [DOI] [PubMed] [Google Scholar]
- Frasca F, Vigneri P, Vella V, Vigneri R, Wang J Y. Tyrosine kinase inhibitor STI571 enhances thyroid cancer cell motile response to hepatocyte growth factor. Oncogene. 2001;20:3845–3856. doi: 10.1038/sj.onc.1204531. [DOI] [PubMed] [Google Scholar]
- Tian M, Schiemann W P. The TGF-β paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann W P. Targeted TGF-β chemotherapies: friend or foe in treating human malignancies? Expert Rev Anticancer Ther. 2007;7:609–611. doi: 10.1586/14737140.7.5.609. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, Leof E, Varga J. A non-Smad mechanism of fibroblast activation by TGF-β via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene. 2009;28:1285–1297. doi: 10.1038/onc.2008.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes M C, Leof E B. TGF-β activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem. 2006;281:27846–27854. doi: 10.1074/jbc.M603721200. [DOI] [PubMed] [Google Scholar]
- Wang S, Wilkes M C, Leof E B, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-β pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- Daniels C E, Wilkes M C, Edens M, Kottom T J, Murphy S J, Limper A H, Leof E B. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S A, Guo W, Liao M J, Eaton E N, Ayyanan A, Zhou A Y, Brooks M, Reinhard F, Zhang C C, Shipitsin M, Campbell L L, Polyak K, Brisken C, Yang J, Weinberg R A. The EMT generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M J, Labarge M A. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, Jaenisch R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiles J W, Gonnella N C, Jeng A Y. The design, structure, and clinical update of small molecular weight matrix metalloproteinase inhibitors. Curr Med Chem. 2004;11:2911–2977. doi: 10.2174/0929867043364018. [DOI] [PubMed] [Google Scholar]
- Skiles J W, Gonnella N C, Jeng A Y. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr Med Chem. 2001;8:425–474. doi: 10.2174/0929867013373417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.