Abstract
Sequence-specific gene silencing with small interfering RNA (siRNA) has transformed basic science research, and the efficacy of siRNA therapeutics toward a variety of diseases is now being evaluated in pre-clinical and clinical trials. Despite its potential value, the highly negatively charged siRNA has the classic delivery problem of requiring transport across cell membranes to the cytosol. Consequently, carrier development for siRNA delivery is one of the most important problems to solve before siRNA can achieve widespread clinical use. An assortment of non-viral carriers including liposomes, peptides, polymers, and aptamers are being evaluated for their ability to shepherd siRNA to the target tissue and cross the plasma membrane barrier into the cell. Several promising carriers with low toxicity and increased specificity for disease targets have emerged for siRNA-based therapeutics. This review will discuss non-viral approaches for siRNA therapeutics, with particular focus on synthetic carriers for in vivo systemic delivery of siRNA.
Introduction
The ability to down-regulate target genes by using double-stranded RNA interference (RNAi) has revolutionized basic science research on signal transduction and gene function 1. RNAi also has tremendous therapeutic potential for treating diseases such as cancer or macular degeneration in which an oncogene or angiogenic growth factor is over-expressed. The scientific community’s commitment to RNAi technology is evidenced by the 2006 Nobel Prize and by high-profile startup biotech companies as well as billion-dollar investments from established pharmaceutical companies.
Two approaches utilize RNAi to inhibit target genes: shRNA (short hairpin RNA) and siRNA (small interfering RNA) 2 (Fig. 1). Whereas the shRNA approach is usually promoter-dependent and can be delivered by both viral (e.g., lentivirus, adeno-associated virus) and non-viral (plasmid-based) methods, siRNA is a chemically synthesized RNA duplex and is generally delivered by non-viral delivery systems. In addition to siRNA, a 29-mer shRNA has also been chemically synthesized and one report showed that the shRNA approach was more potent than the comparable siRNA 3. Both shRNA and siRNA approaches harness the cellular machinery of microRNA for their activity and this provides the basis for efficacy and toxicity of RNAi. In contrast to chemically synthesized siRNA, the promoter-based shRNA approach requires multiple enzymatic and/or transport steps (e.g., transcription, nuclear export, Drosha and Dicer processing) before interaction with RNA-induced silencing complex (RISC) that results in cleavage of the targeted mRNA. Saturation of enzymes or transport systems by viral vectors expressing high levels of shRNA may interfere with endogenous microRNA processing, leading to toxicity 4,5.
Figure 1. Mechanism of mRNA degradation by siRNA.
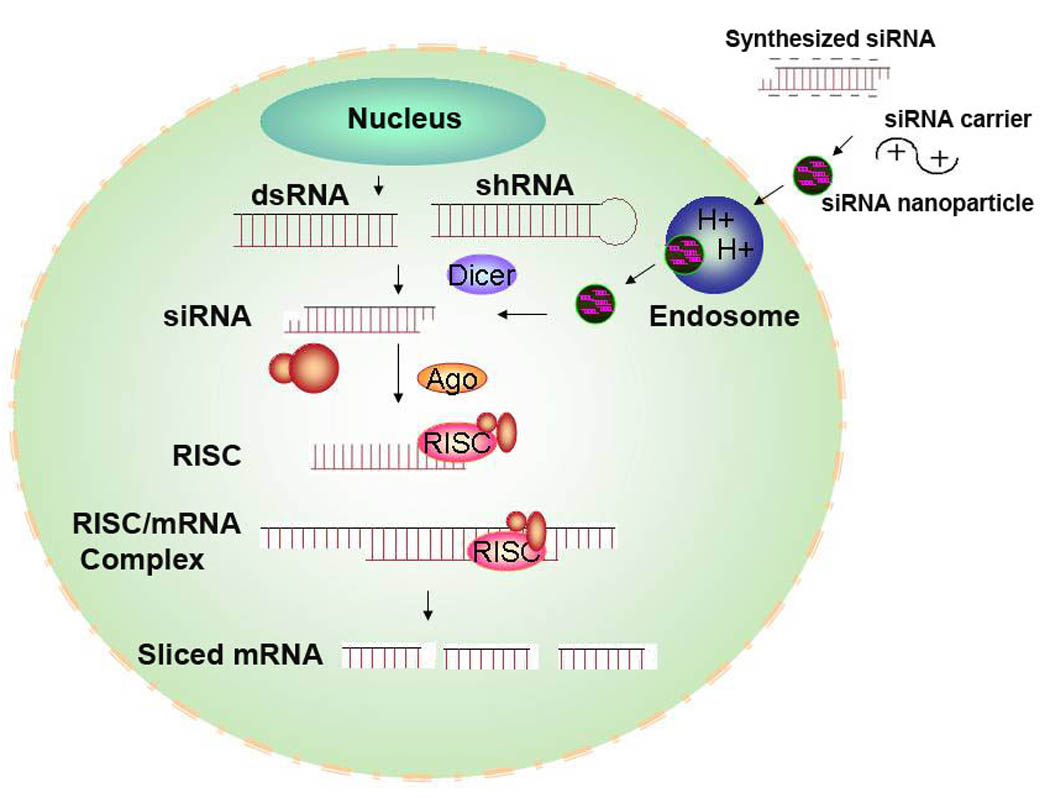
The uptake of siRNA delivered by nanoparticles is by endocytosis. Once released by the nanoparticle into the cytosol, siRNA between 19 and 23 bases is incorporated into RISC, a protein-RNA complex that separates the strands of the RNA duplex and discards the sense strand. The antisense RNA strand then guides the activated RISC to anneal and cleave the target mRNA. The endonuclease, Argonaute 2, plays a key role in unwinding the duplex and down-regulation of the specific mRNA. Following mRNA cleavage, the activated RISC is capable of many rounds of mRNA cleavage. Promoter-based shRNA requires processing by nucleus and by Dicer before incorporation into RISC.
To incorporate within RISC, double-stranded RNA longer than 23 nucleotides are cleaved by Dicer to form 19–23 siRNA duplexes with 5’-phosphorylated ends and 2-nucleotides unpaired and unphosphorylated 3’-ends. Notably, RNA duplexes larger than 30 nucleotides cause nonspecific gene silencing and an inflammatory interferon response. Thus, siRNA applications rely on synthetic 19–29 base pair double-stranded “small interfering RNA” (siRNA) 6–8. Inside the cell, siRNA is incorporated into RISC, a protein-RNA complex that separates the strands of the RNA duplex and discards the sense strand (Fig. 1). The antisense RNA strand then guides the activated RISC to anneal and cleave the target mRNA 9. The endonuclease, Argonaute 2, plays a key role in unwinding the duplex (sense and antisense siRNA strands) and degrading the target mRNA 10. By recycling the target mRNA, the activated RISC complex may show a therapeutic effect for up to 7 days in dividing cells and for several weeks in non-dividing cells. Furthermore, repeated administration of siRNA can result in stable silencing of its target 11.
Despite the promise of efficient and selective siRNA gene inhibition as a targeted therapeutic modality, it shares the classic delivery problem of antisense and gene therapies: nucleic acids are highly negatively charged and cannot easily be transported to the cytosol. The primary focus of this review will be to discuss recent in vivo advances and methods of systemic non-viral delivery of siRNA (Table I).
Table I.
Systemic Delivery of siRNA
| Delivery method Vehicle |
Route | Target organ | Target Gene | Results | References |
|---|---|---|---|---|---|
| Hydrodynamic | IV1 | Liver | Fas Caspase-8 S-gene of HBV S-gene of HBV |
Reduction of fulminant hepatitis Reduction of fulminant hepatitis Inhibition of HBV replication Inhibition of HBV replication |
Song et al, 200377 Zender et al, 200776 Klein et al, 2003 85 Morrissey et al, 200517 |
| Lung | Influenza virus | Protection from lethal influenza challenge |
Tompkins et al, 200474 | ||
| Kidney | Fas | Protection from renal ischemia-reperfusion injury |
Hamar et al, 200475 | ||
| Modified siRNA targeting PTC |
IV | Kidney | p53 | Protection from renal ischemic and nephrotoxic injury |
Molitoris et al, 200990 |
| Transferrin-PEG- cyclodextrin |
IV | Neuro2A Xenograft |
RRM2 | Tumor growth inhibition | Bartlett et al, 200892 |
| RVD-R9 | IV | Brain | GFP | Inhibition of GFP in CNS |
Kumar et al, 200730 |
| PEI | IV | Lung | NP and/or PA genes of influenza virus |
Virus replication inhibition |
Ge et al, 2004121 |
| cRGD-PEG-PEI | IV | Xenograft | VEGFR2 | Inhibition of tumor growth |
Schiffelers et al, 200421 |
| IV | Eye | VEGFA,VEGFR1, VEGFR2 |
Inhibition of angiogenesis |
Kim et al, 2004124 | |
|
Atelocollagen |
IV | PC-3 xenograft | Bcl-lx | Inhibition of tumor growth |
Mu et al, 2009129 |
| IV | Bone | Enhancer of zeste homolog 2 (EZH2) siRNA |
Inhibition tumor bone metastasis |
Takeshita et al, 2005132 | |
| Branched Histidine- lysine peptide |
IV | Breast, squamous cell cancer xenografts |
Human rhomboid family-1 |
Inhibition of tumor growth |
Yan et al, 200829 |
| HIV specific Ab protamine fusion |
IV | HIV expressing- envelope melanoma xenografts |
c-Myc, MDM2, VEGF | Inhibition of tumor growth |
Song et al, 200540 |
| Anti-LFA-1 scFv protamine fusion protein |
IV | K562 cells engrafted in lungs |
Cyclin D1 | Inhibition of lymphocyte proliferation |
Peer et al, 200741 |
| Tf-HoK DOTAP/ DOPE liposome |
IV | Breast cancer | Her-2 | Tumor growth inhibition | Pirollo et al, 2008170 |
| Apo1-DOTAP liposome |
IV | Liver | X-gene of HBV | Reduce HbsAg | Kim et al, 2007154 |
| Lactosylated DOTAP liposome |
IV | Liver | Untranslated region(most effective targeted nucleotides 325–344) |
Decrease HCV replication |
Watanabe et al, 2007155 |
| Cardiolipin/DOPE liposome |
IV | Breast cancer xenograft |
c-raf | Inhibition of tumor growth |
Chien et al, 200515 |
|
SNALP liposome |
IV |
Ebola virus | Ebola Virus polymerase gene |
Animal death protection | Geisbert et al, 200616 |
| Liver | ApoB | ApoB protein reduction | Zimmermann et al, 200618 |
||
| DOPC liposome | IV | Ovarian carcinoma | FAK | Tumor growth inhibition | Halder et al, 2006158 |
| IV or IP | Ovarian carcinoma | EphA2 gene | Tumor growth inhibition | Landen et al, 2005159 Landen et al, 2006160 |
|
| IP | Ovarian carcinoma | IL-8 | Tumor growth inhibition | Merritt et al, 2008161 | |
| IV | Melanoma | PAR-1 | Angiogenesis inhibition | Villares et al, 2008162 | |
| Anti-β-7 Ab conjugated DOPC liposome |
IV | Colon | Cyclin D1 | Suppressing leukocyte proliferation |
Peer et al, 200819 |
| Cholesterol siRNA Conjugate |
IV | Liver | ApoB | Reduce ApoB, Cholesterol and LDL |
Soutschek et al, 200435 |
| Lipophile siRNA Conjugate |
IV | Liver | ApoB1 | Different conjugates, different target organ |
Wolfrum et al, 2007163 |
| α-Tocopherol siRNA Conjugate |
IV | Liver | ApoB | Apo B down-regulation | Nishina et al, 200836 |
| Polyconjugates siRNA |
IV | Liver | ApoB PPARα |
ApoB down-regulation | Rozema et al, 200742 |
| PEG-siRNA Conjugate Micelles |
IV | Prostate cancer xenograft |
VEGF | VEGF down-regulation PEI-core forming agent |
Kim et al, 200833 |
| Apatamer-siRNA conjugate |
IV | Prostate cancer xenograft |
Bcl-2 | Tumor growth inhibition | McNamara et al, 200638 |
Abbreviations: IV, intravenous; HBV, hepatitis B virus; HCV, hepatitis C virus; PTC, proximal tubule cells; RRM2, ribonucleotide reductase subunit M2; RVG-R9, rabies virus glycoprotein peptide conjugated to a 9-mer polyarginine; PEI, polyethylenimine; VEGF, vascular endothelial growth factor; GFP, green fluorescent protein; LFA-1, lymphocyte function-associated antigen-1 integrin; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane ; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; VEGFR2, vascular endothelial growth factor receptor 2; ApoB, apolipoprotein B; PPARα, peroxisome proliferator activated receptor α; HbsAS, hepatitis B surface antigen; LDL, low density lipoprotein
Overview of siRNA Delivery
Non-viral methods for in vivo siRNA delivery can be broadly classified into non-carrier and carrier approaches. Non-carrier siRNA delivery systems (“Naked siRNA”) usually depend on diseases or disease models in which local delivery may be effective. Such diseases that do not necessarily require a carrier include macular degeneration, wound healing, and infectious respiratory diseases 12,13. In addition to localized delivery, systemic delivery of siRNA without a carrier may occur by the hydrodynamic method and/or by heavily modified siRNA. Systemic use of the hydrodynamic method is likely to be confined to animal models, but this approach may be useful in treating diseases localized to particular organs or limbs. Treatment of many diseases (in humans and animal models) will depend on systemic siRNA delivery, and success for this form of therapy requires development of appropriate new carriers. Thus far, there have been no clinically proven effective systemic carriers for siRNA. Nonetheless, several vehicles for systemic delivery of siRNA are currently being tested for their efficacy in animal studies, including liposomes 14–19, cyclodextrin 20, polymers such as polyethylenimine (PEI) 21–23, peptides 24–31, micelles 32,33, siRNA conjugates 18,34–39, antibody-protamine fusion carriers 40,41, and polyconjugates 42. Carriers of siRNA have targeted an array of diseases, including genetic disorders, infectious diseases of the liver, cancer, and ocular diseases.
Modification of siRNA for improved siRNA delivery
Modifications of siRNA may greatly influence its activity and selection of the carrier 16–18. Many factors can affect the success of siRNA-mediated gene silencing, including its modification, the target sequence of siRNA, and the type of carrier. The selection of the siRNA may also affect how its binds to the carrier and the overall stability and toxicity of the carrier. Thus, it is essential to consider the length of siRNA, the type of modification of siRNA, and the siRNA carrier in terms of the carrier and the particular disease being treated.
A. Length
Currently, 21-mer RNA duplexes, mirroring natural siRNAs, are the most commonly used for laboratory research or for clinical development. Other designs include blunt 19-mer 43, blunt 25-mer 44, blunt 27-mer 7 and asymmetric 25/27-mer 8; siRNAs larger than 23-mers are enzymatically processed by the endonuclease Dicer into shorter species before loading into RISC. One of the more interesting alternative siRNAs is the asymmetric 25/27 duplex with 3’-DNA residues on the blunt end (called the ‘R’ duplex); these siRNAs are reported to be usually more potent than the more commonly used 21-mer duplexes 8. Moreover, these ‘R” duplexes potentially have fewer off-target effects than the 21-mer duplex because of increased specificity in targeting the mRNA. Further, Siolas et al. reported that synthetic 29-mer shRNAs were more potent inducers of RNAi than were small interfering RNAs 3. In addition to the biological differences in their siRNA efficacy, the length of the siRNA may have an essential role in stabilizing nanoparticles, particularly with peptide delivery systems.
B. siRNA chemical modification
Small interfering RNA in complex with cationic carriers generally activate proinflammatory cytokines significantly more than siRNA without carriers 45,46. In complex with cationic carriers such as liposomes, specific sequences, such as 5’-UGUGU-3’, or less defined sequences within the siRNA duplex are immunostimulatory both in vitro and in vivo 47 45,48 (Fig. 2). In complex with cationic liposomes, siRNA activate cytokines by binding primarily to TLR7/8 in acidic endosomes. siRNA activate these receptors in a sequence-dependent manner and the pH-buffering agent, chloroquine, is known to suppress this activation 49. Indeed, carriers of siRNA with greater pH-buffering capacity may significantly decrease cytokine activation (unpublished results). Whether the nanoparticles provide an adjuvant template that activates the receptor or whether it is due to mass action of a large number of siRNAs presented to the TLR within endosomes is not known. Nucleoside modification and non-activating sequence selection are strategies currently available to avoid immunostimulation in siRNA technology. Nonetheless, with some therapeutic strategies, it may be desirable for the siRNA to induce cytokines to target viral infections or cancer 49,50. For example, by inducing interferon α, siRNA may prove to be effective in reducing viral titers, including influenza 51 and hepatitis B virus (HBV) 17.
Figure 2. Induction of cytokines by siRNA.
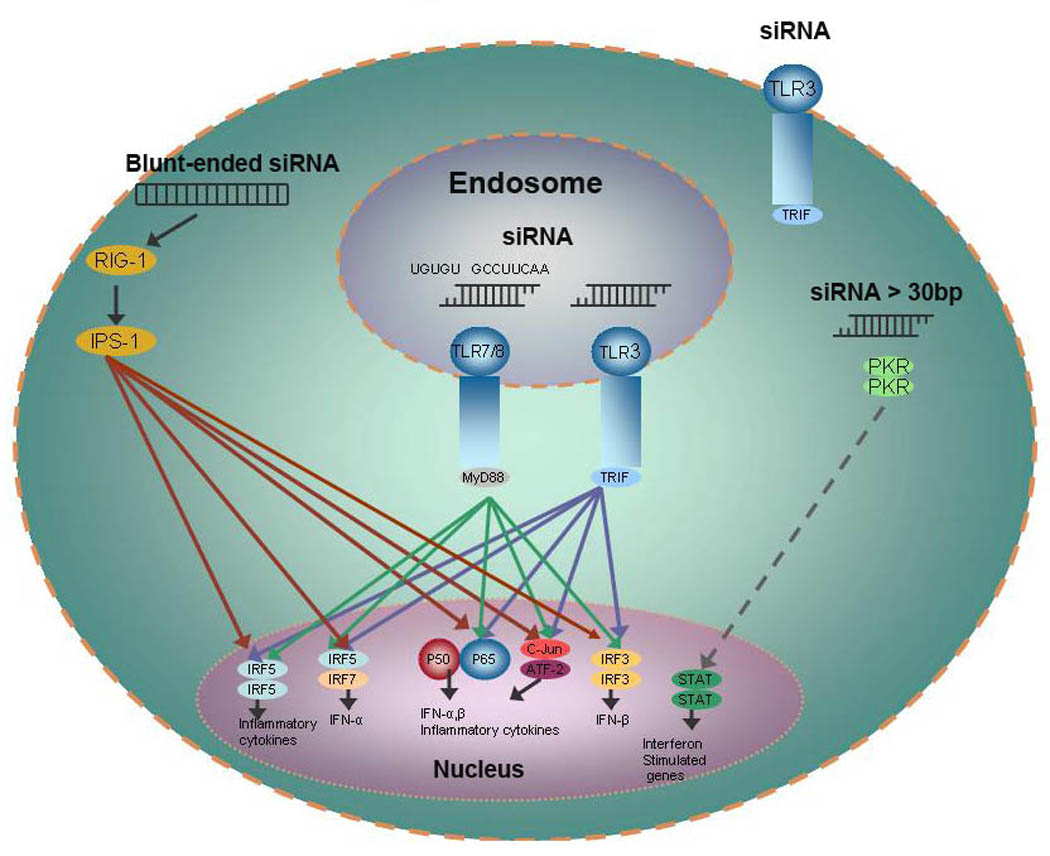
Small interfering RNAs without carriers do not activate or at least poorly activate proinflammatory cytokines in vitro and in vivo. In complex with cationic carriers such as liposomes, specific sequences, such as 5’-UGUGU-3’, 5’-GCCUUCAA-3’, or less defined sequences within the siRNA duplex are immunostimulatory both in vitro and in vivo. siRNA activate cytokines primarily by binding to TLR 7/8 in acidic endosomes, but TLR3 located at the cell surface and within endosomes may also have a role activating cytokines in selected cells. In addition, double-stranded RNA (including siRNA), greater than 30 BP, or blunt-ended siRNA may induce cytokines by binding to PKR and RIG-1, respectively. Whereas TLR 7/8 receptors are activated by specific siRNA sequences, TLR3 and the cytosolic receptors (PKR and RIG-1) are activated by siRNA independent of specific sequences. Abbreviations: RIG-1, retinoic acid inducible gene protein 1; IPS-1, interferon-beta promoter stimulator 1; PKR, double-stranded RNA-dependent protein kinase; TLR, Toll-like Receptor; IRF, Interferon Response Factor; STAT, signal transducer and activator of transcription.
Chemical modification of siRNA can increase the stability of the RNA duplex to nucleases, minimize the possibility of immunostimulatory responses, decrease the possibility of off-target effects, and improve its pharmacodynamic properties 52. Chiu and Rana analyzed the relationship between chemical modification and the efficiency of siRNA silencing by examining 30 different types of siRNA modification 53. These modifications included replacement of the 2’-hydroxyl group of ribose with 2’-fluoro, 2’-O-methyl, and 2’-hydrogen groups or replacement of the phosphate backbone with phosphorothioate or boranophosphates. Compared to an unmodified GFP (green fluorescent protein) siRNA, the majority of siRNA modifications decreased efficacy of silencing GFP. Limited siRNA modifications, however, with 2’-fluoro-, 2’-O-methyl, and phosphorothioate may increase the half-life and stability of siRNA in cells without affecting their silencing efficacy 17,53,54. In addition, 2’-O-methyl modifications significantly reduced cytokine induction by antagonizing the TLR7/8 receptors 17,51,55,56. Initially most groups used unmodified siRNA, but investigator awareness of off-target effects by unmodified siRNA duplexes has made selected modifications of siRNA with 2’-O-methyl and phosphorothioate linkages more common 57.
Delivery Obstacles
The prospects for siRNA-based therapeutics to significantly improve metabolic, cancer, or systemic infection treatment options have been limited by the inability to identify an effective carrier. Recent clinical studies indicate that carrier development is indeed one of the most important problems to address, if not the most important. The in vivo obstacles for the siRNA nanoparticles are, not surprisingly, similar to those of gene therapy nanoparticles, and depend on the route of administration, which can generally be divided into three categories. First, topical administration with siRNA includes treatment of disease of the eye (stromal keratitis), skin (atopic dermatitis, wound healing), vagina (herpes simplex virus), and rectum (inflammatory bowel disease). Second, local or direct administration of siRNA includes diseases of the lungs (SARS, influenza, RSV), or brain (Huntington’s disease, gliomas). Third, systemic delivery may include diseases of the liver (hepatitis B, metabolic) or deep-seated localized or metastatic cancer. Each of these delivery methods has its own challenges dependent on the requirement of the carrier and the targeted disease. For example, with local injections intratumorally of siRNA, tissue specificity is not an issue, but widespread distribution within the tumor is a significant and challenging problem. Convection-enhanced delivery may be at least partially successful for intratumoral injections 58–67, but it is likely that additional advances will be required before this therapy will be successful.
Not surprisingly, there are significant obstacles for systemic delivery of siRNA to their targets including interaction with blood components, entrapment within capillaries, uptake by the reticuloendothelial cells, extravasation from blood vessels to target tissues, and permeation within the tissue (Fig. 3). As long as siRNA remains within the nanoplex, filtration by the glomeruli of the kidney does not occur, but uncomplexed siRNA will be rapidly filtered by the glomeruli (40 kDa is the approx. MW for filtration). As discussed below, glomerular filtration of the siRNA may be advantageous when the proximal tubule cells of the kidney are being targeted. For targeting hepatocytes, nanoparticles should be less than 100 nm to escape the fenestrations in vessels of the liver; this is based on the finding that chylomicrons greater than 100 nm cannot traverse the fenestrations and that the diameter of fenestrations vary between 50 to 150 nm in size in the human liver 68. For tumors, the vasculature may not be well-formed resulting in the so-called “leaky” vessels 69–71, enabling large macromolecules to escape from the vessels into the tumors. In addition, accumulation of nanoparticles within tumors may be due to high microvessel density, increased vascular permeability due to vascular mediators (e.g., VEGF), and impaired lymphatic clearance (collectively these factors are known as Enhanced Permeability and Retention (EPR) 69–71. Thus, unless the siRNA nanoparticle is only targeting the tumor blood vessels, it is important that the nanoparticle enters and pervades within the tumor. Our laboratory often targets tumors with siRNA that have dual inhibitory functions. For example, Raf-1 has an important role in tumor angiogenesis and tumor cell growth, and as a result, the Raf-1 siRNA nanoparticle will likely be more effective if it accumulates both in tumor blood vessels and in perivascular tumor cells.
Figure 3. Barriers for systemic delivery of siRNA.
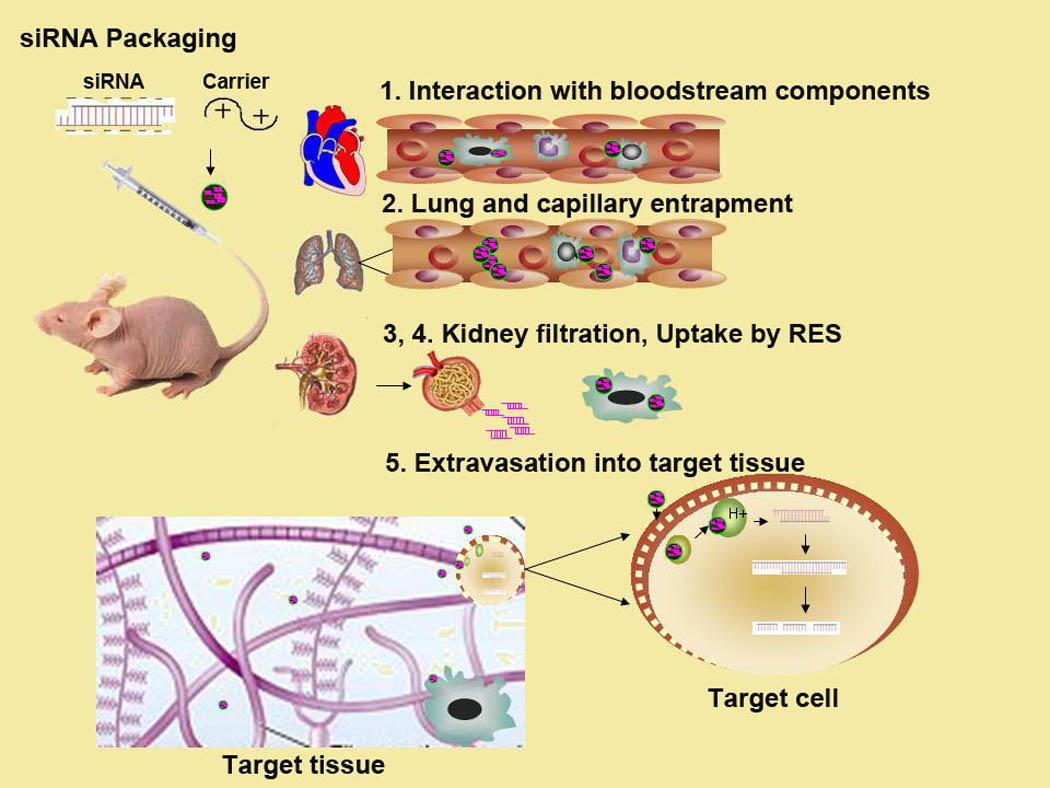
There are five major extracellular barriers that nanoparticles delivering siRNA must overcome to reach its target cell. These obstacles include the following: 1) interaction with blood components, 2) entrapment within capillaries of lungs and other tissues, 3) uptake by RES and by other phagocytic cells, 4) filtration by kidneys, and 5) extravasation from blood to target tissues.
Although considerable overlap exists between requirement for a carrier and different routes of delivery, it is frequently necessary for a carrier to deliver siRNA systemically rather than topically or locally. There are two notable exceptions that may successfully use siRNA injected systemically without a carrier: one is the hydrodynamic delivery method and the other utilizes a modified siRNA that takes advantage of its glomerular excretion. After discussing these two notable exceptions, we will review the current carriers utilized for siRNA therapy.
I. “Naked” siRNA
A. Hydrodynamic Delivery of siRNA
The hydrodynamic injection method involves rapid injection of a large volume of physiologic solutions (about 10% of the body weight administered within 5 to 10 seconds) containing nucleic acid 72,73. After injection of the relatively large volume in the tail vein of rodents, the liver is the primary target for this approach, although other tissues including the lung 74 and kidney 75 have been targeted but with lower efficiency. Originally, this approach was used for plasmids, but it has since been used successfully for transport of siRNA 76–78, protein 79,80, and synthetic compounds to the liver 80,81. The precise mechanism of entry of the siRNA is unclear but volume overload, right ventricular overload, increased hydrodynamic pressure, hepatic congestion, and enlargement of the fenestrae of the liver play an important role in their entry into hepatocytes. In addition, as a result of transient physical injury, siRNA may enter hepatocytes through macropinocytosis 82 or evanescent “pores” 83 in the cell membrane. In contrast, other cells of the liver such as the Kuppfer cells and endothelial cells do not have high uptake of nucleic acid 84. There have been several applications for utilizing the hydrodynamic tail vein (HTV) method with siRNA. For example, by targeting apoptotic genes such as the Fas receptor 77 or caspase-8 76, the incidence of fulminant hepatitis was reduced. Activation of Fas receptor may occur as a result of viral infections or transplantation and caspase 8 has a critical downstream role from Fas and other death receptors. Co-delivery of a plasmid expressing hepatitis B virus (HBV) with an siRNA targeting the S-gene of the virus via the HTV approach resulted in reduction of serum hepatitis B surface and envelope antigens by approximately 80% on day 11 85. With a similar approach targeting the S-gene, modified and unmodified siRNA reduced HBV DNA levels by 3.7 logs and 2.2 logs, respectively 56. Investigators have also used HTV to knock down peroxisome proliferator-activated receptor α (PPARα) in livers of mice and achieve metabolic phenotypes similar to those observed in PPARα(−/−) knockout mice 78. Although 100–150 ml of DNA solution have been administered safely to pigs 86, we doubt that systemic delivery of nucleic acid by the hydrodynamic approach will find therapeutic application in humans. Nevertheless, the hydrodynamic approach with siRNA may gain acceptance for clinical applications when applied locally to diseases of the liver and other organs 87. With insertion of a balloon catheter into the hepatic vein, siRNA injection of a branch of the portal vein could be done repeatedly, avoiding the volume overload problems of HVT 88. Further control of the amount of fluid delivered and associated hydrodynamic pressure may be monitored in real-time, reducing the side effects of this therapy 89.
B. Modified siRNA targeting proximal renal tubules
Systemic delivery of siRNA without carriers is primarily limited to the hydrodynamic approach. The exception is treatment of acute renal injury. In the study by Molitoris and coworkers, acute renal injury was induced by ischemia-reperfusion method or by cisplatin in a rat model 90. Because a large amount of siRNA is excreted by the glomerulus and then reabsorbed in the proximal tubule, the kidney is an excellent organ to target with “naked” siRNA. Indeed, accumulation of free siRNA in the kidney is 40 times higher than in any other organ. In addition, because the proximal tubule cell is most severely affected in acute renal injury, localization of high amounts of siRNA within this cell is ideal for therapy. On the basis of a previous report in which a chemical inhibitor of the pro-apoptotic p53 provided renoprotection 91, it was reasoned that a siRNA targeting p53 might also provide similar protection. With the renal injury induced by ischemia-reperfusion, the investigators determined that a single systemic injection of a p53 siRNA (12 mg/kg) 4 hours after induction of acute renal injury provided significant biochemical and morphologic protection. Similarly, multiple injections of p53 siRNA administered at 4 h, day 2, and day 3 after cisplatin treatment reduced the renal injury compared to that in controls. The p53 siRNA was modified by alternating 2-O-methy modifications within its sequence90, thereby prolonging its half-life in serum and within the cell. Currently, the product, QP-1002, is being developed by Quark Pharmaceuticals for systemic delivery of a siRNA targeting p53 in acute renal injury and delayed graft function.
II. Cationic Polymers
A. Cyclodextrins (CDP)
Considerable effort has been directed toward development of siRNA nanoparticles targeting tumors and, with its entry into clinical trials, the CDP nanoparticle has advanced further than other carriers. As a result, much can be learned from the pre-clinical literature on this carrier and perhaps, applied to other carriers. The cyclodextrin siRNA nanoparticle has been used primarily to target tumors and is composed of three components: the cationic cyclodextrin polymer which binds siRNA, an adamantine-polyethylene glycol (AD-PEG) stabilizing agent, and an adamantine-polyethylene glycol-transferrin (AD-PEG-Tf) targeting component. Because an overabundance of transferrin receptors is found on many tumors, uptake within tumor cells of the CDP labeled with the Tf targeting ligand was increased. Notably, since adamantine has a very high binding affinity toward cyclodextrins (104–105 M−1), it provides a simple method to attach stabilization and targeting components to cyclodextrin. In addition, imidazoles are conjugated to the backbone of cyclodextrins to enhance disruption of endosomes and cellular trafficking of the nanoparticle. Interestingly, cyclodextrin binds to siRNA even when these two components are injected separately into the bloodstream of mice. Initially, investigators demonstrated that the CDP-siRNA nanoparticle targeting the EWS-FLI1 fusion gene inhibited Ewing’s sarcoma xenografts and, more recently, the investigators determined that the CDP carrier of a siRNA targeting ribonucleotide reductase subunit M2 (RRM2) had potent antitumor efficacy 92. Although an excess of cyclodextrin is required to form the nanoparticle with free polymers, the nanoparticle is stable in the blood stream. In contrast to cationic liposomal carriers, Tf-PEG-CDP in complex with siRNA does not elicit an immunostimulatory response in mouse models. Moreover, the Tf-PEG-CDP RRM2 siRNA could be administered safely to non-human primates. The Tf-Peg CDP in complex with RRM2 siRNA (CALAA-01) has been approved for clinical trials.
B. Cationic cell-penetrating peptides (CPPs)
Cationic cell-penetrating peptides (CPPs) have been utilized to carry macromolecules including plasmids, proteins, peptide, and more recently siRNA, across membranes into cells in vitro and in vivo 93–97. CPPs are small arginine-rich peptides that include TAT (human immunodeficiency virus type-1) 98–100, Penetratin (from Antennapaedia) 101, transportan (a hybrid derived from glanin and mastoparan) 102, and polyarginine-synthetic peptides 103–108. These arginine-rich peptides range from 8 to 30 amino acids in length and interact with negatively-charged glycosaminoglycans on the cell surface 109,110. Although CPPs along with their cargos were initially considered to enter cells through a fusogenic mechanism, more recent reports have determined that CPPs enter cells primarily by macropinocytosis, a type of endocytotc pathway 111–113. siRNA has been delivered by CPPs by two methods: 1) conjugating siRNA to the CPP 114–116 and 2) a non-covalent CPP-siRNA polyplex 96. Conjugation of siRNA with CPP has shown conflicting results. Whereas in vitro results have not clearly shown efficacy, animal models utilizing the CPP-conjugates have demonstrated efficacy in down-regulation of the target gene 114–116. Although investigators suggested that these CPP-siRNA conjugates were soluble, more recently it appears that their efficacy may have been due to their forming nanoparticles 96. When larger nanoparticles were removed, the remaining soluble conjugates ineffectively suppressed gene expression. Thus, it appears that early attempts at using soluble CPP-siRNA conjugates may not effectively down-regulate their targets. As a potential solution to address this problem with conjugates, Eguchi et al. developed a CPP containing a siRNA-duplex binding domain (DRBD) 95. CPP-DRBD-delivered siRNA induced rapid RNAi in a large percentage of various primary and transformed cells. At least with in vitro cell culture studies, this appears to be an effective carrier of siRNA but this approach has not been tested in animal models. In contrast to low MW soluble CPP-siRNA conjugates, non-covalent CPP-siRNA polyplexes effectively reduce their target in vitro. Of note, two groups have shown that polyarginine fusions are effective carriers in vivos 117,118. After cholesterol-polyarginine (a 9-mer arginine, R9)/ VEGF siRNA nanoparticle was prepared and added to CT-26 cells, VEGF levels in the medium were reduced by 40% compared to untreated or polyarginine/VEGF siRNA controls 117. This approach was further validated when this cholesterol-polyarginine carrier of VEGF-siRNA administered intratumorally decreased CT-26 xenografts by approximately 7-fold over a 17-day period. The second group created a fusion product between a 9-mer arginine-rich peptide and a short peptide derived from rabies virus glycoprotein (RVG), a protein that recognizes nicotinic acetylcholine receptor on neuronal cells 30. Interestingly, this group found in a GFP transgenic mouse model that RVG-R9/ GFP-siRNA injected intravenously crossed the blood-brain barrier and decreased GFP expression in the brain while not affecting its expression elsewhere. This is the first report demonstrating that targeted CPPs can transport siRNA across the blood-brain barrier.
C. Cationic Synthetic Polymers
Of the synthetic polymers, such as the dendrimer polyamidoamine (PAMAM) and polyethylenimine (PEI), that have shown efficacy as carriers of siRNA 119,120, only PEI has been extensively explored as a carrier of siRNA for in vitro and in vivo studies. PEI is a synthetic cationic polymer with a linear or branched structure. Because PEI effectively binds and condenses nucleic acids into stabilized nanoparticles, it has been widely used as a carrier for oligonucleotides, plasmids, and siRNA. In addition to its ability to condense nucleic acids, the pH-buffering property of PEI disrupts endosomes, thereby enabling nucleic acids to reach the cytosol. Although plasmids must still reach the nucleus, siRNA only needs to reach the cytosol where RISC is formed to degrade mRNA. The commonly used PEI in complex with siRNA has been administered locally and systemically (either ip or iv). Although unmodified branched PEI-nanoparticles have frequently been used for cell culture transfection experiments, a few studies have used unmodified PEI nanoparticles in animal models. For example, Ge et al. found that systemically delivered PEI-siRNA nanoparticles inhibited influenza virus in mouse lungs 121. When 60 ug of siRNA were injected i.v., there was a 10-fold reduction in virus titers in the lungs and when 120ug of siRNA were used, more than a 1000-fold reduction in lung virus titers was observed in some mice. Despite this apparent success, branched 25 kDa PEI in complex with nucleic acids is known to be toxic to the lungs and it is likely that some of the reduction in viral titers in the lungs may have been due to toxicity of the PEI polyplex. Moreover, the commonly used branched PEI in cell culture transfection experiments and/or siRNA delivery is generally known to be toxic to most cells. Thus, there is significant concern regarding toxicity of nanoparticles formed from siRNA and unmodified PEI with high molecular masses (e.g., 25 kDa) and doses, and the clinical use of high molecular weight unmodified PEI will likely be quite limited 122,123.
As a result of its toxicity, strategies to modify the structure of PEI to reduce toxicity while retaining its potent capability to deliver siRNA are in development. One strategy to reduce toxicity of PEI siRNA nanoparticles is to develop particles that would increase the target specificity of the particle. An example of this approach was the study by Schiffelers et al., who developed self-assembling PEI siRNA nanoparticles targeting tumor angiogenesis. The investigators targeted a critical receptor in tumor angiogenesis, VEGFR2, that is upregulated in mitogenic endothelial cells. When injected intravenously through the tail vein of tumor-bearing mice, PEGylated polyethyleneimine (PEI) with an RGD peptide ligand in complex with VEGFR2 siRNA inhibited the growth of neuroblastoma xenografts by about 90%. Biological activity of the siRNA asssoicated with PEGylated PEI was found to be sequence-specific and the specificity of the nanoplex for tumor vessels was dependent on the presence of peptide ligand and could be competed by free peptide 21. Although investigators did not perform toxicity studies, reduced toxicity to non-tumor tissues (e.g., liver, lung) is expected because of the greater specificity. By using these targeted pegylated PEI carriers, siRNA polyplexes targeting VEGFA, VEGFR1, and/or VEGFR2 also reduced angiogenesis in two models of ocular diseases 124. These PEI polyplexes were effective when given locally and/or systemically and interestingly, the mixture of three siRNAs reduced angiogenesis more than a single siRNA inhibitor. By pinpointing several targets in the VEGF pathway in a non-tumor disease, the mixture of siRNAs may be particularly effective in these systems in which compen-satory mechanisms are limited. Furthermore, the ability to direct PEI siRNA nanoparticles with the RGD ligand specifically to diseases with increased angiogenesis makes it likely that other ligands attached to PEI will show equal or greater specificity. In experiments with KB epidermal carcinoma cells that have high levels of folate receptors, a folate-modified PEI 125 in complex with GFP siRNA reduced GFP expression by 80% while unmodified PEI-GFP siRNA reduced GFP expression by 10% (N/P ratio, 16:1). Moreover, the type of PEG and PEGylation pattern should be considered. It is generally accepted that addition of PEG to PEI is required for greater specificity, longer half-life, and reduced immunogenicity. Less appreciated is that the degree and pattern of PEGylation of PEI can reduce toxicity, such as erythrocyte aggregation and hemorrhage 126.
Other groups have been successful in minimizing toxicity by using low molecular weight (LMW) PEI or biodegradable PEI. LMW PEI (4–10 kDa) in complex with plasmids and/or siRNA has minimal toxicity when compared to the 25 kDa PEI 23,127. Furthermore LMW PEI fully protects siRNA against enzymatic degradation, and delivers siRNA into cells where they efficiently induce RNAi. Although the LMW form of PEI is an effective carrier of siRNA in cell culture experiments, the utility of the LMW PEI nanoplexes for systemic delivery will likely depend on their stability to blood components.
A third approach to develop PEI polyplexes with low toxicity was to synthesize a ketalized PEI carrier. These ketalized carriers were found to be significantly less toxic than unketalized PEI. Branches of PEI were modified with acid-degradable amine groups via ketal linkages. Because the ketal linkages are sensitive to mildly acidic conditions around pH 5, this bond rapidly breaks apart in acidic endosomes. Once the ketalized amine-containing groups of PEI are degraded, siRNA efficiently dissociates from the polymer. Notably, only the ketal groups of PEI are biodegradable, while the PEI template is not. Whereas low molecular weight PEI favors plasmid delivery, higher molecular weights increase siRNA import. These three approaches or a hybrid of these approaches suggest the possibility of developing a non-toxic targeted PEI delivery system for siRNA.
D. Atelocollagen
The cationic atelocollagen, prepared from calf dermal collagen, shows low antigenicity and through ionic interactions it forms a macromolecular complex with DNA or RNA. Two groups from Japan have shown the efficacy of atelocollagen as a carrier of siRNA in several animal models targeting bioluminescent bone metastases, anti-apoptotic factors in tumor xenografts, and monocyte chemoattractant protein-1 in inflammatory diseases 128–133. For example, i.v. administration of the atelocollagen Bcl-xL siRNA (100 µg of the siRNA) reduced tumor growth by about 40% and combined therapy of the Bcl-xL siRNA particle with cisplatin showed synergism in reduction of tumor growth by about 75%129. For systemic injections, the complex was first prepared by mixing soluble atelocollagen (0.05%) with siRNA at 4°C, and then upon warming to room temperature, a macromolecular complex between 100 to 300 nm was formed. Initial studies showed that atelocollagen particles can be administered safely without induction of cytokines or observed toxicity to the tissues. Although these complexes have not been modified to target tumors or inflammatory tissues, these particles accumulate selectively within these diseased tissues, because of the enhanced permeability and retention (EPR) effect.
E. Polylysine and Lysine-rich Polymers
Polylysine was one of the earliest carriers of nucleic acids. Hanson et al. demonstrated that galactosylated polylysine effectively delivered plasmids to hepatocytes in vivo 134. By titrating the ionic strength of the solution, polylysine-plasmid polyplexes formed nanoparticles that were between 10 and 20 nm 134,135. Although these initial studies were promising, the use of polylysine as a carrier was associated with cytotoxicity including complement activation 136–139 and RBC lysis 140. Modification of polylysine with PEG reduced the side effects 136 and PEG-polylysine plasmid nanoparticles are currently in Phase I–II clinical trials. As yet, unmodified polylysine carriers have not been effective carriers of siRNA 28. In contrast, various modifications of the polylysine carriers have yielded effective delivery systems for siRNA.
One such modification that increases the ability of lysine-rich peptides to transport nucleic acids is the addition (or incorporation) of histidines with the lysine peptide (for a recent review of histidine-lysine containing peptides, see reference 2626). When a reducible oligolysine was compared to a reducible oligolysine-histidine peptide, the lysine-histidine carrier was a more effective carrier of siRNA 28. While lysine is important for binding DNA/RNA, histidine has an important role in buffering acidic endosomes, thereby leading to endosomal disruption and release of nucleic acid 141. Histidines may also have a role in stabilizing the nanoparticle 142. Furthermore, specific ratios and patterns of histidine and lysine have been found to augment the siRNA delivery 143. With a peptide synthesizer, the specific patterns of histidines (H) and lysines (K) can be varied to optimize the HK peptide carrier for a particular form of nucleic acid. HK peptides with higher lysine content are usually more effective carriers of plasmids, but these peptides are not effective carriers of siRNA. In contrast, carriers with a higher ratio of histidine to lysine content are more effective carriers of siRNA. With branched HK peptides carrying Raf-1 siRNA, there was an approximately 60% inhibition of growth of MDA-MB435 tumor xenografts, without toxicity. In a second study that validated the HK polymer as a useful systemic carrier of siRNA, HK polymer in complex with human rhomboid family-1 siRNA significantly reduced its target expression and tumor growth in a mouse xenograft 29. Recently, Stevenson and colleagues have demonstrated that reducible histidine-lysine peptides of lower molecular weight are effective carriers of siRNA 28. These studies suggest that binding and release of the siRNA (or plasmids) from the carrier are critical and the designs of polymers for siRNA and plasmids may differ.
In place of the endosomolytic histidine-rich peptides, Meyers et al. coupled the endosomolytic agent, mellitin, to pegylated polylysine 144, and also, conjugated siRNA with peptide through a disulfide linkage to prevent extracellular dissociation. Because of the high reducing intracellular potential (about 5 mM levels of glutathione), however, siRNA would be released from the polymer within the cytosol. For cell culture experiments, the polymer-siRNA conjugate formed by disulfide bonds showed superior results when compared to polymer-siRNA nanoplexes formed by ionic interactions. For animal studies, however, these nanoparticles proved to be toxic to liver and lung when administered intravenously.
F. Protamine
Protamines are low molecular weight proteins (50–110 amino acids) that can contain up to 70% arginine 145. Over 40 years ago, protamine was recognized to stimulate the uptake of nucleic acids 146–148. More recently, protamine was noted to interact with plasmid DNA to form nanoparticles for transfecting cells in vitro and in vivo. To augment transfection, protamine has often been added to plasmids or antisense oligodeoxynucleotides to add stability of the nanoparticles, and/or neutralize and condense the nucleic acid to enable encapsulation 149. Similar approaches have been used for siRNA delivery. By neutralizing and condensing siRNA, protamine has been useful in encapsulating siRNA within liposomes 19,150 (see liposome section below for further discussion.)
Antibody-protamine fusion carriers for siRNA have been used in several animal models mirroring human diseases 40. To inhibit tumor growth, investigators implanted a melanoma cell line expressing the HIV envelop protein. A single chain antibody-protamine construct in which Fab fragment targeting the HIV envelope was fused with protamine in complex with a siRNA cocktail (Myc, VEGF, and MDM2 siRNA) reduced melanoma tumor growth. While intratumoral injection of the cocktail siRNA inhibited tumor growth by approximately 80% compared to untreated PBS controls, intravenous injection of the nanoparticle inhibited tumor growth by approximately 60%. This inhibition was specific in that tumors not expressing the HIV envelope were not inhibited. A more clinically relevant ErbB2-protamine fusion protein in complex with siRNA specifically reduced growth of breast cancer cells40. In addition to these in vivo experiments, Song et al. showed that the HIV specific antibody-protamine siRNA nanoparticle targeting the HIV capsid gene, gag, inhibited HIV replication in difficult to transfect T-lymphocytes in vitro40; this data coupled with the in vivo data of tumors expressing the HIV envelope suggest that this carrier might be effectively used in HIV. In another application, an anti-LFA-1 antibody-protamine fusion product carrying cyclin D1 siRNA was able to suppress gene expression and cell proliferation in activated lymphocytes that are usually difficult to transfect with non-viral carriers. One of the anti-LFA-1 antibodies (AL-57) has high affinity for human lymphocyte function-associated antigen (LFA-1) integrin that undergoes a conformational change in activated lymphocytes 41. In a mouse model in which K562 cells expressing activated LFA-1 were engrafted in the lungs, the AL-57 antibody-protamine carrier of siRNA injected intravenously specifically delivered fluorescently labeled siRNA to the K562 cells. Notably, there was no evidence of cytokine induction or toxicity with these fusion siRNA products. Moreover, with six siRNA in complex with the antibody-protamine fusion construct, the molecular weight of the nanoparticle would be about 100 kDa, significantly greater than the 40 kDa cut-off for renal filtration. These experiments utilizing different targets and animal models demonstrate the safety, flexibility, and utility of the antibody-protamine fusion carrier.
III. Liposomal delivery of siRNA
A. Cationic Liposomes
Liposomes have been used for the delivery of nucleic acids for over 25 years as first demonstrated by their ability to transport the preproinsulin gene to the liver 151. Because of initial technical challenges encountered with incorporation of negatively charged DNA within neutral liposomes, this methodology was soon supplanted once cationic liposomes were developed in 1989 152. Cationic liposomes can combine quickly with negatively charged nucleic acids to form lipoplexes and the ease of preparation of these lipoplexes has enabled many researchers to study their favorite gene in vitro and in vivo. Indeed many cationic liposome products developed initially for gene delivery have been modified for siRNA delivery (e.g., Lipofectamine 2000 (Invitrogen), DMRIE-C (Invitrogen), Oligofectamine, (Invitrogen), DOTAP (Roche Applied Science), X-tremeGene (Roche), siPORT NeoFx (Ambion) , RNAifect (Qiagen), GeneSilencer (Genlantis)).
In addition to cationic liposomes being the most commonly used delivery agent in vitro, these cationic carriers have often been used for in vivo studies with siRNA. Several studies have shown that these carriers are effective carriers for systemic delivery of siRNA. Pirollo and co-workers targeted several different xenografts with an HER2-specific siRNA in complex with 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) liposomes 153. These liposomes contain histidine-lysine peptides on their surface to facilitate their escape from endosomes, as well as a single-chain antibody fragment targeting transferrin receptors, which are elevated on the membranes of tumor cells. Although the immunoliposomes-HER2 siRNA significantly inhibited the growth of pancreatic xenografts, combining gemcitabine with these nanoparticles resulted in synergistic interaction and inhibited tumor growth almost completely. In addition to tumors, cationic siRNA lipoplexes have also targeted diseases of the liver. DOTAP/cholesterol liposomes in complex with HBV siRNA successfully reduced viral protein expression of hepatitis B. Eight days after a single intravenous administration of the HBV siRNA nanoparticles (2 mg/kg), HBV surface antigen was reduced by over 70% compared to antigen levels in the control siRNA nanoparticle group 154. Watanabe and colleagues utilized lactosylated cationic siRNA lipoplexes and also showed marked reduction of hepatitis C expression in the liver of a transgenic mouse model 155. Importantly, these lactosylated DOTAP siRNA lipoplexes did not induce interferon-α. Although the above studies with DOTAP lipoplexes show considerable promise, many lipoplexes induce a strong cytokine response 51,156. Perhaps because these lipoplexes were targeted and administered at low dosages, these results showed strong efficacy with lack of toxicity. In addition to these encouraging results, there are three types of cationic liposomes that merit further discussion.
For in vivo studies, DOTAP/cholesterol liposomes were found to be effective for delivery of nucleic acids and only a few alternative lipids have been found to be equivalent or more effective. Interestingly, a liposome comprised of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and cardiolipin in complex with luciferase reporter plasmid administered i.v. resulted in tumors expressing luciferase at seven times higher levels than did DOTAP/cholesterol lipoplex 15. The cardiolipin analog of the liposome was significantly less toxic than the DOTAP/cholesterol liposome: whereas two-thirds of the mice died when injected with 100 mg/kg of DOTAP/cholesterol liposomes, no mice died at a similar dose with the cardiolipin-containing liposome. In addition, c-Raf siRNA in complex with the cardiolipid-containing liposome resulted in about 50% inhibition of breast cancer xenografts compared to the liposome- control mismatch siRNA group. Unfortunately, there was no direct comparison with the DOTAP/cholesterol siRNA lipoplex, and the cardiolipin siRNA mismatch lipoplex inhibited tumor growth about 25% more than the free siRNA control. Despite drawbacks to this study, the significant inhibition of tumor growth with c-Raf-1 siRNA, the lower toxicity of the lipid, and greater in vivo transfection efficiency with the cardiolipin-containing liposomes suggest that further studies of these liposomes are needed 15.
Unlike lipoplexes discussed in previous paragraphs, Li et al. prepared nanoparticles in which the siRNA (targeting luciferase) was internalized within liposomes 157. Minimizing direct ionic interactions between siRNA and cationic lipids may reduce induction of cytokines. siRNA was incorporated within liposomes by first mixing siRNA with carrier thymus DNA and neutralizing the negatively charged nucleic acids with the highly basic protamine. The protamine/nucleic acids complex was then entrapped within DOTAP/cholesterol (1:1, molar ratio) liposomes to obtain LPD (liposome-polycation-DNA) nanoparticles. The positive charge on liposomes from the DOTAP lipids may promote interaction with the negatively charged cell membranes, thereby increasing endocytosis. For increased tumor specificity and stability of the LPD, the preformed nanoparticles were modified with PEG and anisamide. The anisamide ligand has a high affinity for the sigma factor receptor that is expressed on the cell surface of several types of cancers. After one i.v. injection, the targeted Luc siRNA nanoparticle down-regulated luciferase levels by 70 to 80% in lung metastatic model compared to the targeted control siRNA nanoparticle. Interestingly, cytokine induction was minimal with this targeted LPD formulation, particularly when protamine/siRNA or DOTAP/siRNA complexes would likely induce strong cytokine responses.
Another extensively studied form of liposomes for siRNA delivery is the stable nucleic acid-lipid particle (SNALP). SNALP nanoparticles are pegylated liposomes with low cationic lipid content that incorporate nucleic acids, including siRNA, within the lipid envelope. Although SNALP contains a very low cationic lipid content for plasmid DNA delivery (molar percent 5–10%), these vehicles still contain a relatively low amount of cationic lipids for siRNA delivery (molar percent 30%). Morrissey et al. showed that in vivo delivery of siRNA-SNALP complexes that targeted HBV RNA can inhibit HBV replication 17. Three daily intravenous injections (3 mg/kg) reduced HBV levels by about 10-fold for up to 7 days. Moreover, Zimmerman et al. targeted apolipoprotein B with siRNA-SNALP nanoparticles in non-human primates 18. Apolipoprotein B, found in the liver and jejunum, is associated with serum lipid abnormalities, an elevated LDL, and a high incidence of atherosclerotic heart disease. With a single siRNA-SNALP injection, there was maximal silencing of 90% for apolipoprotein B mRNA expression in the liver of nonhuman primates. At the 2.5 mg/kg administered dose of the SNALP-ApoB siRNA nanoparticle, significant reductions in ApoB protein, serum cholesterol, and low density lipoproteins were observed for 11 days. Nevertheless, the SNALP-ApoB siRNA particle at this dosage was associated with transient elevations of liver enzymes. Another study showed that SNALP formulation targeting the polymerase gene of the Zaire strain can protect guinea pigs from lethal challenge of the Ebola virus 16. Although polyethylenimine-siRNA reduced viremia and partially protected guinea pigs from death, the SNALP-siRNA nanoparticles completely protected them from death. In contrast to a previous study 18, SNALP-siRNA nanoparticles did induce interferon α and β16, but it is unlikely that this was the primary factor in their efficacy. The most effective of the siRNAs (EK1) in reducing viremia and preventing death induced the lowest levels of interferon α and β. 16. Although a cytokine response may act in concert with the siRNA, in other cases, high cytokine levels may be deleterious with some therapies in that they can mask the efficacy of the therapeutic gene. Indeed, there has been controversy as to whether the claimed effects in several studies of therapeutic siRNA might really be due instead to cytokine induction 51,156. Although these cationic liposomal siRNA studies appear promising, there are no FDA approved products of cationic liposomes as carriers of nucleic acids despite their long history of development.
B. Neutral Liposomes
Initially neutral liposomes were used for in vitro and in vivo delivery of nucleic acids, but their use as carriers of nucleic acid was limited after the development of cationic liposomes 151. Nevertheless, with the realization that significant toxicities were associated with cationic liposomes, neutral liposomes have re-emerged as promising carriers of siRNA. Recently, several studies from M.D. Anderson have shown the utility of the neutral liposomes, composed of 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), as siRNA carriers in vivo 158–162. The growth of several different tumor xenograft models was inhibited with non-ligand non-peglyated liposomes with siRNA targeting the thrombin receptor (melanoma), interleukin-8 (ovarian), EphA2 (ovarian), and focal adhesion kinase (ovarian). The maximal inhibition of xenograft growth with DOPC siRNA liposomes was observed in a melanoma xenograft. Compared to the control siRNA nanoparticles, the DOPC nanoparticles targeting thrombin receptor (TR) siRNA inhibited by about 80% the growth of melanoma xenografts and number of lung metastases. Marked reduction in TR and angiogenic (VEGF, interleukin 8), and invasive (matrix metalloproteinase-2) factors that TR regulates were also observed with therapy. Similarly but to a lesser extent, the neutral DOPC siRNA nanoparticles reduced the growth of ovarian xenografts; compared to empty liposomes, DOPC siRNA targeting the EphA2 oncogene reduced HeyAP xenografts by 35%; in combination with paclitaxel, DOPC EphA2 siRNA nanoparticles had an additive effect and reduced tumor weight by 75% 159.
Although incorporation of DNA inside neutral liposomes and the stability within the bloodstream were initially problematic, many of these barriers have been overcome by development of new strategies. Freeze-thawing allows a high incorporation of siRNA within liposomes and coating the surface of these liposomes with hyaluronic acid stabilizes these nanoparticles in the bloodstream. These significant advances were highlighted in a recent Science article. To define the role of cyclin D in a colitis-induced mouse model, Peer et al. utilized β-7 antibody conjugated with targeted stabilized liposomal nanoparticles 19. Unlike cationic liposomal carriers of siRNA, the investigators utilized neutral liposomes in which cyclin-D siRNA in complex with protamine was incorporated inside the liposomes. Although β-7 integrins are ubiquitously present on leukocytes, the β-7 antibody had significantly higher affinity toward activated integrin receptor on leukocytes; activation of the receptor occurs in inflammatory states such as colitis and malignancies. Recognition of the activated integrin receptor by the β-7 antibody nanoparticles enabled high specificity and uptake by endocytosis. Notably, the liposomal nanoparticles were stabilized with hyaluronic acid that significantly increased their half-life in the serum. Systemic injection of cyclin D1-siRNA nanoparticles targeted activated leukocytes and reversed experimentally induced colitis in mice by suppressing leukocyte proliferation and T helper cell cytokine expression. This study reveals cyclin D1 to be a potential anti-inflammatory target for siRNA therapy, and suggests that this therapy may not only be applicable toward colitis but also some malignancies. At least for this therapeutic application, targeted neutral liposomes may be more effective than antibody-protamine fusions as carriers for cyclin D siRNA. The authors point out that the immunoliposomal carrier (4000 siRNA/carrier) has a much greater capacity to entrap siRNA compared to the antibody-protamine fusion carrier (6 siRNA/carrier). Nevertheless, no comparisons of their efficacy to deliver siRNA to their targets in vivo were done, and, entrapment (or carrying) capacity is only one of many important factors that determine efficacy of the siRNA nanoparticle.
IV. siRNA conjugates
A. Lipophiles conjugated to siRNA
siRNA conjugated to lipophiles can improve their stability, pharmacokinetics, and biodistribution. The cholesterol-ApoB siRNA had a half-life of 45 minutes and widespread tissue distribution whereas unconjugated siRNA had a half-life of 6 minutes and was not detected in tissues. When 50 mg/kg of a modified ApoB siRNA conjugated to cholesterol was administered iv, ApoB mRNA was decreased 57% and 73% in the liver and jejunum, respectively. Consequently, ApoB protein was reduced by 68%, cholesterol 37%, and LDL 44% 35. No off-target effects were observed despite the high dose of the conjugate administered. Transport and efficient uptake of the cholesterol siRNA conjugates by the liver and jejunum depend on their interaction with lipoproteins 163. The cholesterol-siRNA conjugate targets the liver when it combines with low-density lipoproteins, while the conjugate is directed toward the jejunum if it interacts with very-low-density lipoproteins. In addition to cholesterol, α-tocopherol and bile acids have been conjugated to siRNA 34,36,163. Similar to the cholesterol-siRNA conjugate, the α-tocopherol-ApoB siRNA conjugate similarly reduced hepatic ApoB mRNA and serum cholesterol levels. The dose to achieve maximum inhibition with the α-tocopherol, however, was significantly lower at 2 mg/kg 36.
B. siRNA-Polyconjugates
Rozema and co-workers have developed a siRNA polyconjugate nanoparticle which targets liver hepatocytes to silence ApoB and PPARα genes 42. The siRNA-polyconjugate was synthesized by first conjugating siRNA to the endosomolytic PBAVE polymer through a disulfide bond and then attaching PEG and N-acetylgalactosamine (the liver targeting ligand) to the polymer. The hepatocyte-specific nanoparticle with a size of 10 nm, significantly smaller than SNALP liposomes, reduced ApoB and PPARα mRNA by 76% and 64%, respectively, in a mouse model. As expected, serum cholesterol levels were reduced by 30% in the ApoB siRNA-treated mice relative to control mice. Notably, this study showed no evidence of significant toxicity, with minimal and transient elevations in serum levels of liver enzymes and cytokines.
C. siRNA-PEG Micelles
Kim and co-workers constructed polyelectrolyte complex micelle nanoparticles (PEC micelles) to target VEGF, a growth factor essential for tumor angiogenesis 32,164. The PEC micelles were composed of the PEG-VEGF siRNA conjugate and the core-forming PEI which interacted with siRNA to stabilize the nanoparticles. With atomic force microscopy, the PEC micelles were shown to range in size between 50 to 80 nm. The siRNA was conjugated to polyethylene glycol (PEG) through disulfide linkages and in the highly reducing environment of the cytosol, the siRNA was released to associate with the RISC. In prostate PC-3 cells, PEC silenced VEGF expression more than 95%. Interestingly, these PEC micelles were significantly more effective than PEI/VEGF siRNA polyplexes in silencing VEGF 32. Building on these in vitro results, the growth of PC-3 tumor xenografts was reduced by PEC administered with intratumoral or systemic injections by about 85% 33. Concomitantly, VEGF levels and microvessel density were significantly inhibited in PEC-treated xenografts. Although these systemically delivered non-targeted micelles accumulated in several organs, PEC micelles showed low toxicity and induced low levels of interferon-α.
D. Aptamer-siRNA
Aptamers are small (<15 KDa), highly structured single-stranded RNA or DNA molecules, isolated from combinatorial libraries by a method termed SELEX (systematic evolution of ligands by exponential enrichment). Aptamers can be readily synthesized in large quantities and can be chemically modified to avoid degradation by nucleases. By binding with high affinity to target molecules, aptamers have a number of biological applications including target validation, inhibitors of receptors or enzymes, and as carriers for nucleic acids. An aptamer targeting VEGF (pegaptanib) is effective and clinically approved against neovascular age-related macular degeneration. As carriers of siRNA, aptamers have not been used for systemic delivery, but they offer great promise in their low nanomole binding affinities toward their targets and low immunogenicity.
Chu et al. developed an aptamer that targeted prostate specific antigen highly expressed on LNCaP prostate cancer cells 37. Through a strepavidin-biotin linkage, a laminin A/C siRNA was conjugated to the aptamer. The aptamer has a high affinity toward prostate specific membrane specific antigen (PSMA or PMSA)(2 nM Kd) with rapid internalization of the aptamer-conjugate by clathrin-mediated endocytosis. The siRNA–dependent mediated inhibition of laminin A/C with the aptamer carrier was equivalent to that of Oligofectamine liposomal carriers; inhibition of laminin A/C gene expression was about 70%. Notably, the growth of LNCaP tumor xenografts was inhibited by more 90% with intratumoral injections of anti-PMSA aptamer conjugated to Bcl-2 siRNA 38. In contrast to the previously mentioned anti-PMSA aptamer studies, Wullner and co-workers used an anti-PMSA bivalent aptamer conjugated to an siRNA targeting eukaryotic elongation factor 2 (eEF2) 165. The bivalent aptamer- eEF2 siRNA conjugate inhibited its target mRNA and protein significantly more than did the monovalent aptamer conjugate; perhaps more important, these larger bivalent aptamers conjugates are less likely to be filtered by the glomeruli. These aptamers induced siRNA sequence-specific apoptosis only in PMSA expressing cells and no interferon-α was induced in any of the tested cells.
In addition, the aptamer-siRNA approach has also been utilized to inhibit HIV replication 39. This was the first study in which there was dual inhibitory function of the aptamer-siRNA conjugate on the diseased target: both the anti-gp120 aptamer and the tat/rev siRNA have potent anti-HIV activities in T-cells. Furthermore, the aptamer-siRNA conjugate specifically entered Chinese hamster ovary cells expressing the cell surface gp-160. To augment stability and prevent degradation by nuclease degradation, the senses strand of aptamer had its pyrimidine bases modified by 2’ fluoro ribose bases; aptamers have also been modified by replacing phosphate linkages with boranophosphates 166. Furthermore, the investigators corroborated their earlier study in demonstrating that the 27-base siRNA duplexes were more effective than the 21-base siRNA duplexes. From these tumor and HIV studies, it is difficult to predict whether systemic therapy of aptamer conjugates will be effective. The small size of aptamers increases the likelihood that these would be secreted by the kidney. Moreover, the highly negatively charged RNA may interact with serum proteins and significantly affect biodistribution and specificity of the aptamers. Nevertheless, multimerization of aptamers as done by Wullner and colleagues 165 is expected to reduce renal excretion while selective pegylation will inhibit protein absorption and also reduce renal excretion.
Conclusions
Great advances have been made in developing carriers for siRNA thanks to the efforts of many researchers working to improve delivery methods for antisense oligonucleotides and plasmids. Much of the toxicity of siRNA therapeutics, including off-target effects or cytokine induction, has been circumvented by modification of the bases. Careful evaluation of selected modifications of the siRNA duplex commonly yields a siRNA as effective as the natural siRNA at cleaving the mRNA substrate. These modifications can greatly reduce cytokine induction, off-target effects, and significantly increased intracellular and extracellular stability to nucleases. As we have reported in this review, there are many non-viral carries or approaches that offer exciting opportunities to deliver siRNA to their disease targets. To date, 12 clinical studies are ongoing. Most of these siRNA clinical trials target ocular diseases, but two clinical trials are treating renal disease, and one phase 1 clinical trial is targeting cancer. The clinical trial for cancer patients utilizes a targeted carrier for systemic delivery of therapeutic siRNA. In addition to the ongoing clinical studies, there are many promising pre-clinical carriers that demonstrate efficacy for siRNA delivery.
Despite the diverse number of approaches for siRNA delivery, new strategies such as the siRNA cocktail approach for silencing multiple up-regulated genes are being developed for the treatment of many human diseases. Nonetheless, we need greater understanding between interactions of the nanoparticles and biological milieu to improve siRNA delivery systems further. The physicochemical properties formed by modified or unmodified siRNA with their varied carriers are essential to evaluate to enable tissue targeting and prevent entrapment of the nanoparticles by other non-targeted tissues. The size, surface charge, and 3-D morphology can greatly affect their biodistribution and pharmacokinetics after the nanocomplexes are systemically injected. For example, many factors may affect the formation and stability of the nanocomplexes, such as changes in siRNA length and siRNA modifications, use of different carriers, ratios of siRNA and carriers, and buffers used in their preparation that can further decide the fate of siRNA complexes in vivo. Although biophysical properties (size, charge, etc.) of nanoparticles are frequently reported, these particles are prepared in non-physiological solutions. Few studies have examined the biophysical properties of the nanoparticles in the presence of high levels of serum and their interacting proteins 167–169. Furthermore, we are not aware of any study that has examined the biophysical properties of nanoparticles once they have been exposed to whole blood and the dynamic shear forces of travel through the vasculature. Although half-life studies and incorporation of hydrophilic shields on the surface of nanoparticles partially address the issues of in vivo stability, much remains to be learned about interactions between whole blood and the siRNA delivery system in order to develop a more stable nanoparticle that is clinically relevant. Despite the technical challenges of such experiments, isolation and determination of structure and associated proteins of systemically delivered nanoparticles would no doubt provide insight into development of an improved carrier. With greater insight between the interactions of biological systems and nanoparticles and the resulting effects on their stability and cellular entry, the utility of systemic siRNA in human subjects will become a reality.
Acknowledgments
We are grateful to Dr. Pamela Talalay for her careful reading and useful comments concerning the manuscript. This study was supported by the National Cancer Institute CA136938.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Wong-Staal F, Li QX. Development of new RNAi therapeutics. Histol Histopathol. 2007;22(2):211–217. doi: 10.14670/HH-22.211. [DOI] [PubMed] [Google Scholar]
- 3.Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, et al. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 2005;23(2):227–231. doi: 10.1038/nbt1052. [DOI] [PubMed] [Google Scholar]
- 4.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 5.Snove O, Jr, Rossi JJ. Chemical modifications rescue off-target effects of RNAi. ACS Chem Biol. 2006;1(5):274–276. doi: 10.1021/cb6002256. [DOI] [PubMed] [Google Scholar]
- 6.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 7.Amarzguioui M, Rossi JJ, Kim D. Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS Lett. 2005;579(26):5974–5981. doi: 10.1016/j.febslet.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 8.Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME, et al. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res. 2005;33(13):4140–4156. doi: 10.1093/nar/gki732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404(6775):293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 10.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293(5532):1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett DW, Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34(1):322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YC, Kong LH, Cheng BZ, Li KS. Construction of influenza virus siRNA expression vectors and their inhibitory effects on multiplication of influenza virus. Avian Dis. 2005;49(4):562–573. doi: 10.1637/7365-041205R2.1. [DOI] [PubMed] [Google Scholar]
- 13.Thanik VD, Greives MR, Lerman OZ, Seiser N, Dec W, Chang CC, et al. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14(17):1305–1308. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 14.Aigner A. Cellular delivery in vivo of siRNA-based therapeutics. Curr Pharm Des. 2008;14(34):3603–3619. doi: 10.2174/138161208786898815. [DOI] [PubMed] [Google Scholar]
- 15.Chien PY, Wang J, Carbonaro D, Lei S, Miller B, Sheikh S, et al. Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gene Ther. 2005;12(3):321–328. doi: 10.1038/sj.cgt.7700793. [DOI] [PubMed] [Google Scholar]
- 16.Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, et al. Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference. J Infect Dis. 2006;193(12):1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 19.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319(5863):627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007;104(14):5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32(19):e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12(5):461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 23.Werth S, Urban-Klein B, Dai L, Hobel S, Grzelinski M, Bakowsky U, et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;112(2):257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.DeRouchey J, Schmidt C, Walker GF, Koch C, Plank C, Wagner E, et al. Monomolecular assembly of siRNA and poly(ethylene glycol)-peptide copolymers. Biomacromolecules. 2008;9(2):724–732. doi: 10.1021/bm7011482. [DOI] [PubMed] [Google Scholar]
- 25.Leng Q, Scaria P, Lu P, Woodle MC, Mixson AJ. Systemic delivery of HK Raf-1 siRNA polyplexes inhibits MDA-MB-435 xenografts. Cancer Gene Ther. 2008;15(8):485–495. doi: 10.1038/cgt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Midoux P, Pichon C, Yaouanc JJ, Jaffres PA. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br J Pharmacol. 2009;157(2):166–178. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura Y, Kogure K, Futaki S, Harashima H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J Control Release. 2007;119(3):360–367. doi: 10.1016/j.jconrel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson M, Ramos-Perez V, Singh S, Soliman M, Preece JA, Briggs SS, et al. Delivery of siRNA mediated by histidine-containing reducible polycations. J Control Release. 2008;130(1):46–56. doi: 10.1016/j.jconrel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Yan Z, Zou H, Tian F, Grandis JR, Mixson AJ, Lu PY, et al. Human rhomboid family-1 gene silencing causes apoptosis or autophagy to epithelial cancer cells and inhibits xenograft tumor growth. Mol Cancer Ther. 2008;7(6):1355–1364. doi: 10.1158/1535-7163.MCT-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 31.Moschos SA, Williams AE, Lindsay MA. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochem Soc Trans. 2007;35(Pt 4):807–810. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Jeong JH, Lee SH, Kim SW, Park TG. PEG conjugated VEGF siRNA for anti-angiogenic gene therapy. J Control Release. 2006;116(2):123–129. doi: 10.1016/j.jconrel.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Jeong JH, Lee SH, Kim SW, Park TG. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J Control Release. 2008;129(2):107–116. doi: 10.1016/j.jconrel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz C, Hadwiger P, John M, Vornlocher HP, Unverzagt C. Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells. Bioorg Med Chem Lett. 2004;14(19):4975–4977. doi: 10.1016/j.bmcl.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 36.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, et al. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol Ther. 2008;16(4):734–740. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 37.Chu TC, Twu KY, Ellington AD, Levy M. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006;34(10):e73. doi: 10.1093/nar/gkl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24(8):1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Li H, Li S, Zaia J, Rossi JJ. Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol Ther. 2008;16(8):1481–1489. doi: 10.1038/mt.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23(6):709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 41.Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci U S A. 2007;104(10):4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007;104(32):12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czauderna F, Fechtner M, Dames S, Aygun H, Klippel A, Pronk GJ, et al. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003;31(11):2705–2716. doi: 10.1093/nar/gkg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen N, Kong WJ, Dong JH, Zhang D, Zhang SL, Han YC. SiRNA expression cassettes targeting VEGF effectively inhibit the growth of laryngeal squamous cell carcinoma cell lines. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005;40(10):759–763. [PubMed] [Google Scholar]
- 45.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23(4):457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 46.Kim JY, Choung S, Lee EJ, Kim YJ, Choi YC. Immune activation by siRNA/liposome complexes in mice is sequence- independent: lack of a role for Toll-like receptor 3 signaling. Mol Cells. 2007;24(2):247–254. [PubMed] [Google Scholar]
- 47.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11(3):263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 48.Zamanian-Daryoush M, Marques JT, Gantier MP, Behlke MA, John M, Rayman P, et al. Determinants of cytokine induction by small interfering RNA in human peripheral blood mononuclear cells. J Interferon Cytokine Res. 2008;28(4):221–233. doi: 10.1089/jir.2007.0090. [DOI] [PubMed] [Google Scholar]
- 49.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348(5):1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Schlee M, Hornung V, Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14(4):463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, et al. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19(10):991–999. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- 52.Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18(4):305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 53.Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. Rna. 2003;9(9):1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robbins M, Judge A, MacLachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19(2):89–102. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
- 55.Robbins M, Judge A, Liang L, McClintock K, Yaworski E, MacLachlan I. 2'-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15(9):1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 56.Morrissey DV, Blanchard K, Shaw L, Jensen K, Lockridge JA, Dickinson B, et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41(6):1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- 57.Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. Rna. 2006;12(7):1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 59.Broaddus WC, Gillies GT, Kucharczyk J. Minimally invasive procedures. Advances in image-guided delivery of drug and cell therapies into the central nervous system. Neuroimaging Clin N Am. 2001;11(4):727–735. [PubMed] [Google Scholar]
- 60.Hsich G, Sena-Esteves M, Breakefield XO. Critical issues in gene therapy for neurologic disease. Hum Gene Ther. 2002;13(5):579–604. doi: 10.1089/10430340252837198. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen JB, Sanchez-Pernaute R, Cunningham J, Bankiewicz KS. Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport. 2001;12(9):1961–1964. doi: 10.1097/00001756-200107030-00037. [DOI] [PubMed] [Google Scholar]
- 62.Packer RJ. Alternative treatments for childhood brain tumors. Childs Nerv Syst. 1999;15(11–12):789–794. doi: 10.1007/s003810050472. [DOI] [PubMed] [Google Scholar]
- 63.Palasis M, Luo Z, Barry JJ, Walsh K. Analysis of adenoviral transport mechanisms in the vessel wall and optimization of gene transfer using local delivery catheters. Hum Gene Ther. 2000;11(2):237–246. doi: 10.1089/10430340050015987. [DOI] [PubMed] [Google Scholar]
- 64.Querbes W, Ge P, Zhang W, Fan Y, Costigan J, Charisse K, et al. Direct CNS Delivery of siRNA Mediates Robust Silencing in Oligodendrocytes. Oligonucleotides. 2009;19(1):23–30. doi: 10.1089/oli.2008.0165. [DOI] [PubMed] [Google Scholar]
- 65.Rainov NG, Kramm CM. Vector delivery methods and targeting strategies for gene therapy of brain tumors. Curr Gene Ther. 2001;1(4):367–383. doi: 10.2174/1566523013348445. [DOI] [PubMed] [Google Scholar]
- 66.Ram Z. Advances in the diagnosis and treatment of malignant brain tumors. Isr Med Assoc J. 1999;1(3):188–193. [PubMed] [Google Scholar]
- 67.Walther JL, Bartlett DW, Chew W, Robertson CR, Hostetter TH, Meyer TW. Downloadable computer models for renal replacement therapy. Kidney Int. 2006;69(6):1056–1063. doi: 10.1038/sj.ki.5000196. [DOI] [PubMed] [Google Scholar]
- 68.Le Couteur DG, Warren A, Cogger VC, Smedsrod B, Sorensen KK, De Cabo R, et al. Old age and the hepatic sinusoid. Anat Rec (Hoboken) 2008;291(6):672–683. doi: 10.1002/ar.20661. [DOI] [PubMed] [Google Scholar]
- 69.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 70.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Wu J, Akaike T, Hayashida K, Okamoto T, Okuyama A, Maeda H. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res. 2001;92(4):439–451. doi: 10.1111/j.1349-7006.2001.tb01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6(7):1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 73.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10(10):1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 74.Tompkins SM, Lo CY, Tumpey TM, Epstein SL. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci U S A. 2004;101(23):8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2004;101(41):14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zender L, Kubicka S. Suppression of apoptosis in the liver by systemic and local delivery of small-interfering RNAs. Methods Mol Biol. 2007;361:217–226. doi: 10.1385/1-59745-208-4:217. [DOI] [PubMed] [Google Scholar]
- 77.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9(3):347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 78.De Souza AT, Dai X, Spencer AG, Reppen T, Menzie A, Roesch PL, et al. Transcriptional and phenotypic comparisons of Ppara knockout and siRNA knockdown mice. Nucleic Acids Res. 2006;34(16):4486–4494. doi: 10.1093/nar/gkl609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi N, Kuramoto T, Yamaoka K, Hashida M, Takakura Y. Hepatic uptake and gene expression mechanisms following intravenous administration of plasmid DNA by conventional and hydrodynamics-based procedures. J Pharmacol Exp Ther. 2001;297(3):853–860. [PubMed] [Google Scholar]
- 80.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11(8):675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sebestyen MG, Budker VG, Budker T, Subbotin VM, Zhang G, Monahan SD, et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J Gene Med. 2006;8(7):852–873. doi: 10.1002/jgm.921. [DOI] [PubMed] [Google Scholar]
- 82.Crespo A, Peydro A, Dasi F, Benet M, Calvete JJ, Revert F, et al. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther. 2005;12(11):927–935. doi: 10.1038/sj.gt.3302469. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi N, Nishikawa M, Hirata K, Takakura Y. Hydrodynamics-based procedure involves transient hyperpermeability in the hepatic cellular membrane: implication of a nonspecific process in efficient intracellular gene delivery. J Gene Med. 2004;6(5):584–592. doi: 10.1002/jgm.541. [DOI] [PubMed] [Google Scholar]
- 84.Budker VG, Subbotin VM, Budker T, Sebestyen MG, Zhang G, Wolff JA. Mechanism of plasmid delivery by hydrodynamic tail vein injection. II. Morphological studies. J Gene Med. 2006;8(7):874–888. doi: 10.1002/jgm.920. [DOI] [PubMed] [Google Scholar]
- 85.Klein C, Bock CT, Wedemeyer H, Wustefeld T, Locarnini S, Dienes HP, et al. Inhibition of hepatitis B virus replication in vivo by nucleoside analogues and siRNA. Gastroenterology. 2003;125(1):9–18. doi: 10.1016/s0016-5085(03)00720-0. [DOI] [PubMed] [Google Scholar]
- 86.Yoshino H, Hashizume K, Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13(24):1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Dong X, Sawyer GJ, Collins L, Fabre JW. Regional hydrodynamic gene delivery to the rat liver with physiological volumes of DNA solution. J Gene Med. 2004;6(6):693–703. doi: 10.1002/jgm.595. [DOI] [PubMed] [Google Scholar]
- 88.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15(12):2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 89.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16(6):1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- 90.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, et al. siRNA Targeted to p53 Attenuates Ischemic and Cisplatin-Induced Acute Kidney Injury. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285(5434):1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 92.Bartlett DW, Davis ME. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol Bioeng. 2008;99(4):975–985. doi: 10.1002/bit.21668. [DOI] [PubMed] [Google Scholar]
- 93.Snyder EL, Dowdy SF. Protein/peptide transduction domains: potential to deliver large DNA molecules into cells. Curr Opin Mol Ther. 2001;3(2):147–152. [PubMed] [Google Scholar]
- 94.Snyder EL, Saenz CC, Denicourt C, Meade BR, Cui XS, Kaplan IM, et al. Enhanced targeting and killing of tumor cells expressing the CXC chemokine receptor 4 by transducible anticancer peptides. Cancer Res. 2005;65(23):10646–10650. doi: 10.1158/0008-5472.CAN-05-0118. [DOI] [PubMed] [Google Scholar]
- 95.Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol. 2009 doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliv Rev. 2008;60(4–5):530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10(3):310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 98.Futaki S. Arginine-rich peptides: potential for intracellular delivery of macromolecules and the mystery of the translocation mechanisms. Int J Pharm. 2002;245(1–2):1–7. doi: 10.1016/s0378-5173(02)00337-x. [DOI] [PubMed] [Google Scholar]
- 99.Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276(8):5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 100.Melikov K, Chernomordik LV. Arginine-rich cell penetrating peptides: from endosomal uptake to nuclear delivery. Cell Mol Life Sci. 2005;62(23):2739–2749. doi: 10.1007/s00018-005-5293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271(30):18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 102.Pooga M, Hallbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J. 1998;12(1):67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 103.Cohen JL, Almutairi A, Cohen JA, Bernstein M, Brody SL, Schuster DP, et al. Enhanced cell penetration of acid-degradable particles functionalized with cell-penetrating peptides. Bioconjug Chem. 2008;19(4):876–881. doi: 10.1021/bc700414j. [DOI] [PubMed] [Google Scholar]
- 104.Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62(16):1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Futaki S, Ohashi W, Suzuki T, Niwa M, Tanaka S, Ueda K, et al. Stearylated arginine-rich peptides: a new class of transfection systems. Bioconjug Chem. 2001;12(6):1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- 106.Martins MJ, Negrao MR, Hipolito-Reis C, Azevedo I. Arginine and a polyarginine peptide inhibit alkaline phosphatase activity: possible consequences for cellular transport systems. Clin Biochem. 2001;34(5):435–437. doi: 10.1016/s0009-9120(01)00247-8. [DOI] [PubMed] [Google Scholar]
- 107.Matsui H, Tomizawa K, Lu YF, Matsushita M. Protein Therapy: in vivo protein transduction by polyarginine (11R) PTD and subcellular targeting delivery. Curr Protein Pept Sci. 2003;4(2):151–157. doi: 10.2174/1389203033487270. [DOI] [PubMed] [Google Scholar]
- 108.Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res. 2000;56(5):318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 109.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278(37):35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 110.Zhang W, Smith SO. Mechanism of penetration of Antp(43–58) into membrane bilayers. Biochemistry. 2005;44(30):10110–10118. doi: 10.1021/bi050341v. [DOI] [PubMed] [Google Scholar]
- 111.Futaki S, Nakase I, Tadokoro A, Takeuchi T, Jones AT. Arginine-rich peptides and their internalization mechanisms. Biochem Soc Trans. 2007;35(Pt 4):784–787. doi: 10.1042/BST0350784. [DOI] [PubMed] [Google Scholar]
- 112.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10(6):1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46(2):492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- 114.Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24(45):10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101(51):17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11(8):1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 117.Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim YH, et al. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol Ther. 2006;14(3):343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 118.Kumar LD, Clarke AR. Gene manipulation through the use of small interfering RNA (siRNA): from in vitro to in vivo applications. Adv Drug Deliv Rev. 2007;59(2–3):87–100. doi: 10.1016/j.addr.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 119.Waite CL, Sparks SM, Uhrich KE, Roth CM. Acetylation of PAMAM dendrimers for cellular delivery of siRNA. BMC Biotechnol. 2009;9:38. doi: 10.1186/1472-6750-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou J, Wu J, Hafdi N, Behr JP, Erbacher P, Peng L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem Commun (Camb) 2006;(22):2362–2364. doi: 10.1039/b601381c. [DOI] [PubMed] [Google Scholar]
- 121.Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci U S A. 2004;101(23):8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kircheis R, Schuller S, Brunner S, Ogris M, Heider KH, Zauner W, et al. Polycation-based DNA complexes for tumor-targeted gene delivery in vivo. J Gene Med. 1999;1(2):111–120. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<111::AID-JGM22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 123.Kircheis R, Wightman L, Schreiber A, Robitza B, Rossler V, Kursa M, et al. Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther. 2001;8(1):28–40. doi: 10.1038/sj.gt.3301351. [DOI] [PubMed] [Google Scholar]
- 124.Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165(6):2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim SH, Mok H, Jeong JH, Kim SW, Park TG. Comparative evaluation of target-specific GFP gene silencing efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid complexed with PEI-PEG-FOL conjugate. Bioconjug Chem. 2006;17(1):241–244. doi: 10.1021/bc050289f. [DOI] [PubMed] [Google Scholar]
- 126.Malek A, Merkel O, Fink L, Czubayko F, Kissel T, Aigner A. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharmacol. 2009;236(1):97–108. doi: 10.1016/j.taap.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 127.Hobel S, Aigner A. Nonviral delivery platform for therapeutic RNAi: pegylated siRNA/cationic liposome complexes for targeting of the proto-oncogene bcl-2. Future Oncol. 2009;5(1):13–17. doi: 10.2217/14796694.5.1.13. [DOI] [PubMed] [Google Scholar]
- 128.Fujii T, Saito M, Iwasaki E, Ochiya T, Takei Y, Hayashi S, et al. Intratumor injection of small interfering RNA-targeting human papillomavirus 18 E6 and E7 successfully inhibits the growth of cervical cancer. Int J Oncol. 2006;29(3):541–548. [PubMed] [Google Scholar]
- 129.Mu P, Nagahara S, Makita N, Tarumi Y, Kadomatsu K, Takei Y. Systemic delivery of siRNA specific to tumor mediated by atelocollagen: Combined therapy using siRNA targeting Bcl-xL and cisplatin against prostate cancer. Int J Cancer. 2009 doi: 10.1002/ijc.24382. [DOI] [PubMed] [Google Scholar]
- 130.Nozawa H, Tadakuma T, Ono T, Sato M, Hiroi S, Masumoto K, et al. Small interfering RNA targeting epidermal growth factor receptor enhances chemosensitivity to cisplatin, 5-fluorouracil and docetaxel in head and neck squamous cell carcinoma. Cancer Sci. 2006;97(10):1115–1124. doi: 10.1111/j.1349-7006.2006.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64(10):3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- 132.Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci U S A. 2005;102(34):12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ishimoto T, Takei Y, Yuzawa Y, Hanai K, Nagahara S, Tarumi Y, et al. Downregulation of monocyte chemoattractant protein-1 involving short interfering RNA attenuates hapten-induced contact hypersensitivity. Mol Ther. 2008;16(2):387–395. doi: 10.1038/sj.mt.6300360. [DOI] [PubMed] [Google Scholar]
- 134.Perales JC, Ferkol T, Beegen H, Ratnoff OD, Hanson RW. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc Natl Acad Sci USA. 1994;91(9):4086–4090. doi: 10.1073/pnas.91.9.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferkol T, Perales JC, Mularo F, Hanson RW. Receptor-mediated gene transfer into macrophages. Proc Natl Acad Sci U S A. 1996;93(1):101–105. doi: 10.1073/pnas.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7(12):1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 137.Plank C, Tang MX, Wolfe AR, Szoka FC., Jr Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther. 1999;10(2):319–332. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 138.Boussif O, Delair T, Brua C, Veron L, Pavirani A, Kolbe HV. Synthesis of polyallylamine derivatives and their use as gene transfer vectors in vitro. Bioconjug Chem. 1999;10(5):877–883. doi: 10.1021/bc9900439. [DOI] [PubMed] [Google Scholar]
- 139.Toncheva V, Wolfert MA, Dash PR, Oupicky D, Ulbrich K, Seymour LW, et al. Novel vectors for gene delivery formed by self-assembly of DNA with poly(L-lysine) grafted with hydrophilic polymers. Biochim Biophys Acta. 1998;1380(3):354–368. doi: 10.1016/s0304-4165(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 140.Dobrovolskaia MA, Clogston JD, Neun BW, Hall JB, Patri AK, McNeil SE. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008;8(8):2180–2187. doi: 10.1021/nl0805615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug Chem. 1999;10(3):406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 142.Zhang L, Ambulos N, Mixson AJ. DNA delivery to cells in culture using peptides. Methods Mol Biol. 2004;245:33–52. doi: 10.1385/1-59259-649-5:33. [DOI] [PubMed] [Google Scholar]
- 143.Chen QR, Zhang L, Stass SA, Mixson AJ. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001;29(6):1334–1340. doi: 10.1093/nar/29.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meyer M, Dohmen C, Philipp A, Kiener D, Maiwald G, Scheu C, et al. Synthesis and Biological Evaluation of a Bioresponsive and Endosomolytic siRNA-Polymer Conjugate. Mol Pharm. 2009;6(3):752–762. doi: 10.1021/mp9000124. [DOI] [PubMed] [Google Scholar]
- 145.Balhorn R. The protamine family of sperm nuclear proteins. Genome Biol. 2007;8(9):227. doi: 10.1186/gb-2007-8-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amos H, Kearns KE. Influence of Bacterial Ribonucleic Acid on Animal Cells in Culture. Ii. Protamine Enhancement of Rna Uptake. Exp Cell Res. 1963;32:14–25. doi: 10.1016/0014-4827(63)90064-8. [DOI] [PubMed] [Google Scholar]
- 147.Ryser HJ. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967;32(3):737–750. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ryser HJ. Uptake of foreign proteins by mammalian cells and the functions of pinocytosis. Bull Schweiz Akad Med Wiss. 1969;24(5):363–384. [PubMed] [Google Scholar]
- 149.Mok H, Park JW, Park TG. Antisense oligodeoxynucleotide-conjugated hyaluronic acid/protamine nanocomplexes for intracellular gene inhibition. Bioconjug Chem. 2007;18(5):1483–1489. doi: 10.1021/bc070111o. [DOI] [PubMed] [Google Scholar]
- 150.Li SD, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharm. 2006;3(5):579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- 151.Soriano P, Dijkstra J, Legrand A, Spanjer H, Londos-Gagliardi D, Roerdink F, et al. Targeted and nontargeted liposomes for in vivo transfer to rat liver cells of a plasmid containing the preproinsulin I gene. Proc Natl Acad Sci U S A. 1983;80(23):7128–7131. doi: 10.1073/pnas.80.23.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Felgner PL, Ringold GM. Cationic liposome-mediated transfection. Nature. 1989;337(6205):387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 153.Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, et al. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007;67(7):2938–2943. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 154.Kim SI, Shin D, Choi TH, Lee JC, Cheon GJ, Kim KY, et al. Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol Ther. 2007;15(6):1145–1152. doi: 10.1038/sj.mt.6300168. [DOI] [PubMed] [Google Scholar]
- 155.Watanabe T, Umehara T, Yasui F, Nakagawa S, Yano J, Ohgi T, et al. Liver target delivery of small interfering RNA to the HCV gene by lactosylated cationic liposome. J Hepatol. 2007;47(6):744–750. doi: 10.1016/j.jhep.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 156.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452(7187):591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126(1):77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Halder J, Kamat AA, Landen CN, Jr, Han LY, Lutgendorf SK, Lin YG, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12(16):4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 160.Landen CN, Merritt WM, Mangala LS, Sanguino AM, Bucana C, Lu C, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5(12):1708–1713. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 161.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100(5):359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68(21):9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 164.Kim SH, Jeong JH, Lee SH, Kim SW, Park TG. LHRH receptor-mediated delivery of siRNA using polyelectrolyte complex micelles self-assembled from siRNA-PEG-LHRH conjugate and PEI. Bioconjug Chem. 2008;19(11):2156–2162. doi: 10.1021/bc800249n. [DOI] [PubMed] [Google Scholar]
- 165.Wullner U, Neef I, Eller A, Kleines M, Tur MK, Barth S. Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2. Curr Cancer Drug Targets. 2008;8(7):554–565. doi: 10.2174/156800908786241078. [DOI] [PubMed] [Google Scholar]
- 166.Shaw BR, Moussa L, Sharaf M, Cheek M, Dobrikov M. Boranophosphate siRNA-aptamer chimeras for tumor-specific downregulation of cancer receptors and modulators. Nucleic Acids Symp Ser (Oxf) 2008;(52):655–656. doi: 10.1093/nass/nrn331. [DOI] [PubMed] [Google Scholar]
- 167.Li S, Rizzo MA, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5(7):930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 168.Liu S, Shibata A, Ueno S, Huang Y, Wang Y, Li Y. Translocation of positively charged copoly(Lys/Tyr) across phospholipid membranes. Biochem Biophys Res Commun. 2006;339(3):761–768. doi: 10.1016/j.bbrc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 169.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007;104(7):2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pirollo KF, Chang EH. Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer Res. 2008;68(5):1247–1250. doi: 10.1158/0008-5472.CAN-07-5810. [DOI] [PubMed] [Google Scholar]


