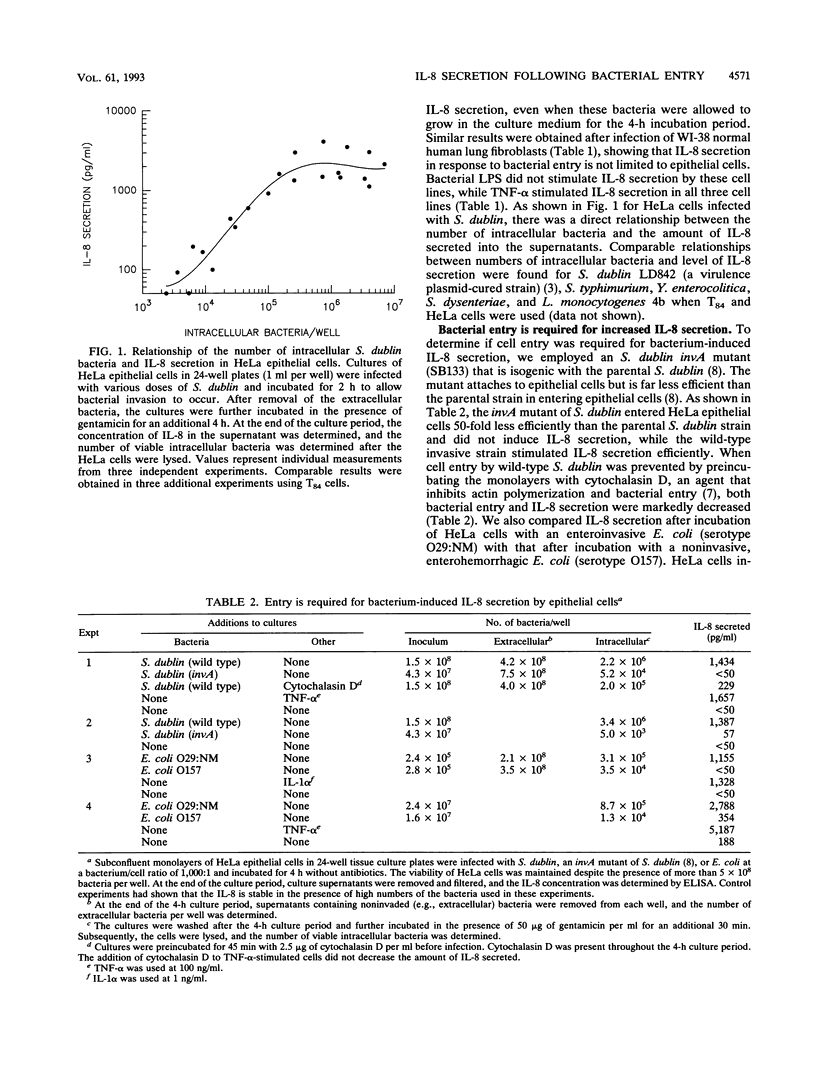
Abstract
Bacterial invasion of mucosal surfaces results in a rapid influx of polymorphonuclear leukocytes. The chemotactic stimulus responsible for this response is not known. Since epithelial cells are among the first cells entered by many enteric pathogens, we investigated the ability of epithelial cells to provide an early signal for the mucosal inflammatory response through the release of chemotactic cytokines. As shown herein, the chemokine interleukin-8 (IL-8), a potent chemoattractant and activator of polymorphonuclear leukocytes, was secreted by intestinal and cervical epithelial cells in response to bacterial entry. Moreover, a variety of different bacteria, including those that remain inside phagosomal vacuoles, e.g., Salmonella spp., and those that enter the cytoplasm, e.g., Listeria monocytogenes, stimulated this response. Increased IL-8 mRNA levels could be detected within 90 min after infection. Neither bacterial lipopolysaccharide nor noninvasive bacteria, including Escherichia coli and Enterococcus faecium, induced an IL-8 response. Moreover, tumor necrosis factor alpha, which is known to be expressed by some epithelial cells, was not detected in the culture supernatants after bacterial entry, and addition of anti-tumor necrosis factor alpha antibodies had no effect on the IL-8 response following bacterial entry. These data suggest the novel concept that epithelial cells serve as an early signaling system to host immune and inflammatory cells in the underlying mucosa following bacterial entry.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agace W., Hedges S., Andersson U., Andersson J., Ceska M., Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993 Feb;61(2):602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. N., Jones M. L., Mitra R. S., Crockett-Torabe E., Fantone J. C., Kunkel S. L., Warren J. S., Dixit V. M., Nickoloff B. J. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am J Pathol. 1991 Oct;139(4):869–876. [PMC free article] [PubMed] [Google Scholar]
- Chikami G. K., Fierer J., Guiney D. G. Plasmid-mediated virulence in Salmonella dublin demonstrated by use of a Tn5-oriT construct. Infect Immun. 1985 Nov;50(2):420–424. doi: 10.1128/iai.50.2.420-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991 Sep;59(9):2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Gillitzer R., Berger R., Mielke V., Müller C., Wolff K., Stingl G. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mRNA in situ. J Invest Dermatol. 1991 Jul;97(1):73–79. doi: 10.1111/1523-1747.ep12478128. [DOI] [PubMed] [Google Scholar]
- Ginocchio C., Pace J., Galán J. E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S., Svensson M., Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992 Apr;60(4):1295–1301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevnikar A. M., Brennan D. C., Singer G. G., Heng J. E., Maslinski W., Wuthrich R. P., Glimcher L. H., Kelley V. E. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int. 1991 Aug;40(2):203–211. doi: 10.1038/ki.1991.201. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Koyama S. Y., Podolsky D. K. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989 May;83(5):1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Terkeltaub R., Villiger P. M. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol. 1992 Jan 15;148(2):466–473. [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., Jaffe H. A., Crystal R. G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991 Oct 15;266(29):19611–19617. [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Pace J., Hayman M. J., Galán J. E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993 Feb 26;72(4):505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- Radema S. A., van Deventer S. J., Cerami A. Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology. 1991 May;100(5 Pt 1):1180–1186. [PubMed] [Google Scholar]
- Ruschkowski S., Rosenshine I., Finlay B. B. Salmonella typhimurium induces an inositol phosphate flux in infected epithelial cells. FEMS Microbiol Lett. 1992 Aug 15;74(2-3):121–126. doi: 10.1016/0378-1097(92)90416-l. [DOI] [PubMed] [Google Scholar]
- Rácz P., Tenner K., Mérö E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab Invest. 1972 Jun;26(6):694–700. [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmouder R. L., Strieter R. M., Wiggins R. C., Chensue S. W., Kunkel S. L. In vitro and in vivo interleukin-8 production in human renal cortical epithelia. Kidney Int. 1992 Jan;41(1):191–198. doi: 10.1038/ki.1992.26. [DOI] [PubMed] [Google Scholar]
- Shirota K., LeDuy L., Yuan S. Y., Jothy S. Interleukin-6 and its receptor are expressed in human intestinal epithelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58(4):303–308. doi: 10.1007/BF02890085. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Imamura K., Rodriguez C., Sariban E., Kufe D. W. Tumor necrosis factor expression in human epithelial tumor cell lines. J Clin Invest. 1988 Feb;81(2):455–460. doi: 10.1172/JCI113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H. Electron-Microscope Studies of Experimental Salmonella Infection in the Preconditioned Guinea Pig: II. Response of the Intestinal Mucosa to the Invasion by Salmonella typhimurium. Am J Pathol. 1967 Jul;51(1):137–161. [PMC free article] [PubMed] [Google Scholar]
- Tesh V. L., O'Brien A. D. Adherence and colonization mechanisms of enteropathogenic and enterohemorrhagic Escherichia coli. Microb Pathog. 1992 Apr;12(4):245–254. doi: 10.1016/0882-4010(92)90043-n. [DOI] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]




