Abstract
Melanoma differentiation associated gene-7/interleukin 24 (mda-7/IL-24) is a unique interleukin (IL)-10 family cytokine displaying selective apoptosis-inducing activity in transformed cells without harming normal cells. The present studies focused on defining the mechanism(s) by which recombinant adenoviral delivery of MDA-7/IL-24 inhibits cell survival of human ovarian carcinoma cells. Expression of MDA-7/IL-24 induced phosphorylation of protein kinase R-like endoplasmic reticulum kinase (PERK) and eukaryotic initiation factor2α (eIF2α). In a PERK-dependent fashion, MDA-7/IL-24 reduced ERK1/2 and AKT phosphorylation and activated c-Jun NH2-terminal kinase (JNK) 1/2 and p38 mitogen-activated protein kinase (MAPK). MDA-7/IL-24 reduced MCL-1 and BCL-XL and increased BAX levels via PERK signaling; cell-killing was mediated via the intrinsic pathway, and cell killing was primarily necrotic as judged using Annexin V/propidium iodide staining. Inhibition of p38 MAPK and JNK1/2 abolished MDA-7/IL-24 toxicity and blocked BAX and BAK activation, whereas activation of mitogen-activated extracellular-regulated kinase (MEK) 1/2 or AKT suppressed enhanced killing and JNK1/2 activation. MEK1/2 signaling increased expression of the MDA-7/IL-24 and PERK chaperone BiP/78-kDa glucose regulated protein (GRP78), and overexpression of BiP/GRP78 suppressed MDA-7/IL-24 toxicity. MDA-7/IL-24-induced LC3-green fluorescent protein vesicularization and processing of LC3; and knockdown of ATG5 suppressed MDA-7/IL-24-mediated toxicity. MDA-7/IL-24 and cisplatin interacted in a greater than additive fashion to kill tumor cells that was dependent on a further elevation of JNK1/2 activity and recruitment of the extrinsic CD95 pathway. MDA-7/IL-24 toxicity was enhanced in a weak additive fashion by paclitaxel; paclitaxel enhanced MDA-7/IL-24 + cisplatin lethality in a greater than additive fashion via BAX. Collectively, our data demonstrate that MDA-7/IL-24 induces an endoplasmic reticulum stress response that activates multiple proapoptotic pathways, culminating in decreased ovarian tumor cell survival.
In the United States, ovarian carcinoma is diagnosed in ∼26,000 patients each year, with ∼15,000 deaths. If the disease is detected at a very early stage, in which a large portion of or the entire ovary can be removed with the tumor, ∼75% of patients survive at least 5 years after diagnosis (Coleman and Sood, 2006,). However, in the majority of cases, if the disease has spread beyond the ovary into the peritoneum with nodal involvement, even under ideal circumstances in which the disease is still only locally advanced and in which essentially all of the tumor can be surgically removed, and the patients are maximally treated with chemotherapies including cisplatin and taxanes, the prognosis is poor, with rapid nadir.
The mda-7/IL-24 gene was isolated from human melanoma cells treated with interferon and mezerein (Jiang et al., 1995; Staudt et al., 2009). The expression of MDA-7/IL-24 is decreased in advanced melanomas, with undetectable levels in metastatic disease (Jiang et al., 1995; Ekmekcioglu et al., 2001; Huang et al., 2001; Ellerhorst et al., 2002; Parrish-Novak et al., 2002; Caudell et al., 2002; Fisher et al., 2003, 2007; Pestka et al., 2004; Lebedeva et al., 2005; Fisher, 2005; Gupta et al., 2006a). Enforced expression of MDA-7/IL-24 inhibits the growth and kills a broad spectrum of cancer cells without exerting deleterious effects in normal human epithelial or fibroblast cells (Su et al., 1998, 2001; Fisher et al., 2003, 2007; Lebedeva et al., 2005; Gupta et al., 2006a). Considering its potent cancer-specific apoptosis-inducing ability and tumor growth-suppressing properties in human tumor xenograft animal models, mda-7/IL-24 was evaluated in a phase I clinical trial in patients with advanced cancers, including melanomas (Fisher et al., 2003, 2007; Lebedeva et al., 2005; Cunningham et al., 2005; Emdad et al., 2009). This study indicated that Ad.mda-7 injected intratumorally was safe, and with repeated injection, significant clinical activity was evident in patients who had not responded to a spectrum of other therapies, including radiation, chemotherapy, and immunotherapy, applied alone or in various combinations.
The apoptotic pathways by which Ad.mda-7 causes cell death are not fully understood; however, current evidence suggests an inherent complexity and an involvement of proteins important for the onset of growth inhibition and apoptosis (Su et al., 1998, 2001; Fisher et al., 2003; Fisher, 2005; Lebedeva et al., 2005; Gupta et al., 2006a; Emdad et al., 2009). In melanoma cell lines, but not in normal melanocytes, Ad.mda-7 infection induces a significant decrease in both BCL-2 and BCL-XL levels, with only a modest up-regulation of BAX and BAK expression (Su et al., 1998, 2001; Lebedeva et al., 2002, 2005; Fisher et al., 2003; Fisher, 2005; Gupta et al., 2006a). The ability of Ad.mda-7 to induce apoptosis in DU-145 prostate cancer cells, which does not produce BAX, indicates that MDA-7/IL-24 can also mediate apoptosis in tumor cells by a BAX-independent pathway (Lebedeva et al., 2003; Su et al., 2006). In prostate cancer cells, overexpression of either BCL-2 or BCL-XL protects cells from Ad.mda-7-induced toxicity in a cell type-dependent fashion (Lebedeva et al., 2003; Su et al., 2006). The combination of radiation and mda-7/IL-24 enhances lethality in ovarian cancer cells (OCCs) (Emdad et al., 2006). In one OCC line, MDA-7/IL24 was reported to kill via the extrinsic apoptosis pathway (Gopalan et al., 2005). Thus, MDA-7/IL-24 lethality seems to occur by multiple distinct pathways in different cell types, but in all of these studies, cell killing is reflected in a profound induction of mitochondrial dysfunction.
MDA-7/IL-24 toxicity has been linked to alterations in endoplasmic reticulum (ER) stress signaling (Gupta et al., 2006a,b). In these studies, MDA-7/IL-24 physically associates with BiP/GRP78 and inactivates the protective actions of this ER-chaperone protein. In addition to virus-administered mda-7/IL-24, delivery of this cytokine as a bacterially expressed glutathione transferase (GST) fusion protein, GST-MDA-7, retains cancer-specific killing, selective ER localization, and induces similar signal transduction changes in cancer cells (Sauane et al., 2004). High concentrations of GST-MDA-7 or infection with Ad.mda-7 kill human glioma cells and do so in a PERK-dependent fashion that is dependent on mitochondrial dysfunction (Yacoub et al., 2004, 2008a,b,c). The precise mechanisms by which GST-MDA-7 or Ad.mda-7 modulates survival in human OCC are unknown.
Our laboratories have demonstrated that Ad.mda-7 kills melanoma cells in part by promoting p38 MAPK-dependent activation of the growth arrest and DNA damage-inducible genes, including GADD153, GADD45, and GADD34 (Sarkar et al., 2002; Yacoub et al., 2003; Mhashilkar et al., 2003; Sauane et al., 2004; Chada et al., 2005). In primary glioblastoma (GBM) cells, we noted p38 MAPK signaling as a protective signal (Yacoub et al., 2008a). Other groups have argued that inhibition of phosphatidyl inositol 3 kinase signaling, but not ERK1/2 signaling, modestly promotes Ad.mda-7 lethality in breast and lung cancer cells (Mhashilkar et al., 2003; Chada et al., 2005). GST-MDA-7 protein, in the 0.25 to 2.0 nM concentration range, causes growth arrest with little cell killing, whereas at ∼20-fold greater concentrations, this cytokine causes profound growth arrest and tumor cell death (Sauane et al., 2004; Yacoub et al., 2008a,c). Using established human OCC lines, we examined the impact of GST-MDA-7 and Ad.mda-7 on viability with a focus on elucidating the molecular mechanisms by which MDA-7/IL-24 enhances tumor cell death.
Materials and Methods
Materials.
SKOVIII and OVCAR OCCs were purchased from the American Type Culture Collection (Manassas, VA). Caspase inhibitors and paclitaxel (Taxol; Bristol-Meyers Squibb Company, Princeton, NJ) were supplied by Calbiochem (San Diego, CA) as powder, dissolved in sterile dimethyl sulfoxide, and stored frozen under light-protected conditions at −80°C. Plasmids expressing dominant-negative PERK, BiP/GRP78, and LC3-GFP were kindly supplied by Drs. A. Diehl (University of Pennsylvania, Philadelphia, PA), A. Lee (University of California, Los Angeles, CA), and S. Spiegel (Virginia Commonwealth University, Richmond, VA). Short hairpin RNA constructs targeting ATG5 (pLVTHM/ATG5) were a generous gift from Dr. Yousefi (Department of Pharmacology, University of Bern, Bern, Switzerland). Commercially available validated short hairpin RNA molecules to knockdown RNA/protein levels were from QIAGEN (Valencia, CA). Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO). Antibody reagents, kinase inhibitors, caspase inhibitors cell culture reagents, and noncommercial recombinant adenoviruses have been described previously by ourselves and others (Guicciardi et al., 2000; Sarkar et al., 2002; Yacoub et al., 2008a,c; Park et al., 2008, 2009; Zhang et al., 2008). The Annexin V/PI kit was from BD Pharmingen (San Diego, CA).
Generation of Ad.5-mda-7 or Ad.5/3-mda-7 and Synthesis of GST-MDA-7.
Recombinant types 5 and 5/3 adenoviruses to express MDA-7/IL-24 (Ad.mda-7) or control (CMV vector) were generated using recombination in human embryonic kidney 293 cells as described elsewhere (Sarkar et al., 2002; Yacoub et al., 2008b). Ad.5/3-mda-7 was prepared as described previously(Dash et al., 2010).
Cell Culture and in Vitro Exposure of Cells to GST-MDA-7 and Drugs.
All established OCC lines were cultured at 37°C [5% (v/v) CO2] in vitro using RPMI supplemented with 5% (v/v) fetal calf serum and 10% (v/v) nonessential amino acids. For short-term cell-killing assays and immunoblotting, cells were plated at a density of 3 × 103/cm2 and 36 h after plating were treated with MDA-7/IL-24 and/or various drugs, as indicated. In vitro small-molecule inhibitor treatments were from a 100 mM stock solution of each drug, and the maximal concentration of vehicle (dimethyl sulfoxide) in media was 0.02% (v/v). For adenoviral infection, cells were infected 12 h after plating, and the expression of the recombinant viral transgene was allowed to occur for 24 h before any additional experimental procedure. Cells were not cultured in reduced serum media during any study.
Cell Treatments, SDS-PAGE, and Western Blot Analysis.
Cells were treated with various GST-MDA-7 concentrations or viral multiplicities of infection, as indicated in the figure legends. For SDS-PAGE and immunoblotting, cells were lysed in either a nondenaturing lysis buffer and prepared for immunoprecipitation as described elsewhere (Yacoub et al., 2008c; Park et al., 2009) or in whole-cell lysis buffer (0.5 M Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, and 0.02% bromphenol blue), and the samples were boiled for 30 min. After immunoprecipitation, samples were boiled in whole-cell lysis buffer. The boiled samples were loaded onto 10 to 14% SDS-PAGE and electrophoresis was run overnight. Proteins were electrophoretically transferred onto 0.22-μm nitrocellulose and immunoblotted with indicated primary antibodies against the different proteins.
Recombinant Adenoviral Vectors: Infection in Vitro.
We generated or purchased previously noted recombinant adenoviruses to express constitutively activated and dominant-negative AKT and MEK1 proteins, dominant-negative caspase-9, X-linked inhibitor of apoptosis protein, c-FLIP-s, CRM A, and BCL-XL (Vector Biolabs, Philadelphia, PA). Cells were infected with these adenoviruses at an approximate m.o.i. of 50. Cells were incubated for 24 h to ensure adequate expression of transduced gene products before drug exposures.
Detection of Cell Death by Trypan Blue, Hoechst, Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling, and Flow Cytometric Assays.
Cells were harvested by trypsinization with trypsin/EDTA for ∼10 min at 37°C. As some apoptotic cells detached from the culture substratum into the medium; these cells were also collected by centrifugation of the medium at 1500 rpm for 5 min. The pooled cell pellets were resuspended and mixed with trypan blue dye. Trypan blue stain, in which blue dye-incorporating cells were scored as being dead, was performed by counting of cells using a light microscope and a hemacytometer. Five hundred cells from randomly chosen fields were counted, and the number of dead cells was counted and expressed as a percentage of the total number of cells counted. For confirmatory purposes the extent of apoptosis was evaluated by assessing Hoechst and terminal deoxynucleotidyl transferase dUTP nick-end labeling-stained cytospin slides under fluorescent light microscopy and scoring the number of cells exhibiting the “classic” morphological features of apoptosis and necrosis. For each condition, 10 randomly selected fields per slide were evaluated, encompassing at least 1500 cells. Alternatively, the Annexin V/propidium iodide assay was carried out to determine cell viability according to the manufacturer's instructions (BD Pharmingen) using a FACScan flow cytometer (BD Biosciences, San Jose, CA).
Colony Formation Assay.
Single cells were plated (250–1500 cells) per 60-mm dish. Twelve hours after plating, cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80, and 24 h after infection, cells were treated with vehicle or cisplatin (CDDP, 3 μM). The media were changed to drug-free media 48 h after CDDP treatment, and colonies of >50 cells/colony were permitted to form for 10 to 14 days.
Plasmid Transfection.
Plasmid DNA (0.5 μg/total plasmid transfected) was diluted into 50 μl of RPMI growth media that lacked supplementation with FBS or with penicillin/streptomycin. Lipofectamine 2000 reagent (1 μl) (Invitrogen, Carlsbad, CA) was diluted into 50 μl of growth media that lacked supplementation with FBS or with penicillin/streptomycin. The two solutions were then mixed together and incubated at room temperature for 30 min. The total mix was added to each well (4-well glass slide or 12-well plate) containing 200 μl of growth media that lacked supplementation with FBS or with penicillin/streptomycin. The cells were incubated for 4 h at 37°C, after which the media were replaced with RPMI growth media containing 5% (v/v) FBS and 1× penicillin/streptomycin.
Microscopy for LC3-GFP Expression.
Where indicated, LC3-GFP-transfected cells, 12 h after transfection, were infected with either Ad.5-cmv or Ad.5-mda-7 and then cultured for 24 h. Cells were then stained with Lysotracker Red Dye (Invitrogen) at the indicated time points for 20 min. Lysotracker Red Dye-stained cells were visualized immediately after staining on a Zeiss Axiovert 200 microscope using the rhodamine filter (Carl Zeiss Inc., Thornwood, NY). LC3-GFP-transfected cells were visualized at the indicated time points on the Zeiss Axiovert 200 microscope using the fluorescein isothiocyanate filter.
Data Analysis.
Comparison of the effects of various treatments was performed using one-way analysis of variance and a two-tailed Student's t test. Differences with a p value of <0.05 were considered statistically significant. Experiments shown are the means of multiple individual points from multiple experiments (± S.E.M.).
Results
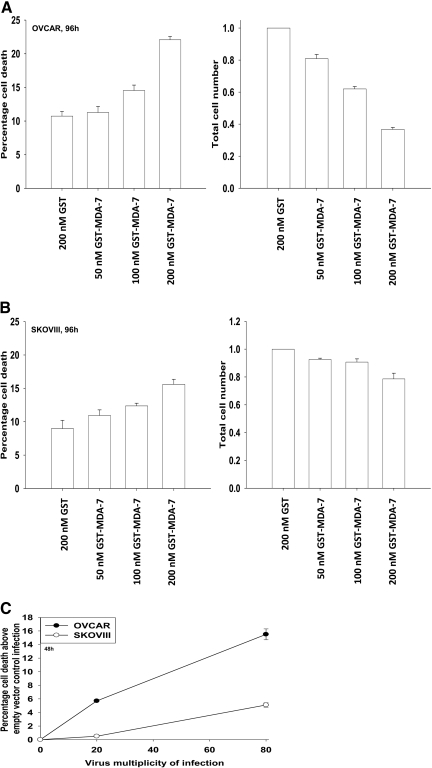
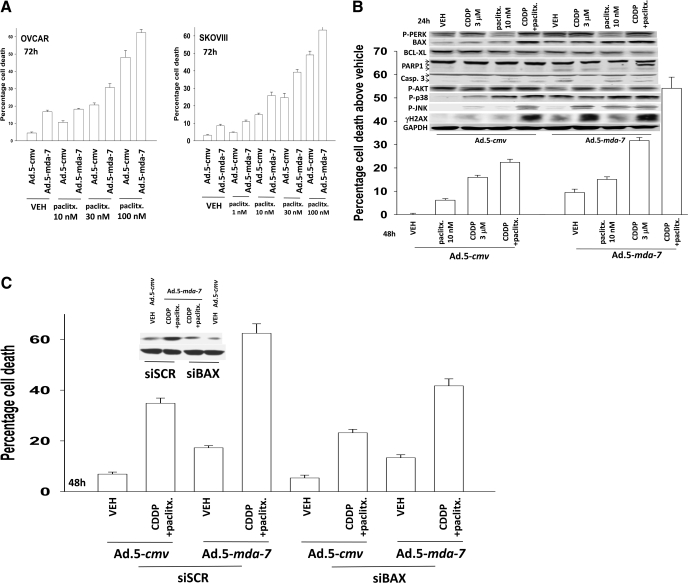
Initial experiments focused on defining the dose-dependent impact of GST-MDA-7 on OCC growth and viability. GST-MDA-7 in a dose-dependent manner suppressed the proliferation of SKOVIII and OVCAR cells (Fig. 1, A and B) with a reduced effect in SKOVIII. These findings correlated with dose-dependent cell killing by GST-MDA-7 in these cells; it is noteworthy that GST-MDA-7 suppressed tumor cell proliferation without causing a large degree of cell death in both OCCs (compare Fig. 1, B with A). In a similar manner as observed with GST-MDA-7 protein, increasing multiplicities of infection of a type 5 recombinant adenovirus expressing MDA-7/IL-24, Ad.5-mda-7, resulted in dose-dependent tumor cell killing (Fig. 1C). In contrast to several prior studies in other tumor cell types, we noted in OCC that infection with a recombinant adenovirus to express MDA-7/IL-24 was at least as efficacious at killing cells as was treatment with GST-MDA-7. As a result of this observation, we focused our studies using Ad.5-mda-7.
Fig. 1.
MDA-7/IL-24 causes a dose-dependent induction of growth arrest and OCC death. A and B, OVCAR cells and SKOVIII cells were treated 24 h after plating with GST-MDA-7 (0–200 nM). At the indicated time point after GST-MDA-7 treatment (96 h), cell viability was determined by trypan blue exclusion assay, and cell numbers were determined using hemocytometer (± S.E.M., n = 3). C, OVCAR and SKOVIII cells were infected with type 5 recombinant adenoviruses empty vector (Ad.5-cmv) or to express MDA-7/IL-24 (Ad.5-mda-7) at an m.o.i. of 20 or 80. Cells were isolated 48 h after exposure, and the loss of cell viability above empty vector Ad.5-cmv infection was determined by using Annexin V propidium iodide staining assays in triplicate using a flow cytometer (± S.E.M., n = 3).
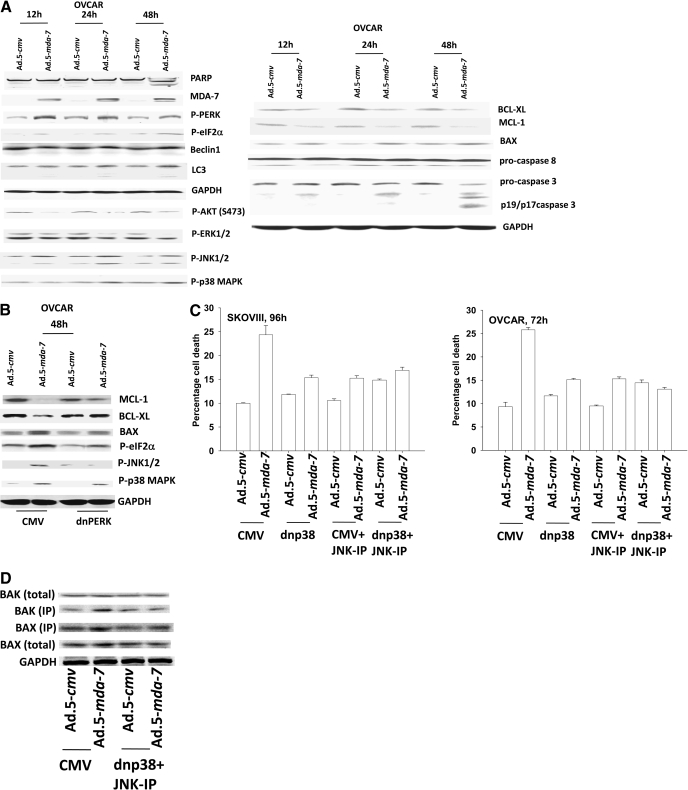
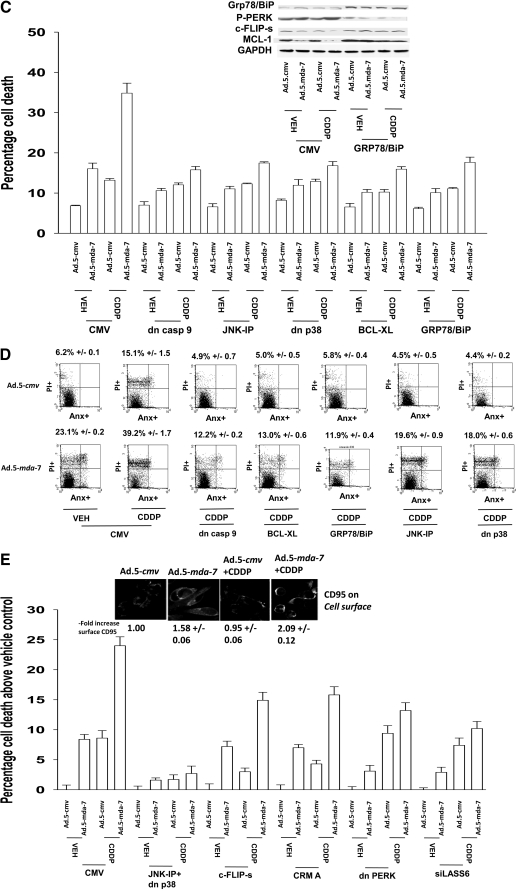
Infection of OCC (OVCAR) with Ad.5-mda-7 promoted cleavage of caspase 3 and PARP1 processing of LC3 within 48 h (Fig. 2A). Similar data were obtained in SKOVIII cells (Supplemental Fig. S1). Expression of MDA-7/IL-24 induced phosphorylation of PERK and eIF2α. In a PERK-dependent fashion, MDA-7/IL-24 also reduced ERK1/2 and AKT phosphorylation and activated JNK1/2 and p38 MAPK (Fig. 2B). MDA-7/IL-24 lowered expression of MCL-1 and BCL-XL and increased BAX levels via PERK signaling. Inhibition of both p38 MAPK and JNK1/2 signaling blocked Ad.5-mda-7 lethality that correlated with reduced activation of BAX and BAK (Fig. 2, C and D). Sustained activation of AKT or of MEK1 using molecular tools suppressed Ad.5-mda-7 toxicity (Fig. 2E). Additional suppression of AKT or MEK1 function using dominant-negative constructs further enhanced MDA-7/IL-24 lethality. In SKOVIII cells that were more resistant to MDA-7/IL-24 lethality than OVCAR cells, we noted that inhibition of the extrinsic pathway using c-FLIP-s or CRM A expression blunted MDA-7/IL-24-induced killing, whereas in OVCAR cells, neither c-FLIP-s nor CRM A had an impact on MDA-7/IL-24 toxicity (Fig. 2E).
Fig. 2.
MDA-7/IL-24 promotes transformed cell killing through ER stress signaling and activation of the JNK/p38 MAPK pathways. A, OVCAR cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 50. Cells were isolated 12–48 h after infection and processed for SDS-PAGE and immunoblotting against the indicated proteins (n = 3). B, OVCAR cells were transfected with empty vector plasmid (CMV) or with a plasmid to express dominant-negative PERK. Twelve hours after transfection, cells were infected with Ad.5.cmv or Ad.5.mda-7 at an m.o.i. of 50. Cells were isolated 48 h after infection and processed for SDS-PAGE and immunoblotting against the indicated proteins (n = 3). C, OVCAR and SKOV3 cells were infected at an m.o.i. of 50 and 100, respectively, with Ad.5-cmv or Ad.5-mda-7. In parallel as indicated, cells were infected with a virus to express dominant-negative p38 MAPK. Twenty-four hours after infection, cells were treated with vehicle or with JNK-IP (10 μM). At the indicated time points in the graphs, cells were isolated, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (±S.E.M., n = 3). D, OVCAR cells were infected with viruses and treated with JNK-IP as in C. Forty-eight hours after infection, cells were isolated, and portions of the lysates were subjected to immunoprecipitation for isolation of activated forms of BAX and BAK. The precipitates were subjected to SDS-PAGE alongside an equal portion of the total cell lysates, and blotting was performed to determine the levels of BAX, BAK, and GAPDH (n = 2). E, OVCAR and SKOVIII cells were infected at an m.o.i. of 50 and 100, respectively, with Ad.5-cmv or Ad.5-mda-7. In parallel as indicated, cells were infected with recombinant adenoviruses to express activated and dominant-negative forms of AKT and MEK1 or with viruses to express apoptosis inhibitory proteins (c-FLIP-s, BCL-XL, dominant-negative caspase 9). Treatment with LY294002 (10 μM) occurred 24 h after virus infection. At the indicated time points in the graphs, cells were isolated, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). F, OVCAR cells were transfected as indicated with expression plasmids (empty vector CMV, dominant-negative PERK, BiP/GRP78, MCL-1) or with siRNA (siSCR, siATG5) and 12 h after transfection, cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 50. Cells were isolated 72 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3).
Prior studies by our laboratories have demonstrated that MDA-7/IL-24-induced activation of PERK was a toxic signal that promoted activation of the JNK pathway as well as a toxic form of autophagy (Yacoub et al., 2008a). In OVCAR cells, Ad.5-mda-7 infection promoted a PERK-dependent vesicularization of a transfected LC3-GFP construct; vesicularization was blocked by knockdown of ATG5 expression (data not shown). Expression of dominant-negative PERK, BiP/GRP78, or knockdown of ATG5 suppressed Ad.mda-7 lethality (Fig. 2F).
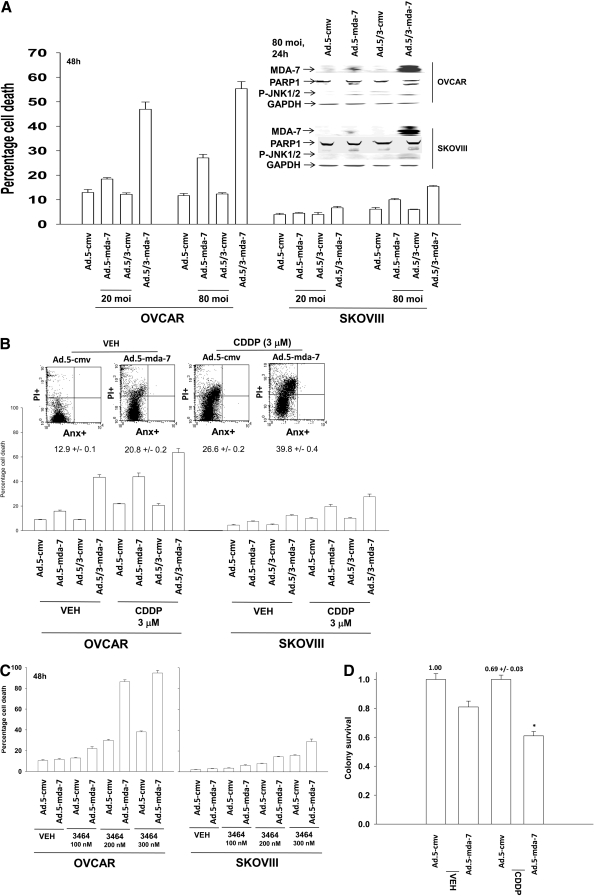
Prior studies from our laboratories, including those in Figs. 1 and 2, have used recombinant type 5 adenoviruses to deliver MDA-7/IL24 to brain cancer cells (Ad.5-mda-7) (Yacoub et al., 2008b). Some OCC tumor cell lines and other tumor cell types such as renal carcinoma and malignant GBM cells are in general not readily amenable to gene therapy approaches using low particle levels of type 5 adenovirus because of their reduced expression of Coxsackie and adenovirus receptor (Park et al., 2009). Because of this, we generated a recombinant adenovirus to express MDA-7/IL24, which contained a modified serotype viral knob from types 5 and 3 adenoviruses (Ad.5/3-mda-7) (Dash et al., 2010). OCCs were poorly killed after exposure to an Ad.5-mda-7 infection at a multiplicity of infection of 20 particles per cell (Fig. 3A). Infection of OCC using an Ad.5/3-mda-7 virus caused significant amounts of cell killing at an m.o.i. of either 20 or 80 (Fig. 3A). These data argue that modified serotype type 5/serotype 3 recombinant viruses may represent useful gene delivery system in ovarian carcinoma.
Fig. 3.
A tropism-modified type 5/type 3 virus infects ovarian cancer cells more readily than a type 5 virus and enhances cisplatin toxicity. A, OVCAR and SKOVIII cells were infected with Ad.5-cmv or Ad.5-mda-7 or with tropism-modified viruses Ad.5/3-cmv or Ad.5/3-mda-7 at an m.o.i. of 20 or 80, as indicated. Cells were isolated 48 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). Inset, cells were infected with the indicated viruses, and 24 h after infection, cells were isolated to determine the expression of MDA-7/IL-24 and the cleavage status of PARP1. B, OVCAR and SKOVIII cells were infected with Ad.5-cmv or Ad.5-mda-7 or with tropism-modified viruses Ad.5/3-cmv or Ad.5/3-mda-7 at an m.o.i. of 80. Twenty-four h after infection, cells were treated with vehicle or cisplatin (CDDP, 3 μM). Seventy-two hours after infection, cells were isolated, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). Inset, identical portions of OVCAR cells, infected as indicated, were isolated 72 h after exposure, and the loss of cell viability was determined by using Annexin V/propidium iodide staining assays in triplicate using a flow cytometer (± S.E.M., n = 3). C, OVCAR and SKOVIII cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80, and 24 h after infection, cells were treated with vehicle or BBR3464 (BBR3464, 100–300 nM). Forty-eight hours after infection, cells were isolated, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). D, OVCAR cells were plated in sextuplicate as single cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80, and 24 h after infection, cells were treated with vehicle or cisplatin (CDDP, 3 μM). The media were changed to drug-free media 48 h after CDDP treatment, and colonies were permitted to form for 10 to 14 days (± S.E.M., n = 2).
Platinum-containing chemotherapeutic drugs are a well established modality for the treatment of ovarian cancer. Infection of tumor cells with Ad.5-mda-7 enhanced the toxicity of cisplatin (Fig. 3B). Data similar to those in OCCs using cisplatin were also observed in primary human GBM cells (Supplemental Fig. S2). Enhanced toxicity of cisplatin, when combined with an oncolytic adenovirus expressing MDA-7/IL-24, has also been observed in hepatocellular carcinoma cells (Wu et al., 2009). BBR3464 is a novel platinum-based drug that has undergone phase II evaluation. In a manner similar to cisplatin, infection of tumor cells with Ad.5-mda-7 enhanced the toxicity of BBR3464 (Fig. 3C). Data similar to those in OCCs using BBR3464 were also observed in primary human GBM cells (Supplemental Fig. S3). In colony-formation assays, Ad.5-mda-7 and cisplatin interacted in a greater than additive fashion to suppress clonogenic survival (Fig. 3D).
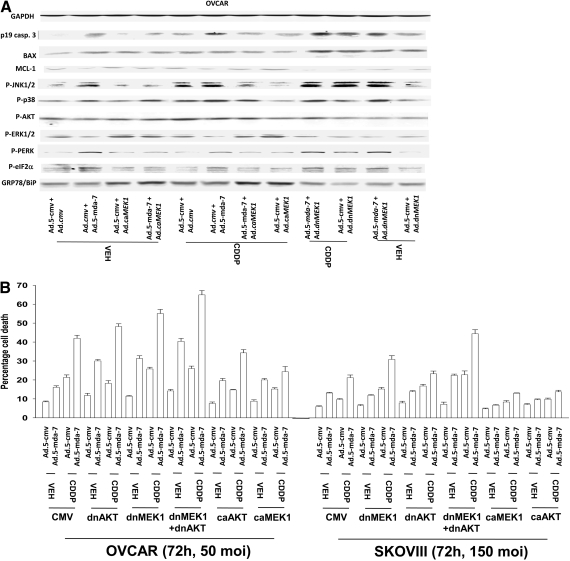
We next determined the impact of Ad.5-mda-7 infection and cisplatin treatment on the activities of survival-signaling kinases and apoptosis-regulatory caspases. Expression of MDA-7/IL-24 promoted the cleavage of caspase 3 and the activation of JNK1/2, p38 MAPK, and PERK (Fig. 4A). Treatment of cells expressing MDA-7/IL-24 with cisplatin caused additional cleavage of caspase 3 and activation of JNK1/2 but not additional activation of PERK. Expression of activated MEK1 EE increased the protein levels of BiP/GRP78 and blocked MDA-7/IL-24-induced activation of PERK. These effects correlated with sustained activation of ERK1/2 and reduced activation of JNK1/2 and of caspase 3. Expression of dominant-negative MEK1 suppressed ERK1/2 activity and BiP/GRP78 levels and enhanced MDA-7/IL-24-induced JNK1/2 and PERK activation and cleavage of caspase 3. Coexpression of dominant-negative MEK1 and dominant-negative AKT significantly enhanced the toxicities of MDA-7/IL-24, cisplatin, and MDA-7/IL-24 + cisplatin treatments (Fig. 4B). Expression of constitutively activated forms of either MEK1 or AKT suppressed MDA-7/IL-24 + cisplatin toxicity. Inhibition of caspase-9 or JNK pathway signaling blocked MDA-7/IL-24 + cisplatin-induced killing (Fig. 4C). Overexpression of BiP/GRP78 also suppressed MDA-7/IL-24 and MDA-7/IL-24 + cisplatin-induced killing as measured in trypan blue dye exclusion assays, which correlated with reduced activation of PERK and sustained expression of MCL-1 and c-FLIP-s (Fig. 4C, inset). Data very similar to those using trypan blue were obtained using Annexin V/PI flow cytometry analyses (Fig. 4D).
Fig. 4.
Ad.5-mda-7 + cisplatin toxicity in OCCs is dependent on PERK, JNK and CD95 signaling. A, OVCAR cells were infected with Ad.5-cmv or Ad.5-mda-7 in parallel with Ad.cmv, Ad.MEK1 EE, or Ad.dnMEK1. Twenty-four hours after infection, cells were treated with vehicle or CDDP (3 μM). Forty-eight hours after infection, cells were isolated and processed for SDS-PAGE and immunoblotting against the indicated proteins (n = 3). B, OVCAR and SKOVIII cells were infected with the indicated viruses at the stated m.o.i., and 24 h after infection, cells were treated with vehicle or cisplatin (CDDP, 3 μM). Cells were isolated 72 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). C, OVCAR cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80 in parallel with viruses to express dominant-negative caspase 9, BCL-XL, or dominant-negative p38 MAPK. In parallel, cells were transfected with empty vector plasmid or plasmid to express Grp78/BiP. Twenty-four hours after infection as indicated, cells were treated with JNK-IP (10 μM) followed by cisplatin treatment (CDDP, 3 μM). Cells were isolated 72 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). Inset, cells infected to express MDA-7/IL-24 and/or BiP/GRP78 were isolated 48 h after infection and processed for SDS-PAGE and blotting to determine the expression of MCL-1, c-FLIP-s, and GAPDH and the phosphorylation of PERK. D, OVCAR cells were infected and treated with cisplatin in a fashion identical with that described in C. Cells were isolated 72 h after infection, and the loss of cell viability was determined by using Annexin V/propidium iodide staining assays in triplicate using a flow cytometer (± S.E.M., n = 3). E, OVCAR cells were infected with caspase 8 inhibitors (c-FLIP-s, CRM A) or dominant-negative p38 MAPK or were transfected to express dominant-negative PERK or to knockdown ceramide synthase 6 (LASS6). Twenty-four hours after infection, cells where indicated were treated with JNK-IP (10 μM). Cells were isolated 72 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). Inset, OVCAR cells growing in glass-chambered slides in triplicate were infected to express MDA-7/IL-24 and were treated 24 h after infection with CDDP (3 μM) for 6 h. Cells were fixed but not permeabilized, and the levels of cell surface CD95 was determined by immunohistochemistry (± S.E.M., n = 2). dn, dominant negative.
In contrast to the minimal effects on altering MDA-7/IL-24 lethality as a single agent, inhibition of caspase-8 function expressing either c-FLIP-s or CRM A prevented cisplatin-enhancing MDA-7/IL-24-induced killing (Fig. 4E). Prior studies in renal cancer cells have shown that ceramide synthase 6 plays a key role in MDA-7/IL-24 lethality, implicating the de novo ceramide synthesis pathway as a mediator of MDA-7/IL-24 effects (Park et al., 2009). Knockdown of ceramide synthase 6 expression suppressed MDA-7/IL-24 and MDA-7/IL-24 + cisplatin lethality (Fig. 4E). Knockdown of ATG5 also suppressed MDA-7/IL-24 + cisplatin-induced killing (Supplemental Fig. S4). Cisplatin treatment activated CD95, and this effect was enhanced by the expression of MDA-7/IL-24 (Fig. 4E, inset). Our data demonstrate that MDA-7/IL-24-induced activation of PERK plays a key role in regulating mitochondrial stability through MCL-1 and that cisplatin enhances MDA-7/IL-24 toxicity via activation of the extrinsic pathway and de novo ceramide synthesis.
Finally, we determined whether Ad.5-mda-7 interacted with another clinically relevant therapeutic agent used in OCC therapy: paclitaxel. In both OVCAR and SKOVIII cells, paclitaxel interacted in an additive fashion to promote Ad.5-mda-7 lethality (Fig. 5A). Cisplatin and paclitaxel interacted to kill OVCAR cells in at least an additive fashion and the drug combination facilitated Ad.5-mda-7 lethality significantly greater than that caused by the cisplatin + Ad.5-mda-7 combination (Fig. 5B). In true percentage terms, Ad.5-mda-7 and cisplatin caused 6.2 ± 0.1% more killing than either agent alone, whereas Ad.5-mda-7 and cisplatin and paclitaxel treatment caused 22.2 ± 2.3% (p < 0.05) more killing than each agent alone. These findings correlated with greater levels of procaspase 3 and PARP1 cleavage (Fig. 5B, top inset). The results also correlated with further increases in BAX levels without any significant further decrease in BCL-XL levels; this suggests that the apoptotic rheostat is further altered toward prodeath signaling by the treatment with paclitaxel. Cisplatin and paclitaxel facilitated Ad.5-mda-7 lethality to kill OVCAR cells in at least a greater than additive fashion (Supplemental Fig. S6). In agreement with the concept that enhanced levels of BAX were causal in the elevated levels of tumor cell killing, knockdown of BAX expression significantly reduced the lethal interaction between Ad.5-mda-7, cisplatin, and paclitaxel (Fig. 5C). Collectively, the data in Figs. 3 to 5 demonstrate that expression of MDA-7/IL-24, via Ad.5-mda-7 or Ad.5/3-mda-7, enhances the toxic effects of established OCC therapeutic drugs in OCCs.
Fig. 5.
Paclitaxel enhances the toxicity of Ad.5-mda-7 + cisplatin in a greater than additive fashion. A, OVCAR and SKOVIII cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80. Twenty-four hours after infection, as indicated, cells were treated with paclitaxel (paclitx., 0–100 nM). Cells were isolated 48 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). B, OVCAR cells were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80. Twenty-four hours after infection, as indicated, cells were treated with paclitaxel (paclitx., 10 nM) and/or cisplatin (CDDP, 3 μM). Cells were isolated 48 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3). Top inset, cells were isolated 24 h after virus infection and processed for SDS-PAGE and immunoblotting against the indicated proteins to determine expression/phosphorylation (n = 2). C, OVCAR cells were transfected with scrambled siRNA (siSCR) or an siRNA to knock down BAX expression and 24 h later were infected with Ad.5-cmv or Ad.5-mda-7 at an m.o.i. of 80. Twenty-four hours after infection, as indicated, cells were treated with paclitaxel (paclitx., 10 nM) and cisplatin (CDDP, 3 μM). Cells were isolated 48 h after infection, and the loss of cell viability was determined by trypan blue exclusion assays in triplicate (± S.E.M., n = 3).
Discussion
Previous studies have noted that GST-MDA-7 (or Ad.5-mda-7) reduces proliferation and causes tumor cell- and transformed cell-specific killing, as well as radiosensitization in malignant glioma, prostate, and ovarian cancer cells (Su et al., 2006; Yacoub et al., 2004, 2008b; Emdad et al., 2006). However, the precise signaling pathways modified by GST-MDA-7 (or Ad.5-mda-7) as a single agent and causally related to its cancer-specific cell killing effects in human ovarian carcinoma cells are still not well understood.
Infection of OCCs with a dose of Ad.5-mda-7 virus particles that caused profound toxicity after ∼72 h correlated with strong activation of the JNK1/2 and p38 MAPK pathways as well as of PERK and eIF2α. This treatment, in parallel, nearly abolished ERK1/2 and AKT signaling. Multiple studies using a variety of cytokine and toxic stimuli document that prolonged JNK1–3 and/or p38 MAPK activation in a wide variety of cell types can trigger cell death (Xia et al., 1995; Matsuzawa et al., 2002; Park et al., 2009). The balance between the readouts of ERK1/2 and JNK1–3 signaling may also represent a common key homeostatic mechanism that regulates cell survival versus cell death processes. Inhibition of JNK1/2 or p38 MAPK reduced MDA-7/IL-24 toxicity, and inhibition of both pathways abolished killing; this was associated with reduced activation of BAX and BAK.
We have published studies arguing that signaling via PERK can promote tumor cell death or alternatively cell survival based on the stimulus (Yacoub et al., 2008a,b; Park et al., 2008). There are three primary unfolded protein response sensors: PERK, activating transcription factor 6, and IRE1. As unfolded proteins accumulate, BiP (GRP78), the 70-kDa heat shock protein ER resident chaperone, dissociates from PERK, activating transcription factor 6, or IRE1. BiP/GRP78 dissociation from PERK allows this protein to dimerize, autophosphorylate, and then phosphorylate eukaryotic translation initiation factor α, the protein required for bringing the initiator methionyl-transfer RNA to the 40S ribosome (Park et al., 2008; Zhang et al., 2008). Phosphorylated eIF2α thus leads to the repression of global translation, helping to allow cells to recover from the accumulation of unfolded proteins. Reduced translation, however, can also lower the expression of some prosurvival proteins such as MCL-1 and c-FLIP-s, as noted in OCCs after the expression of MDA-7/IL-24, leading to increased cell death. MDA-7/IL24 binds to BiP/GRP78, and in OCCs, we hypothesized that this binding would play a central role in regulating PERK activity/activation and regulating MDA-7/IL24-induced cell killing (Gupta et al., 2006a). Indeed, a constitutively active form of MEK1 maintained ERK1/2 phosphorylation in cells expressing MDA-7/IL-24, and increased BiP/GRP78 expression and blocked PERK activation. Overexpression of BiP/GRP78 blocked MDA-7/IL-24–induced PERK activation, and expression of dominant-negative PERK blocked MDA-7/IL-24-induced activation of JNK1/2 as well as reduced expression of MCL-1. Thus, in OCCs, MDA-7/IL-24, by binding to BiP/GRP78, results in the disruption of the BiP/GRP78-PERK chaperone interaction, resulting in enhanced PERK signaling into the eIF2α and JNK downstream pathways that act to promote mitochondrial dysfunction via decreased protective BCL-2 family protein expression and increased activity of toxic BH3 domain proteins, respectively.
In one ovarian cancer cell line, MDA-7/IL-24 lethality has been shown previously to be mediated by CD95-caspase-8 signaling, indicating that the extrinsic pathway to apoptosis could be activated by this cytokine (Gopalan et al., 2005). However, in many of our prior analyses with MDA-7/IL24 in breast, prostate, pancreatic, and brain tumor cells, as well as from the work of others, it was noted that MDA-7/IL-24-induced cell death was mediated by the disruption of mitochondrial function with little or no involvement of death receptors or caspase-8 in the killing process. Our present studies suggest that in SKOVIII cells, but not OVCAR cells, extrinsic pathway signaling also plays a role in MDA-7/IL-24 lethality. In renal carcinoma cells, we have noted recently that overexpression of c-FLIP-s or the caspase-8 inhibitory protein CRM A blocked GST-MDA-7 lethality, and knockdown of CD95 or FADD expression also reduced GST-MDA-7 toxicity (Park et al., 2009).
In addition to JNK pathway-mediated activation of BAX and BAK, our findings in OVCAR cells also argued that MDA-7/IL-24 caused altered ratios in the total expression of proapoptotic BH3 domain-containing proteins, particularly BAX, and antiapoptotic proteins, such as MCL-1 and BCL-XL, with the subsequent activation of caspases-9 and -3; this finding is similar to data obtained by expressing MDA-7/IL-24 in other tumor cell types (e.g., LNCaP prostate cancer cells) (Fisher, 2005). In other prostate cancer cell types, such as DU-145, which lack the expression of BAX, Ad.5-mda-7 is an even more potent inducer of tumor cell death than is observed in LNCaP cells. In GBM cells, our prior data argued that at least five BH3 domain-containing proteins could potentially mediate GST-MDA-7 toxicity downstream of GST-MDA-7-stimulated activation of PERK and JNK1–3 (Yacoub et al., 2008a,c). Together, based on these findings, it is probable that the reason why multiple transformed cell types exhibit MDA-7/IL-24 toxicity regardless of their genetic background is because of the pleiotropic range of proapoptotic proteins and pathways that can be recruited by this cytokine to initiate mitochondrial cell death processes.
Platinum-based chemotherapeutic drugs are a mainstay of ovarian cancer therapy (Coleman and Sood, 2006). MDA-7/IL-24 and cisplatin, as well as the novel platinum-containing drug BBR3464, interacted in a greater than additive fashion to kill OCCs in vitro via CD95. In prior work, treating primary hepatocytes with low doses of bile acids, which cause ligand-independent activation of CD95, we discovered that JNK1/2 activation was CD95-dependent, and our recent studies in renal carcinoma cells also demonstrated that JNK1/2 and, to a lesser extent, p38 MAPK activation after GST-MDA-7 treatment required CD95 signaling. In OCCs, cisplatin further enhanced MDA-7/IL-24-induced JNK1/2 activation, and this enhanced JNK1/2 activation was required for the toxic interaction between the two agents. MDA-7/IL-24-induced PERK activation was required for MDA-7/IL-24 and MDA-7/IL-24 + cisplatin-induced JNK1/2 and p38 MAPK activation. Matsuzawa et al., (2002) have implicated previously a TRAF2-ASK1-JNK cascade downstream of IRE1 in ER stress responses in multiple cell types, and based on our data, PERK-dependent signaling could also feed into this cell survival regulatory process. Further studies are necessary to determine whether the enhanced activity of MDA-7/IL24, when administered by an oncolytic adenovirus, with cisplatin in the context of hepatocellular carcinoma (Wu et al., 2009) involve similar signaling pathway alterations as observed in OCCs.
In our recent studies treating primary hepatocytes with bile acids; in RCC/HCC/pancreatic tumor cells with the drugs sorafenib and vorinostat; and in renal carcinoma cells treated with GST-MDA-7, we discovered that ligand-independent activation of CD95 was dependent in part on the actions of ASMase and the de novo ceramide synthesis pathway (Park et al., 2008; Zhang et al., 2008). Recent studies have suggested that the de novo and ASMase pathways of ceramide generation can cooperate to regulate lipid raft function (Zhang et al., 2009); cisplatin was shown to promote CD95 activation and that ceramide generation may play a role in this process (Min et al., 2007). Determining the molecular mechanisms by which MDA-7/IL-24 enhances cisplatin-induced activation of CD95 and whether cisplatin-induced DNA damage increases expression of ceramide synthase genes require studies beyond the scope of the present article.
The studies in this article were also engaged to determine whether we could enhance the infectivity of adenoviral gene transduction in OCCs, using a chimeric adenovirus containing the knob protein expressing portions of the types 5 and 3 adenoviruses. A type 5/3 recombinant adenovirus was more efficacious at delivering mda-7/IL-24 to tumor cells than a type 5 virus, resulting in greater expression of MDA-7/IL-24. The greater expression of MDA-7/IL-24 using the type 5/3 virus resulted in a greater amount of tumor cell killing, as observed using this virus in low Coxsackie and adenovirus receptor prostate cancer cells (Dash et al., 2010). It is noteworthy that whereas infection using Ad.5/3-mda-7 generated at least 10-fold more MDA-7/IL-24 protein, it only enhanced apoptosis ∼3-fold more than Ad.5-mda-7 infection. This argues that there is a certain threshold at which MDA-7/IL-24 becomes toxic to a tumor cell, and producing more MDA-7/IL-24 may not per se increase killing. We have observed a similar dose-dependent effect of GST-MDA-7 on GBM and renal carcinoma cells in vitro (Yacoub et al., 2008a; Park et al., 2009). However, based on the pleiotropic antitumor effects of MDA-7/IL-24 in vivo, including the inhibition of tumor angiogenesis, potent “bystander” anticancer activity and immune modulating activity, having enhanced the expression of MDA-7/IL-24, may directly contribute to and enhance the anticancer properties of this cytokine in vivo (Fisher, 2005; Sarkar et al., 2005, 2008; Sauane et al., 2008; Emdad et al., 2009; Greco et al., 2009).
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Cancer Institute [Grants P01-CA104177, R01-CA108325, R01-CA63753, R01-CA77141, R01-CA097318, R01-CA12764101]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK52825]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant P01-NS031492]; the V Foundation; and The Samuel Waxman Cancer Research Foundation.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061820
- MDA-7/IL-24
- melanoma differentiation associated gene-7/interleukin-24
- ERK
- extracellular signal-regulated kinase
- MEK
- mitogen-activated extracellular regulated kinase
- JNK
- c-Jun NH2-terminal kinase
- PERK
- protein kinase R-like endoplasmic reticulum kinase
- MAPK
- mitogen-activated protein kinase
- IL
- interleukin
- OCC
- ovarian cancer cell
- GFP
- green fluorescent protein
- ER
- endoplasmic reticulum
- GST
- glutathione transferase
- PAGE
- polyacrylamide gel electrophoresis
- m.o.i.
- multiplicity of infection
- CDDP
- cisplatin [cis-diamminedichloroplatinum(II)]
- FBS
- fetal bovine serum
- GBM
- glioblastoma
- GRP78
- 78-kDa glucose regulated protein
- PARP1
- poly(ADP-ribose) polymerase 1
- siRNA
- small interfering RNA
- IP
- inhibitory peptide
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- MCL-1
- myeloid cell leukemia sequence 1
- IRE1
- inositol requirement 1
- BBR3464
- (SP-4–1)-diamminebis((SP-4–2)-diamminechloroplatinum(π) (μ-hexane-1,6-diamine))platinum tetranitrate
- LY294002
- 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
- eIF2α
- eukaryotic initiation factor 2α.
References
- Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA. (2002) The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol 168: 6041–6046 [DOI] [PubMed] [Google Scholar]
- Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, Zheng M. (2005) mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther 11: 724–733 [DOI] [PubMed] [Google Scholar]
- Coleman RL, Sood AK. (2006) Historical progress in the initial management of ovarian cancer: intraperitoneal chemotherapy. Curr Oncol Rep 8: 455–464 [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, et al. (2005) Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther 11: 149–159 [DOI] [PubMed] [Google Scholar]
- Dash R, Dmitriev IP, Su ZZ, Bhutia SJ, Azab B, Vozhilla N, Yacoub A, Dent P, Curiel DT, Sarkar D, et al. (2010) Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther, in press [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, Chada S, Grimm EA. (2001) Down-regulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int J Cancer 94: 54–59 [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA. (2002) Loss of MDA-7 expression with progression of melanoma. J Clin Oncol 20: 1069–1074 [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D, et al. (2009) Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther 8: 391–400 [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Lebedeva IV, Su ZZ, Gupta P, Mahasreshti PJ, Dent P, Curiel DT, Fisher PB. (2006) Ionizing radiation enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human ovarian cancer. J Cell Physiol 208: 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PB. (2005) Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res 65: 10128–10138 [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. (2003) mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther 2: S23–37 [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, et al. (2007) Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol 224: 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R. (2005) Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells. Cancer Res 65: 3017–3024 [DOI] [PubMed] [Google Scholar]
- Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, et al. (2009) Eradication of therapy-resistant human prostate tumors using an ultrasound guided site-specific cancer terminator virus delivery approach. Mol Ther doi: 10.1038/mt.2009.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. (2000) Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 106: 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, et al. (2006a) mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther 111: 596–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Walter MR, Su ZZ, Lebedeva IV, Emdad L, Randolph A, Valerie K, Sarkar D, Fisher PB. (2006b) BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res 66: 8182–8191 [DOI] [PubMed] [Google Scholar]
- Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ, Alexandre D, et al. (2001) Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene 20: 7051–7063 [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. (1995) Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11: 2477–2486 [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, Reed JC, Fisher PB. (2003) Bcl-2 and Bcl-xL differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene 22: 8758–8773 [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, et al. (2005) mda-7/IL-24: exploiting cancer's Achilles' heel. Mol Ther 11: 4–18 [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. (2002) The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene 21: 708–718 [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. (2002) Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal 4: 415–425 [DOI] [PubMed] [Google Scholar]
- Mhashilkar AM, Stewart AL, Sieger K, Yang HY, Khimani AH, Ito I, Saito Y, Hunt KK, Grimm EA, Roth JA, et al. (2003) MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells. Mol Ther 8: 207–219 [DOI] [PubMed] [Google Scholar]
- Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (2007) (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res 5: 801–812 [DOI] [PubMed] [Google Scholar]
- Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, Sarkar D, Rahmani M, Graf M, Yacoub A, et al. (2009) MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer Ther 8: 1280–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, et al. (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 7: 1648–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, et al. (2002) Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem 277: 47517–47523 [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. (2004) Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22: 929–979 [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, Curiel DT, Fisher PB. (2007) Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res 67: 5434–5442 [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. (2002) mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A 99: 10054–10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, Fisher PB. (2008) A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther 15: 293–302 [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. (2005) Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A 102: 14034–14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, Dent P, Fisher PB. (2004) Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene 23: 7679–7690 [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. (2008) Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci U S A 105: 9763–9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt MR, Depass AL, Sarkar D, Fisher PB. (2009) Model cell culture system for defining the molecular and biochemical events mediating terminal differentiation of human melanoma cells. J Cell Physiol 218: 304–314 [DOI] [PubMed] [Google Scholar]
- Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. (2001) A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A 98: 10332–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, Dent P, Reed JC, Fisher PB. (2006) Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells over-expressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene 25: 2339–2348 [DOI] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. (1998) The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci U S A 95: 14400–14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YM, Zhang KJ, Yue XT, Wang YQ, Yang Y, Li GC, Li N, Wang YG. (2009) Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol Sin 30: 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331 [DOI] [PubMed] [Google Scholar]
- Yacoub A, Gupta P, Park MA, Rhamani M, Hamed H, Hanna D, Zhang G, Sarkar D, Lebedeva IV, Emdad L, et al. (2008a) Regulation of GST-MDA-7 toxicity in human glioblastoma cells by ERBB1, ERK1/2, PI3K, and JNK1–3 pathway signaling. Mol Cancer Ther 7: 314–329 [DOI] [PubMed] [Google Scholar]
- Yacoub A, Hamed H, Emdad L, Dos Santos W, Gupta P, Broaddus WC, Ramakrishnan V, Sarkar D, Shah K, Curiel DT, et al. (2008b) MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther 7: 917–933 [DOI] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, Linehan WM, Su ZS, Sarkar D, Lebedeva IV, et al. (2003) MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther 2: 623–632 [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Hong Y, Gopalkrishnan RV, Su ZZ, Gupta P, Sauane M, Lebedeva IV, Curiel DT, Mahasreshti PJ, et al. (2004) MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther 3: 739–751 [DOI] [PubMed] [Google Scholar]
- Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, Hanna D, Sarkar D, Lebedeva IV, Emdad L, et al. (2008c) Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther 7: 297–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Park MA, Mitchell C, Walker T, Hamed H, Studer E, Graf M, Rahmani M, Gupta S, Hylemon PB, et al. (2008) Multiple cyclin kinase inhibitors promote bile acid-induced apoptosis and autophagy in primary hepatocytes via p53-CD95-dependent signaling. J Biol Chem 283: 24343–24358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Becker KA, Gulbins E. (2009) Ceramide-enriched membrane domains—structure and function. Biochim Biophys Acta 1788: 178–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.