Abstract
Anterior pituitary cells fire action potentials and release cyclic nucleotides both spontaneously and in response to agonist stimulation, but the relationship between electrical activity and cyclic nucleotide efflux has not been studied. In these cells, a tetrodotoxin-resistant background N+ conductance is critical for firing of action potentials, and multidrug resistance proteins (MRPs) MRP4 and MRP5 contribute to cyclic nucleotide efflux. Here, we show that abolition of the background Na+ conductance in rat pituitary cells by complete or partial replacement of extracellular Na+ with organic cations or sucrose induced a rapid and reversible hyperpolarization of cell membranes and inhibition of action potential firing, accompanied by a rapid inhibition of cyclic nucleotide efflux. Valinomycin-induced hyperpolarization of plasma membranes also inhibited cyclic nucleotide efflux, whereas depolarization of cell membranes induced by the inhibition of Ca2+ influx or stimulation of Na+ influx by gramicidin was accompanied by a facilitation of cyclic nucleotide efflux. In contrast, inhibition of cyclic nucleotide efflux by probenecid did not affect the background Na+ conductance. In human embryonic kidney 293 cells stably transfected with human MRP4 or MRP5, replacement of bath Na+ with organic cations also hyperpolarized the cell membranes and inhibited cyclic nucleotide efflux. In these cells, the Na+/H+ antiporter monensin did not affect the membrane potential and was practically ineffective in altering cyclic nucleotide efflux. In both pituitary and MRP4- and MRP5-expressing cells, 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid (MK571) inhibited cyclic nucleotide efflux. These results indicate that the MRP4/5-mediated cyclic nucleotide efflux can be rapidly modulated by membrane potential determined by the background Na+ conductance.
Intracellular cAMP and cGMP concentrations reflect the balance between the rates of their synthesis and elimination. Synthesis of cAMP from ATP is mediated by adenylyl cyclases (ACs), a family of nine plasma membrane-bound enzymes (Willoughby and Cooper, 2007). The production of cGMP from GTP is controlled by both the membrane-bound and soluble guanylyl cyclases (sGCs) (Lucas et al., 2000; Russwurm and Koesling, 2005). On the other hand, phosphodiesterases (PDEs) provide an effective mechanism for the elimination of cyclic nucleotides (Bender and Beavo, 2006). Cyclic nucleotide efflux pathways also contribute to the control of intracellular cAMP and cGMP levels. The multidrug resistance proteins MRP4 (Chen et al., 2001; Lai and Tan, 2002), MRP5 (Jedlitschky et al., 2000), and MRP8 (Guo et al., 2003), also known as ATP binding cassette transporters ABCC4, ABCC5, and ABCC11 (Ritter et al., 2005), have been identified as ATP-dependent export pumps that can also transport cyclic nucleotides, as can the organic anion transporter 2 (SLC22A7) (Cropp et al., 2008). Although some investigators have proposed that cells use these cyclic nucleotide efflux pumps to help actively control their cAMP and cGMP intracellular concentrations (Kruh and Belinsky, 2003; Sager, 2004), this idea has been questioned by others because of the substantial energetic cost of this pathway (Bankir et al., 2002). The uncertainty regarding the participation of MRPs in the control of cyclic nucleotide signaling in part reflects the incomplete knowledge surrounding the nature of their regulation.
In this study, we examined whether and by which mechanism cyclic nucleotide efflux by MRPs can be rapidly regulated. As a cell model for such studies, we selected anterior pituitary cells. Pituitary functions are carried out by five cell types, defined by the hormones they produce and secrete: corticotrophs secrete adrenocorticotropic hormone; thyrotrophs secrete thyroid-stimulating hormone; somatotrophs secrete growth hormone; lactotrophs secrete prolactin; and gonadotrophs secrete luteinizing hormone and follicle-stimulating hormone. Several subtypes of ACs are expressed in the anterior pituitary cells and are responsible for basal and hormone-stimulated cAMP synthesis (Antoni et al., 2003; Gonzalez-Iglesias et al., 2006). Pituitary cells also express the α1β1-sGC dimer, which represents the major pathway for basal cGMP production (Kostic et al., 2001), as well as numerous PDEs (Persani et al., 2001; Ang and Antoni, 2002). Finally, basal cAMP and cGMP release in unstimulated cells is detectable in cells perfused at a flow rate of 0.5 to 1 ml/min, which provides the possibility of studying the dynamics of cyclic nucleotide efflux under different experimental conditions. The same study also indicated the involvement of MRPs in cyclic nucleotide efflux in pituitary cells (Andric et al., 2006).
A common characteristic of normal and immortalized pituitary cell types is spontaneous firing of calcium-dependent and tetrodotoxin (TTX)-insensitive action potentials (APs). Spontaneous excitability in these cells is facilitated by the activation of ACs but not sGC (Gonzalez-Iglesias et al., 2006) and reflects the expression of numerous voltage-gated and ligand-gated channels (Stojilkovic et al., 2005). In lactotrophs and immortalized GH3 pituitary cells, the baseline potential and firing of APs are critically dependent on a TTX-resistant background Na+ (Nab) conductance (Simasko, 1994; Sankaranarayanan and Simasko, 1996). Here, we studied the role of both TTX-sensitive and -insensitive Na+ conductance on spontaneous electrical activity and cyclic nucleotide efflux. We also used nonexcitable human embryonic kidney (HEK) 293 cells stably transfected with human MRP1, MRP4, and MRP5 to study the dependence of cyclic nucleotide efflux on bath Na+.
Materials and Methods
Materials.
Goat polyclonal antibody against MRP5 (P-20) and normal goat serum were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and M4I-80 antibody to detect MRP4 was from Kamiya Biomedical (Tukwila, WA). The secondary antibody, horseradish peroxidase-conjugated rabbit anti-goat antibody, was obtained from Kirkegaard and Perry Laboratories (Gaithersburg, MD). All tissue culture supplies were obtained from Invitrogen (Carlsbad, CA). Specific cyclic nucleotide antisera were provided by Albert Baukal (National Institute of Child Health and Human Development, Bethesda, MD) and 125I-cAMP and 125I-cGMP tracers were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). If not otherwise stated, all drugs and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and Cell Culture.
Experiments were performed on anterior pituitary cells from normal postpubertal female Sprague-Dawley rats obtained from Taconic Farms (Germantown, NY). Euthanasia was performed by asphyxiation with CO2, and the anterior pituitary glands were removed after decapitation. Experiments were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. Anterior pituitary cells were mechanically dispersed after treatment with trypsin and were cultured as mixed cells or enriched lactotrophs in medium 199 containing Earle's salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). A two-stage Percoll discontinuous density gradient procedure was used to obtain enriched lactotrophs. Further identification in single-cell studies was achieved by the addition of dopamine and thyrotropin-releasing hormone (for lactotrophs), gonadotropin-releasing hormone (for gonadotrophs), and GHRH (for somatotrophs). Immortalized GH3 pituitary cells were cultured in Ham's F-12K medium supplemented with 15% heat-inactivated horse serum, 2.5% fetal bovine serum, and gentamicin (100 μg/ml). Parental HEK293 cells, HEK293/5I cells transduced with MRP5 cDNA (Wijnholds et al., 2000), and the MRP4-overexpressing HEK293/4.63 cells (Wielinga et al., 2002) were generous gifts of P. Borst (Division of Molecular Biology and Centre for Biomedical Genetics, The Netherlands Cancer Institute, Amsterdam, the Netherlands). All HEK293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 U of penicillin/streptomycin per milliliter (Invitrogen) at 37°C in 5% CO2 humidified air as described previously (Reid et al., 2003).
Cyclic Nucleotide Measurements.
Anterior pituitary cells (0.25 million/well) and HEK293 cells (0.5 million/well) were plated in 24-well plates and incubated overnight at 37°C under 5% CO2/air and saturated humidity. The following day, medium was removed, and cells were washed and then bathed in 0.1% bovine serum albumin-containing medium under 5% CO2/air and saturated humidity for 15 min. Cyclic nucleotides were measured in incubation medium and in cell extracts. Cyclic nucleotide release was also monitored using cell column perfusion experiments. In brief, 1.5 × 107 pituitary cells or 7.5 × 106 HEK293 cells were incubated with preswollen Cytodex-1 beads (Sigma-Aldrich) in 60-mm Petri dishes for 20 h. The beads were then transferred to 0.5-ml chambers and perfused with Krebs-Ringer medium containing 25 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 0.1% bovine serum albumin, and penicillin (100 U/ml)/streptomycin (100 μg/ml) for 2 h at a flow rate of 0.5 ml/min and at 37°C to establish stable basal secretion. In both static cultures and perfusion experiments, the media were supplemented with 1 mM 3-isobutyl-1-methylxanthine (IBMX) to inhibit PDEs. For sodium-free experiments, NaCl was replaced at a 1:1 ratio by N-methyl-d-glucamine (NMDG), tetramethylammonium (TMA), choline chloride, or sucrose. The osmolarity of the solutions was maintained at 290 to 300 mOsm as determined by a vapor pressure osmometer (VAPRO 5520; Wescor, Logan, UT). Fractions were collected in 1-min intervals, stored at −20°C, and later assayed for cAMP and cGMP contents using radioimmunoassay.
Electrophysiological Measurements.
For electrophysiological recordings, cells were plated on poly(l-lysine)-coated coverslips (15 mm in diameter) at densities of 105 primary cells per coverslip and 104 immortalized cells per coverslip. The cells were then cultured for 1 to 3 days before recording. All recordings were performed at room temperature using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The amphotericin-perforated patch-clamp technique was used to record membrane potentials and whole-cell currents. Cells were continuously perfused with an extracellular solution containing 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose. The pH was adjusted to 7.4 with NaOH. For sodium-free experiments, NaCl was replaced at a 1:1 ratio by NMDG, TMA, or choline chloride. Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) and heat-polished to a tip resistance of 5 to 7 MΩ. Pipette solution contained 90 mM potassium aspartate, 50 mM KCl, 3 mM MgCl2 and 10 mM HEPES. Final pH was adjusted to 7.2 with KOH. Before measurement, amphotericin B was added to the pipette solution from a stock solution (20 mg/ml in dimethyl sulfoxide) to obtain a final concentration of 200 μg/ml. Recordings started when series resistance decreased below 100 MΩ for current-clamp or below 40 MΩ for voltage-clamp recordings. Series resistance was compensated to more than 60%. Drugs dissolved to a final concentration in extracellular solutions were delivered to the recording chamber by a gravity-driven microperfusion system RSC-200 (Bio-Logic USA, Knoxville, TN).
Immunoprecipitation and Western Hybridization.
HEK293 cells were harvested, lysed, and fractionated on a 7.5% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane as described previously (Wu et al., 2005). Each nitrocellulose membrane was incubated for 1 h in blocking buffer (5% milk powder in 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.1% Tween 20). Western hybridization was performed using primary antibodies M4I-80 (1:200) to detect MRP4 and H-100 (1:200) antibody to detect MRP5. The secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit antibody (1:10,000 dilution). Signals were developed using the SuperSignal West Pico Luminol kit (Pierce, Rockford, IL) or the enhanced chemiluminescence kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and exposed to X-ray film.
Results
Effects of Replacement of Bath Na+ with NMDG on Electrical Activity and Cyclic Nucleotide Efflux.
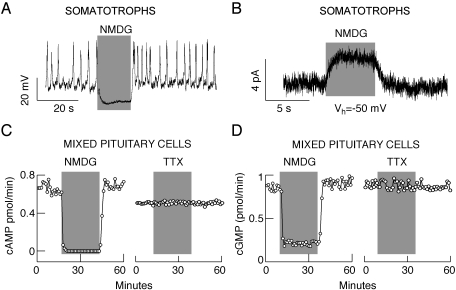
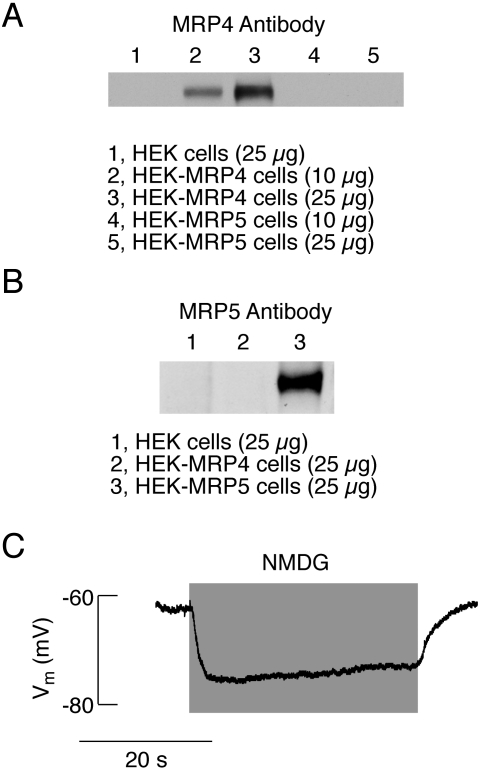
All secretory anterior pituitary cells fire APs. Figure 1A shows spontaneous electrical activity in somatotrophs, and Fig. 2 illustrates the pattern of AP firing in gonadotrophs, lactotrophs, and immortalized GH3 cells. In these cell types, replacement of extracellular Na+ with NMDG led to an instantaneous hyperpolarization of cell membranes that was associated with a cessation of spontaneous firing of APs. Similar effects were also observed in other unidentified pituitary cells, indicating that the dependence of the baseline membrane potential on an Nab conductance is not a unique feature of prolactin-secreting cells (Simasko, 1994; Sankaranarayanan and Simasko, 1996) but represent a common feature of secretory anterior pituitary cells.
Fig. 1.
Spontaneous electrical activity of pituitary cells and basal cyclic nucleotide efflux depend on a background Na+ conductance. A and B, effects of complete replacement of extracellular Na+ with NMDG on electrical activity (A) and whole-cell current (B) in pituitary somatotrophs bathed in IBMX-containing medium. In this and the following figures, traces shown are representative of at least five recordings per experiment. C and D, effects of complete replacement of bath Na+ with NMDG on cAMP (C, left) and cGMP (D, left) efflux and the lack of effects of TTX on cyclic nucleotide efflux (right). Mean ± S.E.M. values are shown under Results and in Table 1. In this and the following figures, gray areas indicate the duration of treatments. Vh, holding potential.
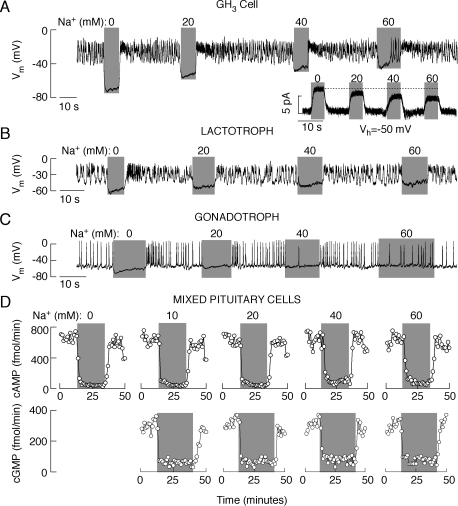
Fig. 2.
Patterns of spontaneous electrical activity and cAMP efflux are determined by Na+ gradient. A to C, effects of partial substitution of bath Na+ with NMDG on the level of hyperpolarization and pattern of electrical activity in GH3 cells (A), lactotrophs (B), and gonadotrophs (C). Inset, the effect of different concentrations of bath Na+ on membrane holding current in GH3 cells clamped at −50 mV. D, Effect of partial substitution of extracellular Na+ with NMDG on cAMP (top) and cGMP (bottom) efflux in perifused pituitary cells in primary culture. During the treatment, the bath perfusion solution was changed from 145 mM Na+ to media containing the Na+ concentrations indicated above gray areas, with the residual substituted with NMDG.
In further experiments, cells were clamped at −50 mV, which was close to the resting potentials from which the slow depolarization and firing of APs occurred in these cells. Under these recording conditions, replacement of extracellular Na+ with NMDG resulted in an outward-like current, which reflected a decrease in the holding membrane current. This response was observed in all pituitary cells and indicated a loss of inward-depolarizing Nab conductance. These experiments were performed in cells bathed in IBMX-containing (Fig. 1A) and IBMX-free medium (Fig. 2, A–C), suggesting that the resting Na+ conductance is not dependent on the intracellular cyclic nucleotide levels.
We also analyzed the effects of replacing bath Na+ with NMDG on cyclic nucleotide efflux. The rate of cAMP release by cells perfused with Na+-containing physiological buffer was 598 ± 51 fmol/min (92 columns in 23 experiments), whereas the rate for cGMP was 874 ± 57 fmol/min (34 columns in 17 experiments). In parallel to electrical activity, the substitution of Na+ with NMDG was immediately associated with a blockade of cAMP release (Fig. 1C, left, and Table 1). On the other hand, TTX treatment was ineffective (Fig. 1C, right), indicating that Na+ influx through voltage-insensitive channels accounts for high basal cyclic nucleotide efflux. Measurements of cGMP levels in the same samples also revealed the presence of high and steady release of this nucleotide, which was abolished by replacing extracellular Na+ with NMDG (Fig. 1D, left, and Table 1) but not by adding TTX (Fig. 1D, right). As a further parallel to changes in resting membrane potential, cAMP and cGMP release recovered after switching the perifusion to Na+-containing extracellular medium.
TABLE 1.
Effect of the replacement of bath Na+ with organic cations and sucrose on basal cAMP and cGMP levels in perifused pituitary cells
Results shown are means ± S.E.M. values of basal cyclic nucleotide efflux in cells. Numbers in parentheses indicate the number of independent experiments (cell preparations done on different days), each performed in two to four columns. The individual values were derived from measurements done in samples collected during 15- to 30-min treatments. All experiments were performed in media containing 1 mM IBMX.
| Treatment | cAMP | cGMP |
|---|---|---|
| fmol/min | ||
| Sodium | 598 ± 5 (23) | 874 ± 57 (19) |
| NMDG | 41 ± 5 (9)* | 73 ± 6 (9)* |
| Choline | 82 ± 7 (5)* | 99 ± 11 (5)* |
| TMA | 121 ± 10 (5)* | 147 ± 13 (5)* |
| Sucrose | 136 ± 9 (5)* | 145 ± 18 (5)* |
P < 0.01; significant difference vs. sodium treatment (control) as estimated by the Student's t test.
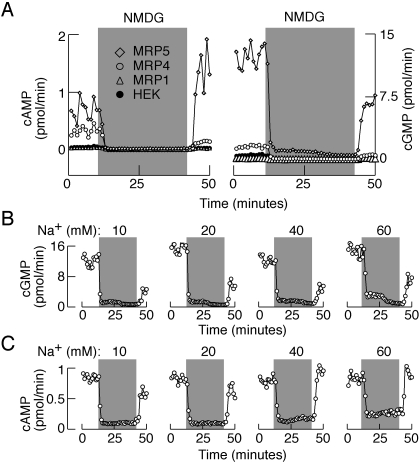
In additional experiments, electrical activity and basal cAMP efflux were recorded in cells perfused with media containing different ratios of NMDG and Na+, whereas the total amount of these cations was kept at 145 mM. In GH3 cells and lactotrophs, extracellular concentrations of up to 60 mM Na+ were not sufficient to preserve the resting potential and firing of APs (Fig. 2, A and B). Consistent with these observations, for GH3 cells held at −50 mV, the amplitudes of outward-like current progressively decreased with increased extracellular Na+ concentrations, but the currents were still not abolished in cells bathed in medium containing 60 mM Na+ (Fig. 2A, inset). There was a partial recovery of spontaneous electrical activity in gonadotrophs bathed in 60 mM Na+-containing medium but not in cells bathed in 20 and 40 mM Na+-containing NMDG buffer (Fig. 2C). Efflux of cAMP (Fig. 2D, top) and cGMP (Fig. 2D, bottom) was also inhibited in mixed anterior cells perifused with medium containing 0 to 60 mM Na+.
Relationship between Intracellular and Released cAMP.
The rapid abolition of cyclic nucleotide efflux in cells bathed in NMDG-containing medium could indicate that the basal AC activity was inhibited, thereby resulting in intracellular cyclic nucleotide levels lower than the threshold needed for the activation of the cyclic nucleotide pump, or that the transporter was inhibited independently of the intracellular cyclic nucleotide levels. To clarify this issue, we measured intracellular cAMP and cGMP levels in pituitary cells in static cultures bathed in different media. The intracellular cAMP contents in cells bathed in physiological Na+-containing and NMDG-containing media for 15 min were comparable: Na+ = 1056 ± 72 versus NMDG = 1148 ± 76 fmol/106 cells, n = 6. The intracellular cGMP levels were also comparable in both groups: Na+ = 452 ± 44 versus NMDG = 528 ± 64 fmol/106 cells, n = 6. These results indicate that the abolition of Nab conductance by substituting extracellular Na+ with organic cations inhibited the cyclic nucleotide efflux transporter independently of the status of AC and sGC activities.
Effects of Replacing Bath Na+ with TMA, Choline, and Sucrose on Cyclic Nucleotide Efflux.
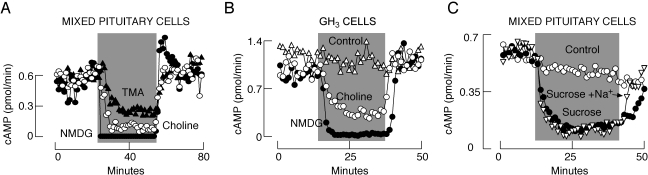
As in the experiments with NMDG, replacement of bath Na+ with choline and TMA resulted in the inhibition of both spontaneous electrical activity (data not shown) and cyclic nucleotide efflux in a reversible manner (Fig. 3). However, choline and TMA were less effective at inhibiting basal cAMP efflux in normal and immortalized pituitary cells than NMDG. This is manifested by the rates of inhibition (Fig. 3) and the steady-state levels reached after 15-min application of organic cations (Table 1). The washout of choline and TMA was accompanied by the full recovery of cyclic nucleotide efflux. Complete and partial replacement of bath Na+ with sucrose also blocked basal cAMP efflux (Fig. 3C and Table 1), indicating that organic cations did not directly inhibit the cyclic nucleotide efflux transporter.
Fig. 3.
Organic cations and sucrose inhibit cAMP efflux. A and B, inhibition of cAMP efflux in normal (A) and immortalized (B) pituitary cells by complete replacement of bath Na+ with NMDG, TMA, and choline. C, inhibition of cAMP efflux in pituitary cells by complete (270 mM sucrose) or partial (75 mM Na+ and 120 mM sucrose) replacement of bath Na+ with sucrose. Mean ± S.E.M. values are shown in Table 1.
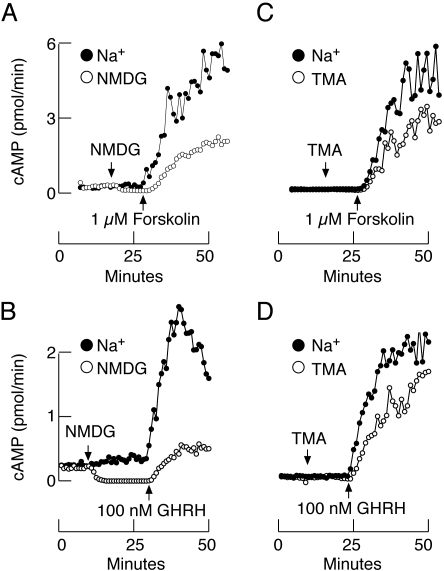
In further experiments, we examined the effects of replacing bath Na+ with organic cations on stimulated cAMP efflux. Our earlier studies showed that the addition of forskolin and GHRH increased cAMP production in a time- and concentration-dependent manner. This was accompanied by a significant increase in cAMP efflux (Andric et al., 2006). Figure 4, A and C, illustrates the effects of 1 μM forskolin on cAMP release. In both experiments, forskolin-induced facilitation of cAMP release was inhibited by replacing the extracellular Na+ with organic cations, but the relative level of inhibition of cAMP efflux was more pronounced in NMDG-perifused cells than in TMA-perifused cells. Activation of Gs-coupled GHRH receptors was also associated with a large increase in cAMP release, which was dramatically reduced in cells bathed in NMDG-containing medium and less notably so in TMA-treated cells (Fig. 4, B and D). These experiments confirmed the dependence of basal and agonist-induced cyclic nucleotide efflux on the status of Nab current in pituitary cells.
Fig. 4.
Stimulated cAMP efflux also depends on the background Na+ conductance. Effect of complete replacement of extracellular Na+ with NMDG and TMA on cAMP release in forskolin- (A and C) and GHRH (B and D)-stimulated cells. In our preparation of mixed populations of pituitary cells, approximately 40% of cells express GHRH receptors (somatotrophs). Data shown are representative of three similar experiments.
Role of MRPs in Cyclic Nucleotide Efflux.
Our earlier studies revealed that mRNA transcripts for MRP4 and MRP5 are expressed in pituitary cells (Andric et al., 2006). To more directly study the impact of replacing bath Na+ with organic cations on cyclic nucleotide efflux, in further experiments we used HEK293 cells stably expressing MRP4 and MRP5, and untransfected and MRP1-transfected HEK293 cells as controls. The overexpression of MRP4 and MRP5 in these cells is shown in Fig. 5, A and B. Like in pituitary cells (Fig. 2), replacement of bath Na+ with NMDG led to a rapid hyperpolarization of HEK293 cell membranes in a reversible manner (Fig. 5C). Together, these data indicate that HEK293 cells expressing recombinant human MRPs represent a good cell model for studying the relationship between the background Na+ influx and MRP-mediated cyclic nucleotide efflux.
Fig. 5.
Characterization of HEK293 cells as a cell model for studies of the dependence of cyclic nucleotide efflux on Nab conductance. A to B, Western blot analyses of MRPs in HEK293 cells stably transfected with MRP4 (A) and MRP5 (B). C, effect of the replacement of bath Na+ with NMDG on membrane potential in HEK293 cells. Numbers in parentheses indicate the amount of proteins used for the Western blot analysis.
Untransfected HEK293 cells released 755 ± 103 fmol/min cGMP, a level comparable with that observed in pituitary cells, whereas cGMP released by MRP1-expressing cells was somewhat lower at 306 ± 31 fmol/min. In contrast, cGMP release was significantly elevated in MRP4- (2.01 ± 021 pmol/min) and MRP5 (16.71 ± 1.68 pmol/min)-expressing cells, 2.6- and 22-fold increases, respectively, compared with untransfected cells. All cells also released cAMP: untransfected cells, 55 ± 9 fmol/min; MRP1-expressing cells, 35 ± 5 fmol/min; MRP4-expressing cells, 361 ± 80 fmol/min; and MRP5-expressing cells, 686 ± 253 fmol/min. The intracellular cyclic nucleotide contents in these four cells types were the following: cAMP, HEK293 = 2.56 ± 0.09, MRP1 = 0.71 ± 0.03, MRP4 = 1.85 ± 0.07, and MRP5 = 1.75 ± 0.04 pmol/106 cells; cGMP, HEK293 = 461 ± 27, MRP1 = 73.2, MRP4 = 599 ± 11, and MRP5 = 462 ± 16 fmol/106 cells. These results indicate that MRP4 and MRP5 but not MRP1 transport cAMP and cGMP, and that under basal conditions and in the presence of 1 mM IBMX MRP5 is a more effective transporter of both cyclic nucleotides than MRP4.
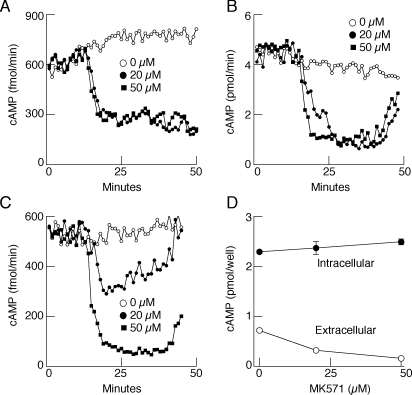
Replacement of bath Na+ with NMDG promptly inhibited cyclic nucleotide effluxes in MRP4- and MRP5-expressing cells in a reversible manner (Fig. 6A). Similar effects were also observed in untransfected and MRP1-transfected cells, indicting that the native cyclic nucleotide transporter(s) of HEK293 cells is also dependent on the presence of bath Na+. In a further parallel with pituitary cells, inhibition of cyclic nucleotide efflux was also observed in MRP5-expressing cells exposed to bath Na+ in the 10 to 60 mM concentration range (Fig. 6, B and C). In contrast, 2′,7′-bis(2-carboxyethyl)-5-(6)-carboxyfluorescein efflux was not affected by replacing bath Na+ with NMDG in MRP5-expressing HEK293 cells (data not shown). In both pituitary cells (Fig. 7A) and HEK293 cells expressing MRP4 (Fig. 7B) or MRP5 (Fig. 7C), cAMP efflux was attenuated in the presence of 20 and 50 μM MK571, a relatively specific inhibitor of MRPs. Inhibition of cAMP efflux by MK571 was also observed in cells in static cultures, whereas the formation of this messenger was not affected (Fig. 7D).
Fig. 6.
Role of MRP transporters in Na+-dependent cyclic nucleotide efflux. A, effect of complete replacement of extracellular Na+ with NMDG on cAMP (left) and cGMP (right) efflux in HEK293 cells stably expressing MRP1, MRP4, or MRP5 proteins. B and C, effect of partial substitution of Na+ with NMDG on cGMP (B) and cAMP (C) efflux in HEK293 cells expressing recombinant MRP5. Data shown are representative of three similar experiments, and mean ± S.E.M. values are shown under Results.
Fig. 7.
Effect of MK571, a specific inhibitor of MRP transporters, on cAMP efflux. A to C, inhibition of cAMP efflux by MK571 in normal pituitary cells (A) and MRP5-(B) and MRP4 (C)-expressing HEK293 cells. Data shown are representative of three similar experiments. D, dose-dependent effect of MK571 on cAMP efflux and intracellular accumulation in MRP4-expressing HEK293 cells in static cultures. Data shown are mean ± S.E.M. values from sextuplicate incubation.
Sodium Influx Is Not Directly Coupled to Cyclic Nucleotide Efflux.
Parallels in the effects of substituting extracellular Na+ with organic cations could suggest a direct coupling between Na+ influx and cyclic nucleotide efflux. Such a coupling would result in hyperpolarization of cell membranes when cAMP efflux transporter is inhibited. To examine this hypothesis, we performed two experiments. First, pituitary cells were perifused with probenecid, another effective inhibitor of cyclic nucleotide efflux in pituitary cells (Andric et al., 2006). Supplemental Fig. 1A shows a dose-dependent effect of probenecid on cAMP efflux. The inhibitory effect of probenecid on cAMP efflux was highly comparable with that observed in cells perifused with NMDG medium. However, inhibition of cAMP efflux did not affect the holding current, in contrast to replacement of Na+ with NMDG (Supplemental Fig. 1B), and thus argues against the direct coupling of Na+ influx to cyclic nucleotide efflux. Furthermore, spontaneous firing of APs was not inhibited but facilitated in cells treated with probenecid (Supplemental Fig. 1C). In the second experiment, we treated MRP5-expressing cells with monensin, an electroneutral Na+/H+ antiporter (Lichtshtein et al., 1979). This compound did not rapidly affect membrane potential at concentrations of 10 or 20 μM, in contrast with replacement of Na+ with NMDG (Supplemental Fig. 2A). Facilitation of Na+ influx by monensin treatment did not result in stimulation of cAMP (Supplemental Fig. 2B) or cGMP (Supplemental Fig. 2C) efflux, again arguing against the hypothesis that Na+ influx is directly coupled to cyclic nucleotide efflux.
Dependence of Cyclic Nucleotide Efflux on Membrane Potential.
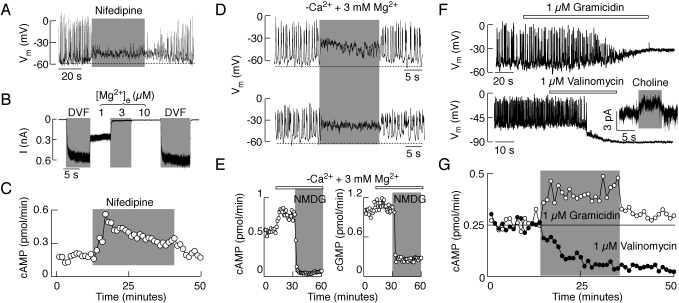
Because Na+ influx was not directly coupled to cyclic nucleotide efflux, parallels in the effects of replacing bath Na+ with NMDG could indicate that changes in baseline membrane potentials affected cyclic nucleotide efflux. To test this hypothesis, we performed several experiments. Abolition of spontaneous firing of APs by adding nifedipine, a blocker of L-type voltage-gated calcium channels, was followed by change in the baseline membrane potential toward depolarizing levels in a reversible manner (Fig. 8A). This was accompanied by an increase in cAMP efflux (Fig. 8C). Likewise, removal of extracellular Ca2+ in the presence of 3 mM Mg2+ depolarized cell membranes (Fig. 8D) and enhanced cAMP and cGMP efflux, which was abolished when bath Na+ was replaced with NMDG (Fig. 8E). To exclude the effects of Na+ influx through L-type channels when cells were bathed in Ca2+-deficient medium (Fig. 8B), the bath Mg2+ concentration was elevated to 3 mM.
Fig. 8.
Dependence of cyclic nucleotide efflux on membrane potential. A and C, effect of nifedipine, an L-type Cav channel blocker, on spontaneous electrical activity in lactotrophs (A) and cAMP efflux (C) in perifused pituitary cells. B, effect of the removal of divalent cations from bath solution (DVF) and addition of Mg2+ on membrane current in lactotrophs held at −60 mV. D and E, effect of the removal of bath Ca2+ on electrical activity in lactotrophs (D, top) and somatotrophs (D, bottom), and cAMP (E, left) and cGMP (E, right) efflux. F and G, effect of gramicidin and valinomycin on spontaneous electrical activity and holding current in lactotrophs (F) and cAMP efflux in perifused pituitary cells (G).
Facilitation of Na+ influx by the addition of gramicidin also depolarized the plasma membrane (Fig. 8F, top) and enhanced cAMP efflux (Fig. 8G). In contrast, facilitation of K+ efflux by valinomycin hyperpolarized the cell membrane in a manner highly comparable with that observed in experiments with organic cations (Fig. 8F, bottom). In valinomycin-treated cells, replacement of bath Na+ with choline still resulted in the abolition of Nab conductance (Fig. 8F, inset). In valinomycin-treated cells, cAMP efflux was also inhibited (Fig. 8G). Similar effects were also observed in MRP4/5-expressing HEK293 cells (data not shown). These results indicate that the MRP4/5 transporters could sense changes in membrane potential, which in turn affects cyclic nucleotide efflux but not other transports.
Discussion
In this study, we showed that replacement of bath Na+ with organic cations resulted in membrane hyperpolarization and thus the abolition of spontaneous AP firing in secretory anterior pituitary cells. Experiments with voltage-clamped cells at resting membrane potential further revealed that replacement of bath Na+ caused a loss of a depolarizing conductance. Such electrophysiological effects of replacing extracellular Na+ with other monovalent organic cations were observed in GH3-immortalized cells and in normal lactotrophs, somatotrophs, gonadotrophs, and other unidentified pituitary cell types. These results are consistent with our earlier studies showing that spontaneous Ca2+ transients in somatotrophs were also abolished by substituting bath Na+ with organic cations (Tomic et al., 1999). Simasko's group also published similar observations in GH3 cells (Sankaranarayanan and Simasko, 1996) and lactotrophs (Simasko, 1994) and termed this current the Nab conductance. Thus, the Nab conductance determines the resting membrane potential and is necessary for the spontaneous firing of Ca2+-dependent APs and the associated Ca2+ transients. The nature of the channels underlying the Nab conductance in these cells has not been clarified, although potential candidates include the neuronal channel NALCN and some transient receptor potential channels, which are expressed in pituitary cells (Riccio et al., 2002; Fonfria et al., 2006).
In parallel to findings for electrical activity and holding current, we show that replacement of extracellular Na+ with NMDG had an instantaneous inhibitory effect on cAMP efflux. Complete removal of bath Na+ also inhibited forskolin- and GHRH-stimulated cAMP efflux. Three additional lines of evidence support the conclusion that cyclic nucleotide efflux depends on Nab conductance: 1) partial substitution of bath Na+ with NMDG had similar concentration-dependent effects on the levels of membrane hyperpolarization and cAMP efflux; 2) as with the Nab conductance, cAMP efflux was also inhibited by replacement of bath Na+ with choline or TMA; and 3) in both electrophysiological and cyclic nucleotide efflux measurements, NMDG was the most effective inhibitor, followed by choline and TMA. On the other hand, cyclic nucleotide efflux was also inhibited by replacing bath Na+ with sucrose, indicating that organic cations are not direct inhibitors of the cyclic nucleotide pump.
Because there was a close correlation between cell content and released cyclic nucleotides in pituitary cells (Gonzalez-Iglesias et al., 2006), it would be reasonable to speculate that the rapid inhibition of cAMP efflux caused by blocking Nab conductance reflected an inhibition of basal AC activity. This would also be consistent with several reports indicating the dependence of AC activity on membrane potential (Schultz et al., 1992; Reddy et al., 1995; Beltrán et al., 1996). However, by measuring intracellular cAMP levels in cells in static culture, we showed that hyperpolarization of cell membranes induced by a blockade of Nab conductance did not overtly affect cyclic nucleotide intracellular levels. Experiments with probenecid, an established inhibitor of cyclic nucleotide efflux (Kruh and Belinsky, 2003; Sager, 2004), further supported this conclusion. Probenecid inhibited cAMP release with a time course that was highly comparable with that observed in experiments involving the replacement of bath Na+ with organic cations. This treatment also rapidly abolished cGMP efflux without obviously affecting its intracellular content. Because basal cGMP production in pituitary cells results from sGC activity (Kostic et al., 2001), not a plasma membrane-associated enzyme, it is obvious that cyclic nucleotide efflux transporter activity was influenced by the removal of bath Na+ independently of the status of de novo production.
Inhibitory effects of organic cations on electrical activity and cyclic nucleotide efflux could also suggest the presence of a common transporter that carries Na+ into cells and cyclic nucleotides out of cells. In general, organic anion transport is indirectly linked to metabolic energy and the Na+ gradient (Zhou and You, 2007). Among these transporters, organic anion transporter 2 is a facilitative transporter of cGMP (Cropp et al., 2008) and is also expressed in cells of the central nervous system (Koepsell and Endou, 2004). However, organic anion transporter 2 is a Na+-independent multispecific organic anion/dimethyldicarboxylate exchanger (Kobayashi et al., 2005). Furthermore, if the above hypothesis was correct, the inhibition of the cyclic nucleotide efflux transporter should hyperpolarize the cell membrane in the same manner as the removal of extracellular Na+. Contrary to that prediction, in our experiments, probenecid completely halted cAMP efflux but did not affect the holding current, thereby facilitating rather than inhibiting the firing of APs; the latter reflects the stimulatory action of elevated intracellular cAMP on AP firing (Gonzalez-Iglesias et al., 2006).
Like other MRPs, MRPs 4, 5, and 8 are also organic anion transporters but have the unique ability to transport cyclic nucleotides (Kruh and Belinsky, 2003). Earlier experiments revealed that transcripts for MRP4 and MRP5 transporters are present in pituitary cells and contribute to cGMP efflux (Andric et al., 2006). To more directly test the effects of bath Na+ on MRP function, we used HEK293 cells stably transfected with human MRP4 and MRP5. We selected HEK293 cells because they also responded to replacement of extracellular Na+ with organic cations by hyperpolarization of cell membranes. Experiments revealed that basal cyclic nucleotide release by MRP4/5-transfected cells was significantly elevated compared with MRP1-expressing and native pituitary and HEK293 cells. It is interesting that both cAMP and cGMP steady-state efflux was higher in MRP5- than in MRP4-expressing cells under basal conditions, suggesting that MRP5 has higher a sensitivity/capacity for cyclic nucleotides than MRP4. Like in pituitary cells, replacement of bath Na+ with NMDG had a rapid and dose-dependent inhibitory effect on cyclic nucleotide efflux in MRP4- and MRP5-expressing cells, clearly indicating that activity of these transporters is coupled to the background Na+ influx pathway. In both pituitary and MRP4/5-expressing cells, MK571 inhibited basal cAMP efflux, supporting the view that these transporters account for cyclic nucleotide efflux in pituitary cells. Replacement of bath Na+ with NMDG also affected basal cyclic nucleotide efflux in untransfected and MRP1-expressing HEK293 cells, which is consistent with the finding that these cells also express MRP4 and MRP5 mRNAs endogenously (Wielinga et al., 2002).
These observations raise the question of which mechanism the Nab-dependent pathway uses to affect the MRP-mediated cyclic nucleotide efflux. The hypothesis that the organic cations used as substitutes for bath Na+ directly inhibited the pump was ruled out because cyclic nucleotide efflux was also inhibited in cells bathed in medium containing sucrose. It is also unlikely that Na+ influx is required for the operation of MRPs because electroneutral movement of Na+ influx by monensin did not stimulate cyclic nucleotide efflux. The parallelism in the effect of removing bath Na+ on electrical activity and cyclic nucleotide efflux is consistent with the functional coupling of two proteins (i.e., MRPs and Nab channels). Specifically, both the firing of APs and MRP4/M5 activity depended on the resting membrane potential determined by the Nab conductance. Experiments with depolarization and hyperpolarization of plasma membranes induced by various means are in accordance with this hypothesis.
The ATP-dependence of cyclic nucleotide transport mediated by MRPs was observed in isolated membrane vesicles in a buffer containing Tris/HCl and sucrose only (Jedlitschky et al., 2000; Chen et al., 2001; Guo et al., 2003). In general, MRPs contain 2 nucleotide binding domains and 12 transmembrane domains that form the essential transporter. It has been suggested that the ATP-dependent alternate opening and closing of the nucleotide binding dimer is directly coupled to the change in the inward-outward orientation of the transmembrane domains that enables substrate translocation (Jones et al., 2009). Because of the rapid effects of NMDG on cyclic nucleotide efflux, it is unlikely that intracellular depletion of ATP accounts for the inhibition of cyclic nucleotide efflux. Consistent with this conclusion, we observed unaltered 2′,7′-bis(2-carboxyethyl)-5-(6)-carboxyfluorescein efflux after replacement of bath Na+ with NMDG. Thus, it is reasonable to conclude that changes in the membrane potential modulate ATP-dependent cyclic nucleotide efflux without altering other functions of this transporter.
MRP4/5 proteins do not have the typical voltage-sensor sequence conserved in voltage-gated ion channels. Both proteins have positively charged sequences, the Lys496 to Lys515 sequence of MRP4 with eight positively charged residues and the Lys511 to Arg531 sequence of MRP5 with 11 positively charged residues, but they are unlikely to represent the transmembrane domains (Ravna et al., 2008). This, however, should not exclude a role for membrane potential in the function of these transporters, because the functions of many carriers and pumps as well as some G protein-coupled receptors are modulated by the membrane potential through other mechanisms (Roepe et al., 1993; Bezanilla, 2008).
In summary, we have shown for the first time that cyclic nucleotide efflux mediated by rat and human MRPs is rapidly modulated in cells. Our results further indicate the importance of the background Na+ influx in the rapid regulation of MRP activity. Sodium influx is not directly coupled to MRP4/5-mediated cyclic nucleotide efflux, is independent of the status of the transporter activity, and determines the baseline membrane potential, which in turns modulates MRP4/5-mediated cyclic nucleotide efflux. Further work is required to identify the channels responsible for the background Na+ influx, the mechanism by which membrane potential affects ATP-dependent cyclic nucleotide efflux, and physiological and clinical relevance of such rapid regulation of cyclic nucleotide efflux.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the Intramural Research Programs of the National Institutes of Health National Institute of Child Health and Human Development and National Institutes of Health National Cancer Institute Center for Cancer Research.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.059386
- AC
- adenylyl cyclase
- AP
- action potential
- GHRH
- growth hormone releasing hormone
- IBMX
- 3-isobutyl-1-methylxanthine
- MRP
- multidrug resistance proteins
- Nab
- tetrodotoxin-resistant background sodium conductance
- PDE
- phosphodiesterase
- sGC
- soluble guanylyl cyclase
- TTX
- tetrodotoxin
- HEK
- human embryonic kidney
- TMA
- tetramethylammonium
- NMDG
- N-methyl-d-glucamine
- MK571
- 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid.
References
- Andric SA, Kostic TS, Stojilkovic SS. (2006) Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology 147: 3435–3445 [DOI] [PubMed] [Google Scholar]
- Ang KL, Antoni FA. (2002) Functional plasticity of cyclic AMP hydrolysis in rat adenohypophysial corticotroph cells. Cell Signal 14: 445–452 [DOI] [PubMed] [Google Scholar]
- Antoni FA, Sosunov AA, Haunso A, Paterson JM, Simpson J. (2003) Short-term plasticity of cyclic adenosine 3′,5′-monophosphate signaling in anterior pituitary corticotrope cells: the role of adenylyl cyclase isotypes. Mol Endocrinol 17: 692–703 [DOI] [PubMed] [Google Scholar]
- Bankir L, Ahloulay M, Devreotes PN, Parent CA.(2002) Extracellular cAMP inhibits proximal reabsorption: are plasma membrane cAMP receptors involved? Am J Physiol Renal Physiol 282: F376–F392 [DOI] [PubMed] [Google Scholar]
- Beltrán C, Zapata O, Darszon A.(1996) Membrane potential regulates sea urchin sperm adenylylcyclase. Biochemistry 35: 7591–7598 [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520 [DOI] [PubMed] [Google Scholar]
- Bezanilla F. (2008) How membrane proteins sense voltage. Nat Rev Mol Cell Biol 9: 323–332 [DOI] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Kruh GD. (2001) Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem 276: 33747–33754 [DOI] [PubMed] [Google Scholar]
- Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, Giacomini KM. (2008) Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol 73: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26: 159–178 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Jiang Y, Tomić M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS. (2006) Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol 20: 2231–2246 [DOI] [PubMed] [Google Scholar]
- Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, Kruh GD. (2003) MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem 278: 29509–29514 [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Burchell B, Keppler D. (2000) The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275: 30069–30074 [DOI] [PubMed] [Google Scholar]
- Jones PM, O'Mara ML, George AM. (2009) ABC transporters: a riddle wrapped in a mystery inside an enigma. Trends Biochem Sci 34: 520–531 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. (2005) Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol 57: 573–578 [DOI] [PubMed] [Google Scholar]
- Koepsell H, Endou H. (2004) The SLC22 drug transporter family. Pflugers Arch 447: 666–676 [DOI] [PubMed] [Google Scholar]
- Kostic TS, Andric SA, Stojilkovic SS. (2001) Spontaneous and receptor-controlled soluble guanylyl cyclase activity in anterior pituitary cells. Mol Endocrinol 15: 1010–1022 [DOI] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG. (2003) The MRP family of drug efflux pumps. Oncogene 22: 7537–7552 [DOI] [PubMed] [Google Scholar]
- Lai L, Tan TM. (2002) Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem J 361: 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D, Dunlop K, Kaback HR, Blume AJ. (1979) Mechanism of monensin-induced hyperpolarization of neuroblastoma-glioma hybrid NG108-15. Proc Natl Acad Sci U S A 76: 2580–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414 [PubMed] [Google Scholar]
- Persani L, Borgato S, Lania A, Filopanti M, Mantovani G, Conti M, Spada A. (2001) Relevant cAMP-specific phosphodiesterase isoforms in human pituitary: effect of Gs(alpha) mutations. J Clin Endocrinol Metab 86: 3795–3800 [DOI] [PubMed] [Google Scholar]
- Ravna AW, Sylte I, Sager G. (2008) A molecular model of a putative substrate releasing conformation of multidrug resistance protein 5 (MRP5). Eur J Med Chem 43: 2557–2567 [DOI] [PubMed] [Google Scholar]
- Reddy R, Smith D, Wayman G, Wu Z, Villacres EC, Storm DR. (1995) Voltage-sensitive adenylyl cyclase activity in cultured neurons. A calcium-independent phenomenon. J Biol Chem 270: 14340–14346 [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. (2003) Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 63: 1094–1103 [DOI] [PubMed] [Google Scholar]
- Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. (2002) Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem 277: 12302–12309 [DOI] [PubMed] [Google Scholar]
- Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Köck K, Kroemer HK. (2005) Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev 37: 253–278 [DOI] [PubMed] [Google Scholar]
- Roepe PD, Wei LY, Cruz J, Carlson D. (1993) Lower electrical membrane potential and altered pHi homeostasis in multidrug-resistant (MDR) cells: further characterization of a series of MDR cell lines expressing different levels of P-glycoprotein. Biochemistry 32: 11042–11056 [DOI] [PubMed] [Google Scholar]
- Russwurm M, Koesling D. (2005) Purification and characterization of NO-sensitive guanylyl cyclase. Methods Enzymol 396: 492–501 [DOI] [PubMed] [Google Scholar]
- Sager G. (2004) Cyclic GMP transporters. Neurochem Int 45: 865–873 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Simasko SM. (1996) A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. Am J Physiol 271: C1927–1934 [DOI] [PubMed] [Google Scholar]
- Schultz JE, Klumpp S, Benz R, Schürhoff-Goeters WJ, Schmid A. (1992) Regulation of adenylyl cyclase from Paramecium by an intrinsic potassium conductance. Science 255: 600–603 [DOI] [PubMed] [Google Scholar]
- Simasko SM. (1994) A background sodium conductance is necessary for spontaneous depolarizations in rat pituitary cell line GH3. Am J Physiol 266: C709–C719 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Zemkova H, Van Goor F. (2005) Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab 16: 152–159 [DOI] [PubMed] [Google Scholar]
- Tomić M, Koshimizu T, Yuan D, Andric SA, Zivadinovic D, Stojilkovic SS. (1999) Characterization of a plasma membrane calcium oscillator in rat pituitary somatotrophs. J Biol Chem 274: 35693–35702 [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Reid G, Challa EE, van der Heijden I, van Deemter L, de Haas M, Mol C, Kuil AJ, Groeneveld E, Schuetz JD, et al. (2002) Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol 62: 1321–1331 [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, et al. (2000) Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A 97: 7476–7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. (2007) Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010 [DOI] [PubMed] [Google Scholar]
- Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. (2005) Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 272: 4725–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, You G. (2007) Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res 24: 28–36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.