Abstract
Retinoic acid receptor-related orphan receptors (RORs) regulate a variety of physiological processes including hepatic gluconeogenesis, lipid metabolism, circadian rhythm, and immune function. Here we present the first high-affinity synthetic ligand for both RORα and RORγ. In a screen against all 48 human nuclear receptors, the benzenesulfonamide liver X receptor (LXR) agonist N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide (T0901317) inhibited transactivation activity of RORα and RORγ but not RORβ. T0901317 was found to directly bind to RORα and RORγ with high affinity (Ki = 132 and 51 nM, respectively), resulting in the modulation of the receptor's ability to interact with transcriptional cofactor proteins. T0901317 repressed RORα/γ-dependent transactivation of ROR-responsive reporter genes and in HepG2 cells reduced recruitment of steroid receptor coactivator-2 by RORα at an endogenous ROR target gene (G6Pase). Using small interference RNA, we demonstrate that repression of the gluconeogenic enzyme glucose-6-phosphatase in HepG2 cells by T0901317 is ROR-dependent and is not due to the compound's LXR activity. In summary, T0901317 represents a novel chemical probe to examine RORα/γ function and an excellent starting point for the development of ROR selective modulators. More importantly, our results demonstrate that small molecules can be used to target the RORs for therapeutic intervention in metabolic and immune disorders.
Several members of the nuclear receptor (NR) superfamily regulate the expression of key genes involved in carbohydrate and lipid metabolism in response to ligands such as fatty acids, bile acids, cholesterol metabolites, and steroid hormones. For example, the nuclear receptors liver X receptors (LXRs) α and β (NR1H3 and NR1H2) bind oxidized cholesterol and function as sensors for excess intracellular oxysterols (Janowski et al., 1996; Kalaany et al., 2005). Many studies have demonstrated that the LXRs are involved in the regulation of a wide variety of physiological processes, including cholesterol metabolism and transport, lipogenesis, gluconeogenesis, and inflammation, making these receptors attractive targets for the development of synthetic ligands for the treatment of disorders such as dyslipidemia, atherosclerosis, and diabetes (Mohan and Heyman, 2003). An early result of drug discovery efforts on LXRs was the discovery of two potent synthetic agonists, the benzenesulfonamide T0901317 and the tertiary amine GW3965, which have been used extensively to help expand our understanding of the physiological roles of the LXRs (Schultz et al., 2000; Collins et al., 2002). However, recently, it has been shown that in addition to potent modulation of the LXRs, T0901317 but not GW3965 is a potent agonist of both the farnesoid X receptor and the xenobiotic receptor pregnane X receptor (Houck et al., 2004; Mitro et al., 2007), bringing into question conclusions drawn from pharmacological studies using this compound.
The promiscuity of T0901317 and other nuclear receptor ligands indicates that there are privileged structures (chemotypes) that bind to a range of these receptors. Because it is possible to use these promiscuous ligands as points to initiate the development of receptor-selective ligands, we set out to profile the activity of a collection of well characterized NR ligands against all human nuclear receptors. Our laboratory has developed a GAL4 nuclear receptor library containing all 48 human receptors to facilitate the selectivity profiling of putative NR modulators that emerge from HTS campaigns at the Scripps Research Molecular Screening Center. In an effort to demonstrate the usefulness of the NR library, a collection of 65 well characterized NR modulators including the LXR agonist T0901317 was assembled. It is interesting that when this chemical set was tested against the GAL4 NR library, it was discovered that in addition to its expected activity, T0901317 was a potent inhibitor of the nuclear receptors retinoid-related orphan receptors α and γ (RORα and RORγ; NR1F1 and NR1F3) yet afforded little or no activity on RORβ (NR1F2).
The RORs are orphan nuclear receptors for which the endogenous ligand has yet to be described. Because the RORs are constitutive activators of transcription in the absence of ligands, it has been suggested that the coactivator binding surface, or activation function 2, is locked in the holo-conformation (Harris et al., 2002), circumventing the need for ligand interaction to transactivate target genes. However, the cocrystal structures of RORα LBD bound to cholesterol and cholesterol sulfate have been solved, suggesting that like the LXRs, the RORs can bind and may respond to metabolites of cholesterol (Kallen et al., 2002, 2004).
The RORs have emerged as attractive drug targets for the treatment of metabolic disorders and inflammatory disease. Here we demonstrate, for the first time, that a synthetic ligand can bind directly to and modulate the transcriptional activity of RORα and RORγ. T0901317 was found to directly bind to RORα and RORγ with high affinity (Ki = 132 and 51 nM, respectively), resulting in the modulation of the receptor's ability to interact with transcriptional cofactor proteins. T0901317 repressed RORα/γ-dependent transactivation of ROR-responsive reporter genes and in HepG2 cells reduced the recruitment of steroid receptor coactivator-2 (SRC2) by RORα at an endogenous ROR target gene. Using small interference RNA (siRNA), we demonstrate that repression of the gluconeogenic enzyme glucose-6-phosphatase in HepG2 cells by T0901317 is ROR-dependent and is not due to the compound's LXR activity.
In summary, T0901317 represents a novel chemical probe to examine RORα/γ function. In addition, this compound, with a chemically tractable scaffold, represents an excellent starting point for medicinal chemistry toward the development of ROR-selective modulators. More importantly, our results demonstrate for the first time that small molecules can be used to target the RORs for potential therapeutic intervention in metabolic and immune disorders.
Materials and Methods
Reagents
T0901317 was purchased from Sigma-Aldrich (St. Louis, MO). 25-Hydroxycholesterol was purchased from MP Biomedicals (Irvine, CA). Radioligand 25-[26,27-3H]hydroxycholesterol was from PerkinElmer Life and Analytical Sciences (Waltham, MA). Fifty-five endogenous and synthetic ligands from the Sigma Nuclear Receptor Signaling Ligand set (Sigma-Aldrich) were used to build the 65-compound chemical set run against the Gal4NR library. For ligand binding studies, RORα ligand binding domain (amino acids 304–556) was PCR-amplified and cloned into a pGEX-2T (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) encoding an N-terminal GST-Tag according to the manufacturer's instructions. The protein was induced with 1 mM isopropyl β-d-thiogalactoside in BL21 gold (DE3) cells (Invitrogen, Carlsbad, CA) and purified by affinity chromatography with Protino GST/4B column (Macherey-Nagel, Bethlehem, PA) followed by size-exclusion chromatography with HiLoad 26/60 Superdex 200 column (GE Healthcare). The protein was eluted, concentrated, and stored in 20 mM Tris, pH 8.0, 150 mM NaCl, 2 mM dithiothreitol, and 10% glycerol. For AlphaScreen studies (PerkinElmer Life and Analytical Sciences), recombinant His-RORα-LBD protein and Biotin-RIP140 box B peptide containing the LYYML motif were gifts from Eli Lilly and Company (Indianapolis, IN). The histidine (Nickel) detection kit was purchased from PerkinElmer Life and Analytical Sciences.
GAL4 NR Library Compound Profiling
The GAL4 NR library was built by replacing the endogenous N terminus and DNA binding domain (DBD) of all 48 receptors with a GAL4 DBD. The fusion constructs consist of the GAL4 DBD, the hinge domain, and LBD (and F domain if applicable) of the human receptors. The library was plated in triplicate on 384-well plates, and HEK293T cells were reverse-transfected with the well-specific construct and the UAS luciferase reporter pGL4.31 (Promega, Madison, WI) using Fugene6 transfection reagent (Roche Applied Sciences, Indianapolis, IN) in a final volume of 40 μl. Control wells contained constructs encoding for the GAL4 DBD alone (pBind) or GAL4 fused to VP16 were also analyzed. After 24 h, optimized compounds (2 μM final concentration) or DMSO was added to the plates and allowed to incubate for 20 h before the addition of 40 μl of BriteLite (PerkinElmer Life and Analytical Sciences) to measure luciferase activity. Compounds that attenuate the GAL4-VP16-dependent luciferase activity in the positive control were considered promiscuous or cytotoxic. Each compound was evaluated using two plates of the GAL4 NR library providing six replicates and was averaged for luciferase and compared with DMSO-only controls. Compounds with mean signals 3 S.D. from the DMSO controls were considered hits in this assay.
Radioligand Receptor Binding Assay
Forty-five or 90 ng of purified GST-RORα or GST-RORγ was incubated with various concentrations of [ 3H]25-hydroxycholesterol in assay buffer (50 mM HEPES, pH 7.4, 0.01% bovine serum albumin, 150 mM NaCl, and 5 mM MgCl2) to determine the Kd value. Nonspecific binding was defined in the absence of protein and excess of nonradioactive 25-hydroxycholesterol and was shown to be identical. The assays were terminated by rapid filtration through presoaked Whatman GF/B filters (0.5% polyethylenimine in phosphate-buffered saline) in Multiscreen plates (Millipore, Billerica, MA) and were washed (3 × 0.1 ml) with ice-cold assay buffer. The radioligand binding results were analyzed using Prism software (GraphPad Software, Inc., San Diego, CA). For the competition assay, various concentrations of T0901317 were incubated with receptor in the presence of 3 nM [ 3H]25-hydroxycholesterol.
AlphaScreen
The assays were performed in triplicate in white opaque 384-well plates (PerkinElmer Life and Analytical Sciences). The final volume was 20 μl for the generation of compound dose-response curves (0.5–7.5 μM). All dilutions were made in assay buffer (100 mM NaCl, 25 mM HEPES, and 0.1% bovine serum albumin, pH 7.4). The final DMSO concentration was 0.25%. A mix of 12 μl of His-RORα-LBD (75 nM), beads (30 μg/ml of each), and 4 μl of increasing concentrations of compound (0.02–8 μM) was added to the wells, and the plates were sealed and incubated for 1 h at room temperature in the dark. After this preincubation step, 4 μl of Biotin-RIP140B (25 nM) was added, the plates were sealed and further incubated for 2 h at room temperature in the dark. The plates were read on a PerkinElmer Envision 2104, and data were analyzed using Prism software.
Cell Culture and Transcriptional Assays
RIP140 Modulation of RORα Activity.
Luciferase reporter assays were conducted using a pBind Gal4-tagged RORα LBD construct, UAS luciferase reporter, and pSport6 full-length RIP-140 cotransfected into HEK293T cells. Reverse transfections were performed in bulk using 106 cells in 6-cm plates; 3 μg of total DNA in a 1:1:1 ratio of receptor, reporter, and corepressor, respectively; and FuGene6 (Roche) in a 1:3 DNA/lipid ratio. As controls, separate transfections containing either reporter only or receptor/reporter were performed using pBind or pSport6 empty vectors in place of receptor and corepressor, respectively. After 24-h bulk transfection, cells from different transfection conditions were counted and plated in 384-well plates at a density of 104 cells/well. After additional 24-h incubation, luciferase levels were assayed by one-step addition of 20 μl of BriteLite (PerkinElmer Life and Analytical Sciences) and read using an Envision multilabel plate reader (PerkinElmer Life and Analytical Sciences). Data were normalized to luciferase signal from UAS luciferase reporter/pBind control empty vector and displayed as the -fold change over UAS luciferase reporter. Unpaired t tests were performed on all data sets, and significance of differences between Gal4RORα and Gal4RORα/RIP140 was determined at p < 0.001.
RORα Modulation of Glucose 6-Phosphatase Wild-Type and Mutant Reporters.
For the glucose 6-phosphatase promoter, wild-type promoter or ROR-response element (RORE) mutant was used to transfect HEK293T cells with SRC2 as coactivator in the presence or absence of full-length RORα and were treated as defined in the figure legends (Chopra et al., 2008). Likewise, for Cyp7B1, the wild-type or RORE mutant promoter was transfected in HEK293T cells in the presence or absence of full-length RORα. The Cyp7B1 promoter constructs were a gift from Dr. Wen Xie (University of Pittsburgh, Pittsburgh, PA) and have been described previously (Wada et al., 2008).
RORα Modulation of IL-17 Reporter.
HEK293 cells were grown in 96-well plates (1 × 106/well) and were transiently transfected using Lipofectamine (Invitrogen) according to the manufacturer's protocol. Cells were transfected with a total of 200 ng of DNA per well consisting of the pGL4 mIL-17 firefly luciferase reporter construct, the pGL4 mIL-17 + CNS-5 firefly luciferase reporter construct, or the pGL4 mIL-17 2kB RORE mutant (100 ng/well) (Addgene, Cambridge, MA), an actin promoter Renilla reniformis luciferase reporter (50 ng/well), and either control vector alone or the test DNA (full-length RORα or full-length RORγ at 50 ng/well). The IL-17 reporters have been described previously (Zhang et al., 2008) and were obtained from Addgene. Cells were treated with T0901317 for 24 h and then lysed and read using the Dual-Glo Luciferase assays system (Promega) 48 h after transfection. These results were normalized (firefly/R. reniformis ratio).
Reduction of Endogenous Gene Expression by Small Interference RNAs
To reduce endogenous ROR expression, HepG2 cells were seeded onto a 12-well plate (2.5 × 105/well) and transfected the next day with siRNAs against human RORα and RORγ (Dharmacon RNA Technologies, Lafayette, CO) at 50 nM according to the instructions for Dharma-FECT 1 transfection reagent. Forty-two hours after transfection, cells were treated with vehicle (DMSO) or T0901317 (10 μM) for 6 h. Cells were harvested, and total RNA was isolated. Quantitative reverse transcriptase PCR was performed to analyze mRNA levels of human RORα, RORγ, GAPDH, and glucose 6-phosphatase (G6Pase) using SYBR Green technology. The primers used for quantitative PCR analysis are as follows: human RORα, GTAGAAACCGCTGCCAACA (forward) and ATCACCTCCCGCTGCTT (reverse); human RORγ, CCCCTGACCGATGTGGACT (forward) and CAGGATGCTTTGGCGATGA (reverse); human G6Pase, TCATCTTGGTGTCCGTGATCG (forward) and TTTATCAGGGGCACGGAAGTG (reverse); and GAPDH, TGCACCACCAACTGCTTAGC (forward) and GGCATGGACTGTGGTCATGAG (reverse).
ChIP/re-ChIP
HepG2 cells were infected with Flag-RORα adenovirus for 24 h and then treated with vehicle (DMSO) or T0901317 (10 μM) for another 24 h. Re-ChIP assays were performed by using the kit from Active Motif Inc. (Carlsbad, CA). Anti-FLAG (Sigma-Aldrich) antibody was used to do the first immunoprecipitation for all of the samples. The second immunoprecipitation was performed by using anti-mouse IgG (Millipore), anti-RNA Pol II (Millipore), or anti-SRC2 (Bethyl Laboratories, Montgomery, TX). The G6Pase primers used in PCR were CCCTGAACATGTTTGCATCA (forward) and CATTCCTTCCTCCATCCTCA (reverse).
Results
Using a cell-based GAL4-NR LBD cotransfection assay, we found that T0901317 (2 μM) was a potent repressor of both GAL4-RORα and GAL4-RORγ (Fig. 1). It is interesting that T0901317 inhibited the constitutive transactivation activity of both GAL4-RORα and GAL4-RORγ with little or no activity on GAL4-RORβ (Fig. 1). We observed that 1 μM T0901317 repressed RORα by almost 70% and by approximately 90% at 10 μM (see Fig. 1, inset). In control cells transfected with GAL4-VP16 and the UAS reporter, no repression of GAL4-VP16 transactivation of the luciferase gene was observed, suggesting that the repression induced by T0901317 is not a result of nonspecific luciferase effects or cellular toxicity (data not shown). As illustrated in Fig. 2, A and B, treatment of cells expressing GAL4-LXRα, GAL4-RORα, or GAL4-RORγ with increasing concentrations of T0901317 demonstrated an excellent dose response, with an estimated EC50 of 0.25 μM (LXRα) and estimated IC50 values of 2.0 (RORα) and 1.7 μM (RORγ), respectively.
Fig. 1.
Gal4 nuclear receptor profiling of T0901317. RORα and RORγ transactivation is repressed by T0901317 in a GAL4 NR library selectivity panel. Gal4 NR clones were reverse-transfected with a UAS reporter construct into HEK293T cells. After 24 h, the LXRα agonist T0901317 (2 μM final concentration) or DMSO was added and incubated for 20 h. The luciferase activity of each construct was measured and normalized to the mock (vector alone), then the -fold change in signal compared with DMSO was calculated (n = 6). Inset, HEK293T-transfected cells were separately treated with either 1 or 10 μM T0901317 or vehicle for 20 h followed by luciferase activity measurement (data shown is mean ± S.E.M., n = 6). Horizontal dashed lines represent ±5 S.D. of the control (mock transfected).
Fig. 2.
Dose-response curves for transactivation of LXRα and suppression of RORα and RORγ by T0901317. 293T cells were cotransfected with UAS-luciferase and Gal4-LXRα (A) or Gal4-RORα/γ (B) and were treated with various concentration of T0901317 for 20 h followed by luciferase activity measurement. Relative change was determined by normalizing to control vector treated with vehicle. Each data point was performed in eight replicates and is represented as the mean ± S.E.M., n = 8.
To determine whether the repression of RORα by T0901317 is due to direct binding to the receptor, we carried out a competitive radioligand binding assay. Previous studies using mass spectroscopy indicated that 25-hydroxycholesterol binds to RORα (Bitsch et al., 2003), and we developed a radioligand binding assay using [ 3H]25-hydroxycholesterol. We demonstrate that 25-hydroxycholesterol binds to RORα and RORγ with a Kd value of 3.3 ± 0.89 and 5.1 ± 0.71 nM, respectively, as determined from a saturation binding curve (Fig. 3, A and C). More importantly, T0901317 dose-dependently competed with [ 3H]25-hydroxycholesterol for RORα and RORγ binding with an approximate IC50 of 254 and 81 nM (Ki = 132 and 51 nM, respectively) (Fig. 3, B and D).
Fig. 3.
Radioligand binding assay with GST-RORα and GST-RORγ. Saturation curves for [3H]25-hydroxycholesterol was generated with 45 ng of GST-RORα (A) or 90 ng of GST-RORγ (C) in the assay buffer as mentioned in Materials and Methods. Kd value was 3.3 and 5.1 nM for GST-RORα and GST-RORγ, respectively. Competition assay was performed to determine the Ki value of T0901317 for RORα and GST-RORγ. Increasing concentrations of T0901317 were incubated with 3 nM [3H]25-hydroxycholesterol and 45 ng of GST-RORα (B) or 90 ng of GST-RORγ (D). Estimated IC50 values for T0901317 were 254 nM and 81 nM for GST-RORα and GST-RORγ, respectively. Data shown are representative results from two independent experiments performed in duplicate. The results were analyzed using Prism software.
The activity of nuclear receptors can be modulated by ligand-induced cofactor protein interaction where, for example, agonist recruits coactivator protein, and antagonist either blocks coactivator interaction or facilitates the recruitment of corepressor. Corepressors such as NCoR1, NCoR2, and RIP140 have been shown to interact with the RORs (Jetten, 2009). RIP140, also known as nuclear receptor-interacting protein 1, is a nuclear protein that has been shown to specifically interact with the activation function 2 domain of nuclear receptors and repress their activity. A screen of peptides derived from the NR boxes of coactivators and corepressors using Luminex technology revealed that two NR box peptides representative of the ligand-dependent nuclear receptor binding domain of RIP140 (RIP140-B and RIP140-9) interacted strongly with the RORs (data not shown). Using AlphaScreen technology, we sought to determine the ability of T0901317 to modulate receptor-corepressor interaction. The interaction of the NR box peptide, RIP140-B, with the histidine-tagged LBD of RORα was monitored in response to increasing concentrations of T0901317. As shown in Fig. 4A, T0901317 modulated the interaction of RIP140-B with RORα in a dose-dependent fashion. In the absence of receptor, the AlphaScreen counts are at baseline. The AlphaScreen data along with the radioligand binding results demonstrate that T0901317 binds directly to RORα and can induce a conformational change in the LBD that modulates interaction with the NR box peptide derived from the repressor RIP140. We then sought to confirm the ability of RIP140 to repress RORα activity in cells. In cotransfection studies, full-length RIP140 effectively represses the transactivation activity of Gal4RORα on the UAS luciferase reporter (Fig. 4B).
Fig. 4.
RORα is repressed by RIP140, and T0901317 modulates RORα interaction with RIP140. A, increasing concentrations of T0901317 were incubated with RORα (75 nM) and 30 μg/ml AlphaScreen beads for 1 h at room temperature in the dark as described under Materials and Methods. The Biotin-RIP140B peptide (25 nM) was added to each well, and the plates were incubated in the dark for an additional 2 h at room temperature. Representative data are shown as mean ± S.D. of three independent experiments. B, cell-based luciferase reporter assays measuring the effects of RIP140 on RORα-dependent transactivation. HEK293T cells were reverse-transfected with 3 μg of total DNA composed of either UAS luciferase reporter only, Gal4RORα and UAS luciferase reporter, or Gal4RORα, UAS luciferase reporter, and full-length RIP140. After bulk transfections, cells were replated, and assays were conducted in 384-well format with n = 6. Data were normalized to luciferase signal from UAS/pBind vector control. Differences in transactivation between Gal4RORα and Gal4RORα/RIP140 were determined to be significant with p < 0.001 (***), as determined by unpaired t test.
It has been shown that G6Pase gene expression is regulated by RORα along with the coactivator SRC2 (Chopra et al., 2008). Therefore, we examined the ability of T0901317 to modulate a G6Pase reporter in an RORα-dependent fashion in the presence of the coactivator SRC2. As expected, cotransfection of RORα strongly stimulated the G6Pase reporter gene and, as shown in Fig. 5A, T0901317 dose-dependently repressed the G6Pase promoter activity with approximately 31% repression at 10 μM. In the absence of RORα or in the presence of a G6Pase promoter-reporter containing a mutation of RORE binding site, the repressive effect of T0901317 was eliminated (data not shown), suggesting strongly that this effect was mediated via RORα. Likewise, we analyzed yet another RORα-responsive gene, the cytochrome P450 7B1 (Cyp7B1) (Wada et al., 2008). Again, cotransfection of RORα stimulated Cyp7B1 reporter gene activity by more than 8-fold, and T0901317 dose-dependently repressed the Cyp7B1 promoter activity with approximately 35% repression at 10 μM (Fig. 5B). The repressive effect of T0901317 was nearly abolished in the absence of RORα or when the RORE binding site of the Cyp7B1 promoter was mutated (data not shown).
Fig. 5.
Modulation of RORα-mediated G6Pase promoter activity and CYP7B1 promoter activity by T0901317 (T1317). 293T cells were cotransfected with pS6 control plasmid or pS6 containing full-length RORα along with G6Pase promoter (A) or CYP7B1 promoter (B) as detailed under Materials and Methods. SRC2 as a coactivator was also cotransfected with G6Pase promoter. Dose-response curve was determined by treating the transfected cells with varying concentrations of T0901317 for 20 h. Luciferase activity was measured, and relative change was determined by normalizing to cells treated with vehicle only. Each data point was performed in eight replicates and is represented as mean ± S.E.M., n = 8.
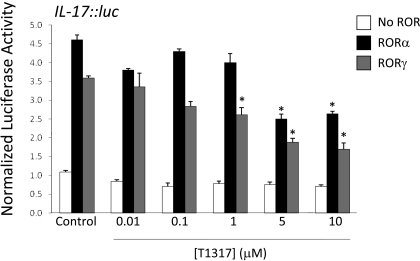
To examine the RORα/γ component of T0901317 pharmacology, we identified a target gene that is regulated by RORα and RORγ but not LXR. IL-17 is a well characterized target gene for RORα and RORγ, harbors an essential RORE in the promoter (Yang et al., 2008; Zhang et al., 2008), and displays no responsiveness to LXR (data not shown). HEK293 cells were transfected with an IL-17 promoter-driven luciferase reporter containing the RORE, and these cells were treated with compound or DMSO for 24 h. As shown in Fig. 6, T0901317 was able to repress activation of the IL-17 promoter induced by either RORα or RORγ in a dose-dependent fashion. Unfortunately, there are no good T-cell-derived cell lines that are readily able to be transfected, and there are no cell lines derived from Th17 cells that express significant levels of RORα/RORγ. However, as reported previously, HEK293 cells have been shown to be a good model to study ROR-dependent regulation of the IL-17 promoter (Ichiyama et al., 2008).
Fig. 6.
T0901317 (T1317) suppresses RORα- and RORγ-mediated IL-17 transcription. HEK293 cells were transiently transfected with the IL-17-dependent reporter construct, R. reniformis luciferase, and vectors containing full-length RORα (RORα), full-length RORγ (RORγ), or empty vector alone (endogenous). Twenty-four hours later, cells were treated with DMSO or increasing concentrations of T0901317. Twenty-four hours after treatment, IL-17 activity was determined by dual luciferase assay. The data are normalized to the vehicle (DMSO)-treated cells. Differences in transactivation were determined to be significant with p < 0.05 (*), as determined by unpaired t test.
With the recent finding that the gluconeogenic enzyme G6Pase gene expression is regulated by RORα (Chopra et al., 2008), we examined the ability of T091317 to suppress endogenous G6Pase expression in HepG2 cells and to determine whether the effects of T091317 on G6Pase are ROR-dependent. Therefore, we monitored mRNA levels of G6Pase before and after knockdown of endogenous RORα and RORγ by siRNA. As shown in Fig. 7A, expression of endogenous G6Pase was reduced in HepG2 cells by treatment with T091317 (10 μM). Transfection of these cells with a nontargeting siRNA did not interfere with the ability of T091317 to reduce the expression of G6Pase. Transfection with the nontargeting siRNA did not have an effect on the expression levels of either RORα or RORγ (data not shown). Treatment of HepG2 cells with siRNAs targeting RORα and RORγ reduced the expression of both receptors by more than 60% but had no effect on GAPDH expression levels (data not shown). In agreement with previous studies (Chopra et al., 2008), reduction of expression levels of RORα and RORγ reduced the expression of G6Pase (Fig. 7A). More importantly, the ability of T0901317 to repress G6Pase expression in HepG2 cells was lost when the RORs were knocked down (Fig. 7A). These results demonstrate that T091317 modulation of G6Pase is ROR-dependent and is not related to the compound's LXR activity, as has been suggested previously.
Fig. 7.
Modulation of endogenous G6Pase mRNA expression and endogenous G6Pase promoter activity by T0901317 in HepG2 cells. A, expression of endogenous G6Pase in HepG2 cells was reduced by treatment with siRNA against both human RORα and RORγ. siRNA oligonucleotides against RORs were transfected into HepG2 cells at 50 nM. Forty-two hours after transfection, cells were treated for 6 h with either vehicle (DMSO) or T091317 (10 μM). Total RNA was prepared from these cells and was subjected to reverse transcriptase PCR to measure the mRNA levels. B, sequential chromatin immunoprecipitation (ChIP/reChIP) assay illustrating that T0901317 (10 μM) treatment reduces the ability of RORα to recruit SRC2 to a G6Pase gene promoter. HepG2 cells overexpressing Flag-tagged RORα were treated with vehicle (DMSO) or 10 μM T0901317 for 24 h, followed by sequential ChIP. The first immunoprecipitation was performed using α-Flag antibody, and the second immunoprecipitation was performed using α-SRC2 antibody. Mouse IgG was used as a negative control, and anti-RNA pol II antibody was used as a positive control.
Finally, the ability of T0901317 to modulate RORα recruitment of the p160 coactivator SRC2 to the G6Pase promoter was assessed using a sequential ChIP assay (ChIP/reChIP). Treatment with T0901317 did not affect the level of RORα occupancy of the G6Pase promoter (Fig. 7B); however, in the reChIP using the SRC2 antibody, substantial decrease in the amount of SRC2 occupancy was noted in the presence of the T0901317. These results demonstrate that T0901317 decreases the ability of RORα to recruit the SRC2 coactivator to the G6Pase promoter and thus decreases the expression of the gene.
Discussion
The first ROR (RORα) was discovered in the early 1990s based on sequence similarities to the retinoic acid receptor and the retinoid X receptor, hence the name “retinoic acid receptor-related orphan receptor” (Beckerandre et al., 1993; Giguere et al., 1994), soon followed by the identification of RORβ and RORγ (Carlberg et al., 1994; Hirose et al., 1994). Each ROR gene generates multiple isoforms as a result of alternative promoter usage and splicing. In humans, four forms of RORα have been detected (α1–α4), yet only α1 and α4 are found in the mouse (Jetten et al., 2001). Two forms of RORβ are found in the mouse (β1 and β2), but only β1 is present in humans (Jetten et al., 2001). Two forms of RORγ are found in human and mouse (γ1 and γ2), with the γ2 form often referred to as RORγt because it is primarily expressed in the immune system. This isoform has garnered much attention lately because of its role in Th17 cells (Jetten et al., 2001; Miller and Weinmann, 2009). All three isoforms display a high degree of sequence similarity, yet surprisingly, as we demonstrate here, T0901317 can modulate the activity of both RORα and RORγ but not that of RORβ, suggesting the possibility for the development of synthetic molecules that would be ROR isoform-selective modulators.
The three RORs display distinct patterns of expression, suggesting nonredundant functions. RORα is expressed in the liver, skeletal muscle, skin, lungs, adipose tissue, kidney, thymus, and brain (Hamilton et al., 1996; Steinmayr et al., 1998), whereas RORβ expression is restricted to the central nervous system (Andre et al., 1998a,b). RORγ is highly expressed in the thymus; however, significant expression is also found in the liver, skeletal muscle, adipose tissue, and kidney (Medvedev et al., 1996). All RORs recognize and bind to specific sequences of DNA, termed ROREs as monomers, and these ROREs typically consist of an AGGTCA “half-site” with a 5′ AT-rich extension (Carlberg et al., 1994; Giguere et al., 1994; Hirose et al., 1994). When bound to their element within the promoter of a target gene, all three RORs constitutively recruit coactivators such as the p160 coactivator SRC2, resulting in constitutive activation of transcription of their target genes (Atkins et al., 1999).
A role for RORα in the regulation of metabolic pathways was revealed by studies in the staggerer (RORαsg/sg) mouse. This natural mutant mouse strain carries an intragenic insertion within the RORα gene that results in a frameshift and a premature stop codon, rendering RORα inactive (Hamilton et al., 1996). Detailed examination of the staggerer mouse revealed alterations in lipid metabolism evidenced by low levels of total plasma cholesterol, triglycerides, apoCIII (an apolipoprotein involved in triglyceride-rich metabolism), high-density lipoprotein, and apoA1 (the major apolipoprotein in high-density lipoprotein) (Mamontova et al., 1998). Staggerer mice are less susceptible to hepatic steatosis and have a reduced body fat index relative to wild-type mice, despite higher food consumption (Lau et al., 2008). The size of both brown and white adipose cells are smaller in these animals, and hepatic triglyceride content is lower (Lau et al., 2008). Consistent with this phenotype, the animals are less susceptible to high-fat diet-induced obesity and hepatic steatosis (Lau et al., 2008).
RORγ-null mice exhibit normal levels of plasma cholesterol and triglycerides (Kang et al., 2007). An interesting metabolic phenotype was revealed when staggerer mice were crossed with RORγ-null mice, effectively creating an RORα/γ double knockout. Although neither individual strain showed significant alterations in plasma glucose levels, the double knockout was hypoglycemic, illustrating a role for these receptors in maintaining glucose homeostasis (Kang et al., 2007). This study also demonstrated that RORα and RORγ display significant redundancy in function, which is consistent with plasma glucose levels remaining unaffected unless both receptors are lost. More recently, a role for RORα in the regulation of glucose metabolism was characterized when Chopra et al. (2008) found that loss of the p160 family coactivator SRC2 in mice led to a phenotype similar to von Gierke's disease, which is associated with severe hypoglycemia and abnormal accumulation of glycogen in the liver. Loss of expression of the enzyme G6Pase is responsible for 80% of the diagnosed von Gierke's disease cases. It is noteworthy that SRC2 was required for RORα to regulate this gene in a normal manner (Chopra et al., 2008).
Both RORα and RORγ regulate key physiological pathways and are also involved in pathogenic processes. RORα regulates lipid and glucose metabolism and is believed to play a role in protection against the development of atherosclerosis (Jetten, 2009). This receptor also is critical for normal function of the mammalian clock and is involved in the modulation of immune function (Yang et al., 2008; Jetten, 2009). The most prominent role for RORγ is the regulation of immune function, especially in the development of the Th17 cells that are believed to play an important role in autoimmunity (Ivanov et al., 2007). RORγ also helps to coordinate lipid and glucose metabolism in concert with RORα (Kang et al., 2007; Jetten, 2009). RORα and RORγ have also been implicated in bone development and cancer (Jetten, 2009). Thus, the development of small-molecule ligands that modulate the activity of these two orphan receptors has held significant interest for those pursuing the role of RORα/γ in the areas of metabolic diseases, autoimmunity, osteoporosis, and cancer. Our finding that T0901317 binds directly to both RORα and RORγ and modulates their transcriptional activity provides the first step toward the development of chemical tools to determine the ability to pharmacological target these receptors for these diseases.
The pharmacology of T0901317 has been characterized in detail in the literature with much of its activity attributed to the activation of LXRα and LXRβ (Michael et al., 2005). However, we described previously that this compound also activates farnesoid X receptor (Houck et al., 2004), and it was later reported that it also activates pregnane X receptor (Mitro et al., 2007). This degree of receptor promiscuity that provided us a critical advantage for the identification of a synthetic ligand for an orphan nuclear receptor creates difficulties for the interpretation of results obtained with this compound, especially in animal models. For example, T0901317 has been used to show that activation of LXR may lead to decreased severity of experimental autoimmune encephalomyelitis (Hindinger et al., 2006) by decreasing Th17 function (Xu et al., 2009). Based on our results, these effects may be due to the ability of T0901317 to suppress the activity of RORα and RORγ that is required for Th17 cell proliferation and IL-17 production.
It is unclear what the relative contribution of inhibition of RORα/γ activity is to the pharmacology to the array of animal studies examining the role of T0901317 on lipid and glucose metabolism. The results presented here demonstrate that the T0901317 effects on repression of G6Pase are in fact ROR-dependent and are not a result of the compound's LXR activity.
Conclusion
RORs regulate a variety of physiological processes, including hepatic gluconeogenesis, lipid metabolism, circadian rhythm, and immune function. Here we demonstrate that T0901317 represents the first synthetic ligand for RORα and RORγ, and this compound is a potent inverse agonist of these two orphan nuclear receptors. This was demonstrated by competitive radioligand binding assay and cell-based assays in which T0901317 repressed RORα/γ-dependent transactivation of reporter genes driven by the ROR-responsive promoters from the G6Pase and Cyp7b1 genes. Moreover, repression of G6Pase by T0901317 was relieved after knockdown of both RORs, concluding that this compound's effects on this gluconeogenic enzyme are ROR-dependent. Finally, we show that T0901317 reduces recruitment of the p160 coactivator SRC2 by RORα at the G6Pase promoter, thus providing a mechanism for control of this important enzyme by the RORs.
The pharmacology of T0901317 has been extensively studied in animal models, with the compound exhibiting acceptable pharmacokinetic properties. More importantly, the benzenesulfonamide scaffold is amenable to a modular synthetic chemistry optimization (Michael et al., 2005). Therefore, T0901317 represents a novel chemical tool to examine RORα/γ function, and our findings offer an excellent starting point for the design of potent and selective ROR ligands with potential application in the treatment of metabolic and immune disorders.
This work was supported in part by the National Institutes of Health National Institute of Mental Health [Grant U54-MH074404]; the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM084041]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Disease [Grant R01-DK080201]; and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS066417].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.060905.
- NR
- nuclear receptor
- ROR
- retinoic acid receptor-related orphan receptor
- ChIP
- chromatin immunoprecipitation
- SRC2
- steroid receptor coactivator 2
- LXR
- liver X receptor
- G6Pase
- glucose 6-phosphatase
- T0901317
- (2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide
- GW3965
- 3-[3-[[[2-chloro-3-(trifluoromethyl)phenyl]methyl](2,2 -diphenylethyl) amino]propoxy]benzeneacetic acid hydrochloride
- siRNA
- small interference RNA
- PCR
- polymerase chain reaction
- DBD
- DNA binding domain
- LBD
- ligand binding domain
- HEK
- human embryonic kidney
- DMSO
- dimethyl sulfoxide
- RORE
- retinoic acid receptor-related orphan receptor response element
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- IL
- interleukin.
References
- André E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-André M. (1998a) Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J 17: 3867–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E, Gawlas K, Becker-André M. (1998b) A novel isoform of the orphan nuclear receptor RORbeta is specifically expressed in pineal gland and retina. Gene 216: 277–283 [DOI] [PubMed] [Google Scholar]
- Atkins GB, Hu X, Guenther MG, Rachez C, Freedman LP, Lazar MA. (1999) Coactivators for the orphan nuclear receptor RORalpha. Mol Endocrinol 13: 1550–1557 [DOI] [PubMed] [Google Scholar]
- Becker-André M, André E, DeLamarter JF. (1993) Identification of nuclear receptor messenger RNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun 194: 1371–1379 [DOI] [PubMed] [Google Scholar]
- Bitsch F, Aichholz R, Kallen J, Geisse S, Fournier B, Schlaeppi JM. (2003) Identification of natural ligands of retinoic acid receptor-related orphan receptor alpha ligand-binding domain expressed in Sf9 cells–a mass spectrometry approach. Anal Biochem 323: 139–149 [DOI] [PubMed] [Google Scholar]
- Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLamarter JF, Becker-André M. (1994) RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol 8: 757–770 [DOI] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, et al. (2008) Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322: 1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, et al. (2002) Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem 45: 1963–1966 [DOI] [PubMed] [Google Scholar]
- Giguère V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. (1994) Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev 8: 538–553 [DOI] [PubMed] [Google Scholar]
- Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, et al. (1996) Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 379: 736–739 [DOI] [PubMed] [Google Scholar]
- Harris JM, Lau P, Chen SL, Muscat GE. (2002) Characterization of the retinoid orphan-related receptor-alpha coactivator binding interface: a structural basis for ligand-independent transcription. Mol Endocrinol 16: 998–1012 [DOI] [PubMed] [Google Scholar]
- Hindinger C, Hinton DR, Kirwin SJ, Atkinson RD, Burnett ME, Bergmann CC, Stohlman SA. (2006) Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J Neurosci Res 84: 1225–1234 [DOI] [PubMed] [Google Scholar]
- Hirose T, Smith RJ, Jetten AM. (1994) ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun 205: 1976–1983 [DOI] [PubMed] [Google Scholar]
- Houck KA, Borchert KM, Hepler CD, Thomas JS, Bramlett KS, Michael LF, Burris TP. (2004) T0901317 is a dual LXR/FXR agonist. Mol Genet Metab 83: 184–187 [DOI] [PubMed] [Google Scholar]
- Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. (2008) Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem 283: 17003–17008 [DOI] [PubMed] [Google Scholar]
- Ivanov, Zhou L, Littman DR. (2007) Transcriptional regulation of Th17 cell differentiation. Semin Immunol 19: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383: 728–731 [DOI] [PubMed] [Google Scholar]
- Jetten AM. (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7: e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten AM, Kurebayashi S, Ueda E. (2001) The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol 69: 205–247 [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. (2005) LXRs regulate the balance between fat storage and oxidation. Cell Metab 1: 231–244 [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. (2004) Crystal structure of the human RORα ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem 279: 14033–14038 [DOI] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. (2002) X-ray structure of the hRORalpha LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORalpha. Structure 10: 1697–1707 [DOI] [PubMed] [Google Scholar]
- Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM. (2007) Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics 31: 281–294 [DOI] [PubMed] [Google Scholar]
- Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE. (2008) The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem 283: 18411–18421 [DOI] [PubMed] [Google Scholar]
- Mamontova A, Séguret-Macé S, Esposito B, Chaniale C, Bouly M, Delhaye-Bouchaud N, Luc G, Staels B, Duverger N, Mariani J, et al. (1998) Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORalpha. Circulation 98: 2738–2743 [DOI] [PubMed] [Google Scholar]
- Medvedev A, Yan ZH, Hirose T, Giguère V, Jetten AM. (1996) Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene 181: 199–206 [DOI] [PubMed] [Google Scholar]
- Michael LF, Schkeryantz JM, Burris TP. (2005) The pharmacology of LXR. Mini Rev Med Chem 5: 729–740 [DOI] [PubMed] [Google Scholar]
- Miller SA, Weinmann AS. (2009) Common themes emerge in the transcriptional control of T helper and developmental cell fate decisions regulated by the T-box, GATA and ROR families. Immunology 126: 306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro N, Vargas L, Romeo R, Koder A, Saez E. (2007) T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett 581: 1721–1726 [DOI] [PubMed] [Google Scholar]
- Mohan R, Heyman RA. (2003) Orphan nuclear receptor modulators. Curr Top Med Chem 3: 1637–1647 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. (2000) Role of LXRs in control of lipogenesis. Genes Dev 14: 2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmayr M, André E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crépel F, Mariani J, Sotelo C, et al. (1998) Staggerer phenotype in retinoid-related orphan receptor α-deficient mice. Proc Natl Acad Sci U S A 95: 3960–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Kang HS, Angers M, Gong H, Bhatia S, Khadem S, Ren S, Ellis E, Strom SC, Jetten AM, et al. (2008) Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3). Mol Pharmacol 73: 891–899 [DOI] [PubMed] [Google Scholar]
- Xu J, Wagoner G, Douglas JC, Drew PD. (2009) Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol 86: 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Meng G, Strober W. (2008) Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol 9: 1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]