Abstract
The thyrotropin-releasing hormone (TRH) receptor undergoes rapid and extensive agonist-dependent phosphorylation attributable to G protein-coupled receptor (GPCR) kinases (GRKs), particularly GRK2. Like many GPCRs, the TRH receptor is predicted to form an amphipathic helix, helix 8, between the NPXXY motif at the cytoplasmic end of the seventh transmembrane domain and palmitoylation sites at Cys335 and Cys337. Mutation of all six lysine and arginine residues between the NPXXY and residue 340 to glutamine (6Q receptor) did not prevent the receptor from stimulating inositol phosphate turnover but almost completely prevented receptor phosphorylation in response to TRH. Phosphorylation at all sites in the cytoplasmic tail was inhibited. The phosphorylation defect was not reversed by long incubation times or high TRH concentrations. As expected for a phosphorylation-defective receptor, the 6Q-TRH receptor did not recruit arrestin, undergo the typical arrestin-dependent increase in agonist affinity, or internalize well. Lys326, directly before phenylalanine in the common GPCR motif NPXXY(X)5–6F(R/K), was critical for phosphorylation. The 6Q-TRH receptor was not phosphorylated effectively in cells overexpressing GRK2 or in in vitro kinase assays containing purified GRK2. Phosphorylation of the 6Q receptor was partially restored by coexpression of a receptor with an intact helix 8 but without phosphorylation sites. Phosphorylation was inhibited but not completely prevented by alanine substitution for cysteine palmitoylation sites. Positively charged amino acids in the proximal tail of the β2-adrenergic receptor were also important for GRK-dependent phosphorylation. The results indicate that positive residues in helix 8 of GPCRs are important for GRK-dependent phosphorylation.
The type 1 TRH receptor is a GPCR expressed in the anterior pituitary gland. In response to TRH, the receptor activates Gq/11, which in turn stimulates phospholipase C. The resulting increases in inositol trisphosphate and diacylglycerol cause rapid release of intracellular calcium and activation of protein kinase C. Like many GPCRs, the TRH receptor contains a canonical NPXXY at the end of transmembrane 7 in a NPXXY(X)5–6F(R/K) motif (Mirzadegan et al., 2003; Okuno et al., 2005; Swift et al., 2006) and is predicted to form an amphipathic eighth helix with a positively charged face ending at a downstream pair of cysteine residues, where palmitoylation occurs (Du et al., 2005).
When TRH binds, the receptor undergoes rapid and quantitative phosphorylation at multiple sites in the cytoplasmic tail (Jones and Hinkle, 2005, 2008; Jones et al., 2007). Agonist-stimulated phosphorylation of the receptor drives arrestin binding and arrestin-dependent receptor desensitization and internalization. The receptor tail is not detectably phosphorylated before activation.
Agonist-dependent phosphorylation of GPCRs is usually carried out by either GRKs that recognize the activated state of the receptor or downstream kinases that are activated by receptor signaling. The cytoplasmic tail of the TRH receptor is phosphorylated by GRKs, and GRK2 small interfering RNA and dominant-negative GRK2 inhibit phosphorylation in pituitary cells expressing endogenous receptor and transfected HEK293 cells (Jones and Hinkle, 2005; Jones et al., 2007). GRK2 translocates to the plasma membrane in response to TRH addition (Jones and Hinkle, 2005). Protein kinase C inhibitors and calcium chelators do not interfere with agonist-dependent receptor phosphorylation, suggesting that diacylglycerol- and calcium-activated kinases are not involved (Jones and Hinkle, 2005; Jones et al., 2007).
There are seven GRKs in mammalian cells, four of which are widely expressed (Krupnick and Benovic, 1998; Pitcher et al., 1998; Premont and Gainetdinov, 2007). GRKs are remarkable in their ability to distinguish between agonist-occupied and inactive receptors and in their ability to phosphorylate hundreds of GPCRs. These enzymes do not have a rigid sequence requirement for substrates but prefer to phosphorylate at clusters of serines and threonines surrounded by acidic residues. Most GRK phosphorylation sites are in the cytoplasmic tails or third intracellular loops. GRKs 2 and 3 have C-terminal pleckstrin homology domains and interact directly with Gβγ made available by receptor activation, whereas GRKs 5 and 6 associate with membranes by virtue of lipid modifications and/or hydrophobic helices in their C-terminal segments (Jiang et al., 2007).
The helix 8 region of GPCRs moves significantly upon receptor activation (Cai et al., 1999; Fritze et al., 2003; Li et al., 2007; Altenbach et al., 2008; Scheerer et al., 2008), making it a candidate for recognition by GRKs. Because the TRH receptor undergoes such rapid and extensive phosphorylation, we tested the hypothesis that helix 8 is required for the activation of GRK and report here that the highly conserved positively charged residues play a critical role in receptor phosphorylation.
Materials and Methods
Materials.
Embryonic fibroblasts from mice lacking arrestins 2 and 3 were from Dr. Robert Lefkowitz (Duke University, Durham, NC). Plasmids encoding GRKs 3, 5, and 6 were from Dr. Jonathan Willets (University of Leicester, Leicester, UK), arrestin3-GFP was from Dr. Marc Caron (Duke University), arrestin3 was from Dr. Vsevolod Gurevich (Vanderbilt University, Nashville, TN), HA-tagged human β2-adrenergic receptor was from Missouri S&T cDNA Resource Center (Rolla, MO), and GRK2 was from Dr. Jeffrey Benovic (Thomas Jefferson University, Philadelphia, PA), who also generously provided purified GRK2. Mutant receptors were prepared using QuikChange reagents from Stratagene (La Jolla, CA) and were confirmed by sequencing.
Cell Growth and Transfection.
HEK293 cells from the American Type Culture Collection were maintained in Dulbecco's modified Eagle's medium/F-12 media containing 5% fetal bovine serum and transfected with Lipofectamine (Invitrogen, Carlsbad, CA) or FugeneHD (Roche Diagnostics, Indianapolis, IN) 24 to 48 h before use. Cells were plated on uncoated glass coverslips for microscopy and on poly(l-lysine)-coated culture dishes for other experiments.
Phosphoreceptor ELISA.
Phosphorylated TRH receptor was quantified using an ELISA protocol with affinity-purified phosphosite-specific antibodies directed against phosphopeptides from different regions of the cytoplasmic tail of the receptor, as previously described in detail (Jones et al., 2007). In brief, dishes were rinsed, fixed with MeOH/acetone, air-dried, washed with PBS, blocked with RIPA/milk buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 10 mM NaF, 100 nM sodium orthovanadate, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, pH 8.0, and 5% nonfat dried milk), and incubated with 1:1000 primary antibody for 90 min. Plates were then washed three times with PBS, incubated with 1:7500 horseradish peroxidase-linked anti-rabbit antibody in RIPA/milk for 45 min, washed three times, and incubated for 5 to 20 min with ELISA TMB reagent (Sigma-Aldrich, St. Louis, MO). The reaction was terminated with 5% sulfuric acid. GRK-dependent phosphorylation of the β2-adrenergic receptor was measured identically using rabbit antibody against the pSer355 and pSer356 in the human receptor from Santa Cruz Biotechnology (Santa Cruz, CA) at 1:500.
Cell Surface ELISA.
Surface receptors containing aminoterminal V5- or HA-epitope tags were quantified in modified ELISA protocols. In one, cells were washed with PBS and then fixed for 10 min with 2% paraformaldehyde and processed as described above for phosphoreceptor ELISA, except that a nonpermeabilizing buffer, PBS plus 5% milk, was used in place of RIPA/milk. In another, primary antibody in Hanks' balanced salt solution with 20 mM HEPES, pH 7.4 (HBSS/HEPES) was added to intact cells, and incubation was carried out at 37°C for 20 to 30 min, when cells were washed three times and then fixed and incubated with secondary antibody. Primary antibodies, monoclonal anti-V5 (AbD Serotec, Raleigh, NC) or anti-HA (Covance Research Products, Princeton, NJ), were used at 1:5000 in both protocols, which gave equivalent results. When phosphorylation data were normalized to receptor expression, for each receptor, the A450 from the phosphoreceptor ELISA was divided by the A450 from the cell surface ELISA. Where noted, these ratios were expressed as a percentage of the wild-type value determined in the same experiment.
Receptor Internalization.
Transfected HEK293 cells in 12-well plates were washed once with PBS and then equilibrated in HBSS/HEPES for 20 to 60 min at 37°C when vehicle or agonist was added from a thrice-concentrated solution in HBSS/HEPES, and incubation continued for 5 to 60 min. The plate was put on ice, and 200 μl of 1:1000 mouse anti-V5 antibody in PBS with 5% milk was added per well. After 20 min, each well was washed three times for 5 min with PBS, fixed with 2% paraformaldehyde for 10 min, washed twice for 5 min with PBS at room temperature, incubated for 30 min with 200 μl of 1:5000 horseradish peroxidase-conjugated goat anti-mouse antibody, washed three times for 5 min with PBS, and analyzed as described above.
In Vitro Phosphorylation.
Crude membranes were prepared from 10-cm dishes of HEK293 cells expressing either wild-type or mutant V5-TRH receptor as follows. Cells were rinsed with ice-cold TM buffer (20 mM Tris and 2 mM MgCl2, pH 7.4), scraped into ice-cold TM buffer supplemented with 1:1000 protease inhibitors (Calbiochem), and incubated for 20 min. Cells were then homogenized on ice in a Dounce homogenizer (Wheaton, Millville, NJ) and centrifuged at 10,000g for 10 min at 4°C. The membrane pellets were resuspended in 50 μl in vitro kinase buffer (20 mM Tris, 2 mM EDTA, 5 mM MgCl2, 2 mM ATP, and 1 mM GTP, pH 7.5) supplemented with phosphatase inhibitors (10 mM NaF, 10 mM Na3PO4, and 100 nM sodium orthovanadate). GRK2 (100 nM) was added to some samples before the addition of 100 nM TRH for 5 min at 30°C. After incubation, 1 ml of ice-cold lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100, pH 8.0) with phosphatase and protease inhibitors was added. The samples were rotated for 20 min at 4°C and then centrifuged at 10,000g for 10 min. The supernatant was incubated with 1:100 affinity-purified Ab6959 overnight at 4°C in lysis buffer, 20 μl of Protein A/G beads (Santa Cruz Biotechnology) was added for 1 h at 4°C, and immune complexes were collected by centrifugation. The beads were washed twice with ice-cold lysis buffer and resuspended in LDS sample buffer (Invitrogen) supplemented with dithiothreitol. Electrophoresis and immunoblotting were carried out as described previously (Jones and Hinkle, 2005).
GRK2 Coimmunoprecipitation.
HEK293 cells were cotransfected with DNA encoding V5 epitope-tagged TRH receptors and a kinase-dead mutant of GRK2 (K220R). Live cells were incubated at room temperature for 1 h in HBSS/HEPES with 1:1000 anti-V5 antibody to label surface receptors. Cells were washed and then incubated with TRH for the times noted. After incubation, cells were lysed on ice with 0.1% n-dodecyl-β-d-maltoside in PBS (0.1% NDM buffer) supplemented with protease inhibitors for 20 min at 4°C and centrifuged at 10,000g for 10 min. Protein A/G beads (20 μl) were added for 1 h at 4°C, and immune complexes were collected by centrifugation and washed twice with 0.01% NDM buffer. Electrophoresis and immunoblotting were carried out as described above using 1:1000 rabbit anti-GRK2 (Santa Cruz Biotechnology) or 1:5000 anti-V5 antibody.
Arrestin3-GFP Recruitment.
HEK293 cells grown on untreated coverslips in 12-well plates were transiently transfected with arrestin3-GFP and either V5-WT or V5–6Q-TRH receptors. After 24 h, cells were rinsed once with PBS and incubated with or without 1 μM TRH for various times at 37°C in HBSS/HEPES. After agonist stimulation, the plates were fixed with 2% paraformaldehyde in PBS for 10 min at room temperature with shaking. Wells were then washed twice for 5 min with PBS, and coverslips were mounted on glass slides using ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole (Invitrogen) for imaging.
Other Methods.
[3H]Inositol labeling and inositol phosphate determination and [3H]MeTRH binding, measurement of acid/salt resistance, and Scatchard analysis were carried out as described previously (Jones and Hinkle, 2005). For equilibrium binding studies, cells were incubated for 90 min with [3H]MeTRH at concentrations from 0.6 to 40 nM and washed three times with ice-cold saline before cells were removed in 0.1% SDS and counted. Nonspecific binding was determined on parallel dishes containing a 100-fold or greater excess of unlabeled TRH or on mock-transfected cells and subtracted.
Statistical Analysis.
Data are shown as mean ± S.E.M. of triplicate determinations representative of at least three independent experiments unless otherwise noted. Where not visible, error bars fell within the symbol size. Data were statistically analyzed, as appropriate, with one-way analysis of variance and post hoc Tukey's test or Student's unpaired t test.
Results
Receptor Signaling in Helix 8 Mutant Receptors.
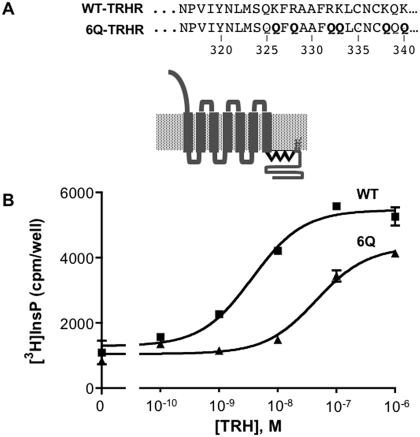
To examine the importance of positive charges in the helix 8 region of the receptor, we mutated six arginine and lysine residues to glutamines (6Q-TRH receptor). Four of these residues were between the NPXXY motif at the cytoplasmic end of the seventh transmembrane domain and the palmitoylation sites (Cys335 and Cys337), and two were at residues 338 and 340 (Fig. 1A). The helix prediction program Jpred3 (Cole et al., 2008) predicts that wild-type (WT) and all mutant receptors tested have a helical probability of more than 80%. Receptors were tagged at the amino terminus with a V5 epitope. The relative concentration of different receptor constructs at the cell surface was determined by ELISA; anti-V5 antibody was added to intact cells, such that antibody only had access to extracellular epitopes.
Fig. 1.
Signaling by TRH receptors. A, sequence of the helix 8 region of the WT and mutant rat TRH receptors. Helix 8 is depicted in boldface type. B, HEK293 cells were transfected with DNA encoding WT or 6Q-TRH receptors with N-terminal V5 epitope tags. Cells were metabolically labeled overnight with [3H]inositol and incubated with 10 mM LiCl and vehicle or TRH for 30 min, when total [3H]inositol phosphates were quantified. TRH receptor density was measured by cell-surface ELISA and was identical for both receptors. Results are from a representative experiment performed in duplicate.
The ability of WT and 6Q-TRH receptors to signal was tested in HEK293 cells metabolically labeled with [3H]inositol. [3H]Inositol phosphate formation was measured with a range of TRH concentrations. As shown in Fig. 1B, TRH increased inositol phosphate production strongly via both the WT and 6Q-TRH receptors, which were expressed at the same concentration. The 6Q receptor displayed a higher EC50 (45 nM) than the WT receptor (3.6 nM), consistent with its lower ligand affinity, as described below. The maximal response of the 6Q receptor, measured at 1 or 10 μM TRH, was between 60 and 90% of the WT response in different experiments.
We also tested a receptor in which Cys335 and Cys337 were converted to serine. The TRH receptor is normally palmitoylated at these sites. In agreement with published findings (Du et al., 2005), we found that this mutant signaled strongly, with an EC50 of 0.9 nM and a maximal response averaging 109% of the WT response (data not shown). These results indicate that neither positive charge in the helix 8 region nor acylation is required for robust receptor expression and signal transduction.
Importance of Helix 8 Region for Receptor Phosphorylation.
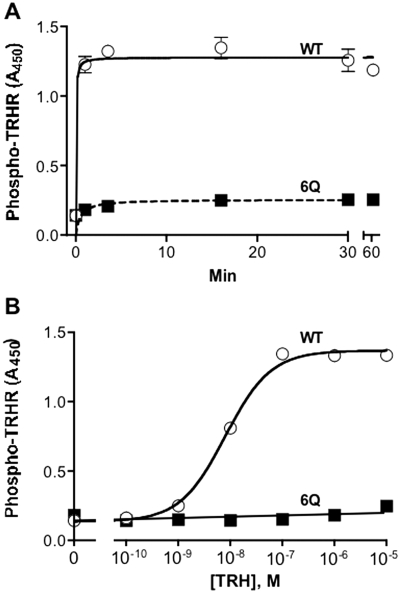
We quantified receptor phosphorylation by ELISA using a highly specific phosphosite-specific antibody that has been characterized extensively (Jones et al., 2007). As shown in Fig. 2, TRH caused more than a 10-fold increase in phosphorylation of the WT receptor but less than a 50% increase in the 6Q-TRH receptor. Phosphorylation of the WT receptor was half-maximal at 8 nM TRH (Fig. 2A). Phosphorylation of the 6Q-TRH receptor remained low even with long incubation periods and high concentrations of TRH (Fig. 2B).
Fig. 2.
Rate and concentration-dependence of TRH-induced receptor phosphorylation. Cells were transfected with WT or 6Q-TRH receptors. A, cells were incubated with 100 nM TRH for the times shown. B, cells were incubated for 5 min with the concentrations of TRH shown. Cells were fixed, and phosphorylated receptor was measured by ELISA using antibody 6959. Receptor levels were 32% lower for 6Q than for WT receptors. Backgrounds were identical in mock-transfected cells and cells not exposed to TRH. Results show the mean and range of duplicates in a representative experiment.
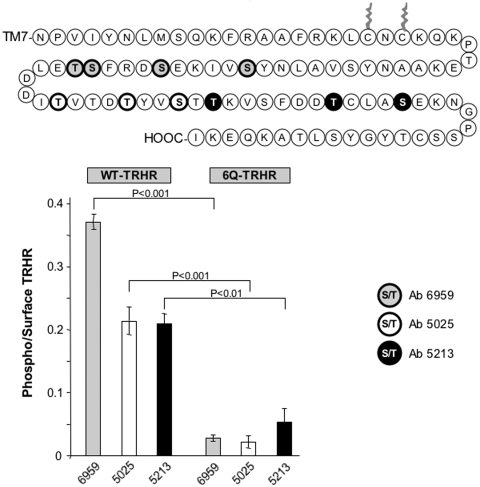
Agonist-induced phosphorylation of the WT-TRH receptor occurs at several different sites in the receptor tail (Jones et al., 2007). We determined whether positively charged residues in the helix 8 region were important for phosphorylation at different positions using a panel of phosphosite-specific antibodies (Fig. 3). The 6Q-TRH receptor was poorly phosphorylated at all regions of the cytoplasmic tail, including sites quite distal to helix 8.
Fig. 3.
Phosphorylation of different sites in the TRH receptor cytoplasmic tail. Cells were mock-transfected or were transfected with WT or 6Q-TRH receptor and then incubated for 5 min with vehicle or 100 nM TRH and fixed. Phosphorylation was measured with three different affinity-purified antibodies directed against phosphopeptides from the receptor tail (Jones et al., 2007). Antibodies were raised against peptides with the following residues phosphorylated: Ab6959: Ser355, Ser360, Ser364, and Thr365; Ab5025: Thr371, Thr375, and Ser378; and Ab5213: Thr380, Thr387, and Ser391. Results are normalized to correct for differences (<20%) in surface receptor expression and show mean and S.E.M. values of triplicates in a representative experiment. Signals in mock-transfected cells were equal to or slightly higher than signals in cells treated with vehicle, indicating that there was no constitutive phosphorylation.
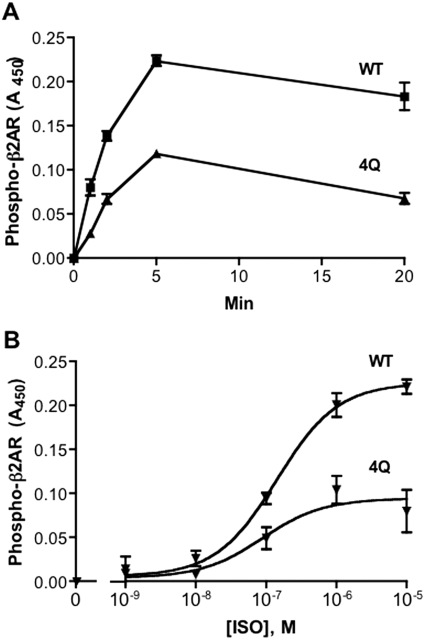
To learn whether the helix 8 region has the potential to influence receptor phosphorylation events in other GPCRs, we examined GRK site phosphorylation of the prototypical class A β2-adrenergic receptor using an antibody directed against two established GRK sites, phospho-Ser355 and phospho-Ser356 (Seibold et al., 2000). We made analogous mutations to those in the 6Q-TRH receptor by converting two arginine residues (328 and 333) between the NPXXY motif and the palmitoylation site (Cys341) and two arginine residues (343 and 344) just after the cysteine to glutamine. Glutamine substitution of the four arginine residues in the β2-adrenergic receptor did not reduce the isoproterenol-stimulated cAMP response (data not shown). As shown in Fig. 4, the 4Q-mutant β2-adrenergic receptor was phosphorylated only half as much as the wild-type receptor in response to all concentrations of isoproterenol. Furthermore, this defect, like that of the 6Q-TRH receptor, was not overcome with time, because stimulation with saturating amounts of agonist for as long as 20 min did not elicit steady-state phosphorylation greater than half of that of wild-type receptor.
Fig. 4.
Rate and concentration-dependence of isoproterenol-induced β2-adrenergic receptor phosphorylation. Cells were transfected with HA-tagged WT or 4Q-β2-adrenergic receptors. A, cells were incubated with 1 μM isoproterenol for the times indicated. B, cells were incubated for 5 min with the concentrations of isoproterenol (ISO) shown. Phosphorylated receptor was measured by ELISA using antibodies against phospho-β2-adrenergic receptor (pSer355/pSer356) and surface receptor expression with anti-HA antibody. 4Q receptors were expressed at 95% of the level of WT receptors. Results of two independent experiments performed in duplicate are shown.
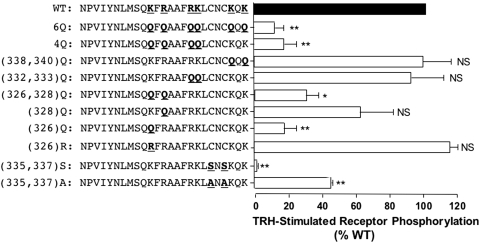
Identification of Critical Residues.
To determine whether a specific segment of helix 8 is required for efficient receptor phosphorylation, we mutated pairs of lysine and arginine residues and measured phosphorylation in response to TRH, again correcting for differences in receptor expression at the plasma membrane (Fig. 5). The two lysine residues distal to the palmitoylation site (338 and 340) were not involved, because mutating them to glutamine had no effect. Mutation of the four positive residues between transmembrane domain 7 and the palmitoylation site (4Q) greatly impaired TRH-dependent phosphorylation. It is interesting that the two lysine residues at positions 332 and 333 seemed to be unimportant, whereas positive charges at residues 326 and 328 were required. In fact, mutation of a single amino acid, Lys326, caused a marked decrease in TRH-dependent receptor phosphorylation. Conversion of Lys326 to arginine did not reduce receptor phosphorylation, ruling out mechanisms depending on ubiquitination and emphasizing the importance of positive charge at this position. A minor fraction of WT TRH receptor is ubiquitinated, apparently as part of a quality control mechanism, but no ubiquitinated receptor is found on the plasma membrane (Cook et al., 2003).
Fig. 5.
Role of positively charged residues in helix 8 and palmitoylation sites. V5-tagged TRH receptors with the mutations shown were expressed in HEK293 cells, which were incubated with or without 100 nM TRH for 5 min. Phosphorylated and cell surface receptor levels were quantified by ELISA. For each receptor, the level of phosphorylation was normalized to the level of the cell surface receptor. Results are expressed relative to the amount of phosphorylated WT receptor in the same experiment. Shown are the mean ± S.E.M. values of results obtained in three or four experiments, each performed in duplicate or triplicate. ∗∗, P < 0.001, ∗, P < 0.01; NS, not significant versus WT.
Mutation of the cysteine residues at positions 335 and 337 to alanine inhibited receptor phosphorylation by almost 60%, whereas mutation to serine completely prevented phosphorylation. The same results were obtained when the cysteine-substituted receptors were tested with antibodies against more distal phosphosites (data not shown). Our antibodies would not detect phosphorylation at Ser335 or Ser337, which are potential protein kinase C sites. Phosphorylation at the serine residues might explain the failure of the Cys(335,337)Ser receptor to undergo phosphorylation at the major sites used in the WT receptor. In fact, inclusion of 10 μM GF109203X, a protein kinase C inhibitor, increased phosphorylation of the Cys(335,337)Ser receptor at GRK sites, although not to levels observed with Cys(335,337)Ala or WT receptors (data not shown). Because the Cys(335,337)Ala mutant was significantly phosphorylated, it is clear that palmitoylation is not an absolute requirement for receptor phosphorylation.
Arrestin-Dependent Events.
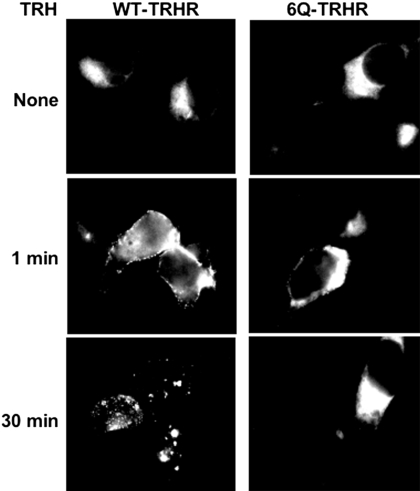
Arrestin is recruited to the activated TRH receptor when either the proximal or distal region of the cytoplasmic tail is phosphorylated. Requirements are more stringent for TRH receptor internalization and desensitization, which take place only if sites between amino acids 355 and 365 are phosphorylated (Jones and Hinkle, 2008). We characterized arrestin-receptor interactions by coexpressing different TRH receptors with arrestin3-GFP (Fig. 6). The addition of TRH to cells expressing WT receptor resulted in rapid translocation of arrestin-GFP to the plasma membrane and internalization of the arrestin-GFP-receptor complex into vesicles. In contrast, arrestin3-GFP was not recruited to activated 6Q-TRH receptors, and it did not move into vesicles during the next 30 min.
Fig. 6.
Interaction of TRH receptors with arrestin3-GFP. HEK293 cells expressing arrestin3-GFP and either WT or 6Q-TRH receptor were stimulated with 1 μM TRH for the times shown, fixed, and imaged.
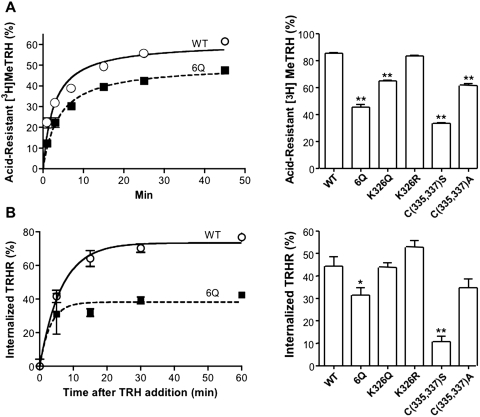
We also determined the ability of the 6Q and other mutant TRH receptors to form an acid-stable complex with agonist (Fig. 7A) and to undergo internalization, based on the amount of N-terminal V5 receptor epitope accessible to antibodies in intact cells before and after addition of TRH (Fig. 7B). The 6Q-TRH receptor formed a less acid-resistant complex with agonist and internalized less extensively than the WT receptor. The acid resistance of Lys326 and Cys335,337 mutants paralleled their ability to undergo phosphorylation, whereas internalization of the K326Q mutant was surprisingly high. The results are reminiscent of previous studies showing that the TRH receptor retains some ability to form an acid-resistant complex and undergo endocytosis in arrestin-null cells (Jones and Hinkle, 2005), pointing to the existence of an uncharacterized arrestin-independent pathway.
Fig. 7.
Internalization of TRH receptors and formation of an acid-resistant agonist-receptor complex. HEK293 cells were transfected with DNA encoding WT or mutant TRH receptors. A, cells were incubated with 5 nM [3H]MeTRH for the times shown (left) or with 10 nM [3H]MeTRH for 45 min (right), when the fraction of specifically bound hormone resistant to ice-cold 0.2 N acetic acid/0.5 M NaCl, pH 2.5, was measured. B, cells were stimulated with 100 nM TRH for the times shown (left) or with 1 μM TRH for 45 min (right), when the amount of receptor on the cell surface was measured by ELISA. Results represent the mean ± S.E.M. of four determinations. ∗, P < 0.05, ∗∗, P < 0.01 versus WT.
The arrestin-TRH receptor complex has much higher affinity for TRH than the nonphosphorylated receptor (Jones and Hinkle, 2005, 2008), just as other GPCR-arrestin complexes bind agonists with higher affinity than the GPCRs alone (Gurevich et al., 1997). When equilibrium [3H]MeTRH binding was measured in live HEK293 cells, the 6Q-TRH receptor had significantly lower apparent agonist affinity than the WT receptor (Table 1). It seemed likely that the low agonist affinity of the 6Q-TRH receptor resulted from its weak interaction with arrestin due to a lack of receptor phosphorylation. To test this idea, we measured [3H]MeTRH binding in mouse embryonic fibroblasts lacking endogenous arrestins (arrestin2/3knockout mouse embryonic fibroblasts) with and without transfection of arrestin3. Coexpression of arrestin3 had no significant effect on the affinity of the 6Q-substituted receptor for [3H]MeTRH, whereas arrestin caused a 14.4-fold increase in agonist affinity of the WT-TRH receptor.
TABLE 1.
TRH receptor affinity
Either HEK293 or arrestin2/3 knockout MEFs were transfected with WT or 6Q-TRH receptors. Equilibrium binding of [3H]MeTRH was measured, and Kd values were determined by Scatchard analysis. Values shown are the mean ± S.E.M. from three experiments. Data for WT receptor in arrestin-null cells are from Jones and Hinkle (2008).
| Cell Type |
Kd |
|
|---|---|---|
| WT-TRH Receptor | 6Q-TRH Receptor | |
| nM | ||
| HEK293 | 2.2 ± 0.2 | 9.1 ± 0.1 |
| Arrestin2/3KO MEF | ||
| Mock | 20.1 ± 2.6 | 13.8 ± 3.6 |
| +Arrestin3 | 1.4 ± 0.2 | 11.9 ± 1.5 |
Analysis of Phosphorylation Defect.
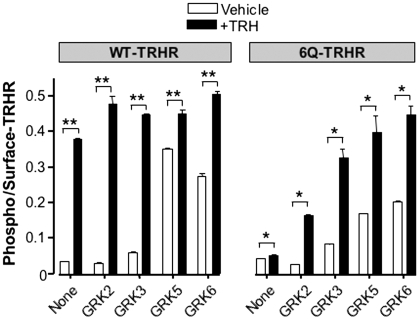
As discussed above, GRK2 has been implicated in phosphorylation of the TRH receptor cytoplasmic tail. We overexpressed GRK2 to determine whether this would overcome the deficit in phosphorylation observed with the 6Q-TRH receptor. GRK2 increased the phosphorylation of 6Q and WT-TRH receptors, but overexpression of the kinase did not fully restore phosphorylation of the mutant receptor (Fig. 8). GRK3 caused a small increase in basal receptor phosphorylation of the 6Q receptor and, like GRK2, increased phosphorylation in response to TRH. Overexpression of GRKs 5 and 6, which are membrane-localized all the time (Jiang et al., 2007), increased basal phosphorylation of WT and 6Q-substituted receptors.
Fig. 8.
Effect of GRK overexpression. Cells were transfected with WT or 6Q TRH receptors together with empty vector or GRK2, 3, 5, or 6 and then stimulated with vehicle or 100 nM TRH for 5 min. Phosphorylated and cell-surface receptor were measured by ELISA, and phosphorylation values were normalized to receptor expression. Results show the mean and S.E.M. of triplicates in a representative experiment. ∗∗, P < 0.001, ∗, P < 0.01 versus vehicle-treated control.
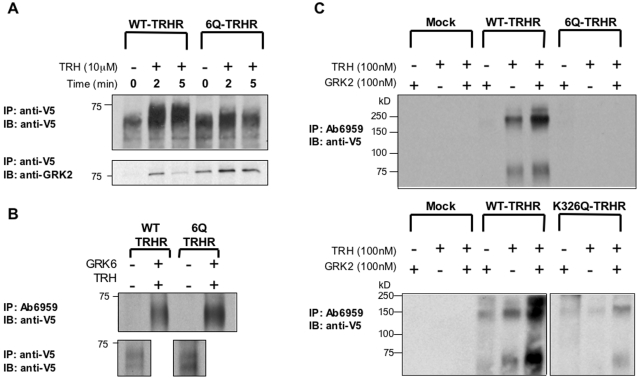
Interactions of the WT and 6Q receptors with GRK2 were characterized in cells transfected with receptor and kinase-dead K220R-GRK2. Surface receptors were labeled with anti-V5 antibodies, and cells were exposed to TRH for various times. Receptors were solubilized in n-dodecyl-β-d-maltoside, and immunoblots were probed for total receptor and GRK2 (Fig. 9A). TRH rapidly increased the amount of kinase associated with the WT receptor but exerted a much smaller effect on the 6Q receptor, which showed stronger association with the kinase in the absence of agonist. On average, TRH increased GRK2 coprecipitated with receptor 6.9-fold for WT and 1.5-fold for 6Q receptors at 2 min, when responses peaked. TRH caused a strong shift in the mobility of the WT receptor but only a small change in the mobility of the 6Q receptor, consistent with differences in phosphorylation.
Fig. 9.
In vitro phosphorylation of TRH receptors and interactions with GRK2. A, cells expressing WT or 6Q-TRH receptors with or without K220R-GRK2 were incubated with antibody to V5 to label surface receptors, washed, and incubated with or without 10 μM TRH for 0 to 5 min. Receptors were solubilized with dodecyl-β-d-maltoside, and immune complexes were collected on protein A/G beads. Immunoprecipitates were resolved on SDS-polyacrylamide gel electrophoresis and blotted for either total receptor (top) or GRK2 (bottom). B, cells were transfected with WT or 6Q receptors with or without GRK6 and exposed to vehicle or 100 nM TRH for 5 min. Proteins were solubilized with Triton X-100, and phosphorylated and total receptors were immunoprecipitated with antibodies to phosphoreceptor (Ab6959) (top) and V5 epitope (bottom), respectively, resolved on gels and blotted with anti-V5 antibody. C, membranes were isolated from cells expressing WT, 6Q, or K326Q-TRH receptors and incubated in in vitro kinase buffer with or without 100 nM TRH and 100 nM GRK2 for 5 min. Immunoprecipitation and immunoblotting were carried out as in B. Densitometric analysis of blots of total receptor in lysates from the same experiment (data not shown) revealed that 6Q and K326Q receptors were expressed at 92 and 65% of WT, respectively.
Similar results were obtained when the recruitment of endogenous GRK2 to the membrane was monitored. Most endogenous GRK2 was already membrane-associated in nontransfected cells. The ratio of membrane/soluble GRK2 reached a maximum 30 s after TRH addition, increasing 2. 8 ± 1.1-fold in cells expressing WT receptor and 1.5 ± 0.7-fold (n = 3) in cells expressing the 6Q-substituted receptor, but these differences were not statistically significant (data not shown). Both approaches suggest that GRK2 associates less strongly with activated receptor when charged residues in helix 8 are replaced. This deficit could result from either failure of the 6Q receptor to recruit the kinase or weaker association because the receptor does not bind well to the enzyme active site.
To test whether the 6Q-TRH receptor could serve as a substrate for GRK2 under conditions in which kinase recruitment was not an issue, we carried out in vitro phosphorylation assays using membranes from cells expressing 6Q, K326Q, and WT-TRH receptors and purified GRK2. The extent of phosphorylation was analyzed by solubilizing receptors and then immunoprecipitating with phosphosite-specific antibody 6959, which recognizes TRH receptors phosphorylated at residues between 355 and 365. The experiment was designed in this manner because phosphosite-specific antibodies against the TRH receptor work well for immunoprecipitation but not for immunoblotting. To verify that antibody 6959 can immunoprecipitate phosphorylated 6Q-TRH receptors, we overexpressed GRK6 and treated cells expressing either WT or 6Q receptors with TRH to drive phosphorylation. Antibody 6959 efficiently immunoprecipitated WT and 6Q-TRH receptors under these conditions (Fig. 9B).
The WT receptor was partially phosphorylated by endogenous kinases when membranes were incubated with TRH in ATP-containing buffer and more extensively phosphorylated when purified GRK2 was added (Fig. 9C). Phosphorylation of the WT receptor was completely dependent on agonist stimulation. In contrast, the 6Q-TRH receptor was not phosphorylated alone or in the presence of GRK2, and the K326Q receptor was only slightly phosphorylated. Receptors were expressed at comparable concentrations in these experiments. Results from the in vitro phosphorylation assays establish that GRK2 is not active toward receptors lacking critical lysine and arginine residues in helix 8.
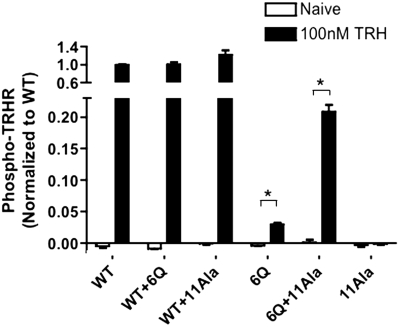
Activation of a single rhodopsin molecules leads to the phosphorylation of hundreds of others, a phenomenon referred to as high-gain or trans-phosphorylation (Hurley et al., 1998). Trans-phosphorylation occurs with the TRH receptor too, because a nonactivatable mutant TRH receptor that binds agonist but does not become phosphorylated can undergo phosphorylation if it is coexpressed with an activatable receptor lacking phosphorylation sites (Song et al., 2007). Here we tested whether the 6Q-TRH receptor, which possesses all serine and threonine residues in the receptor tail, becomes phosphorylated when expressed with a mutant TRH receptor (11Ala) lacking all 11 conserved serine and threonine residues in the C-terminal domain, including the four residues recognized by the phosphosite-specific antibody. If the 11Ala mutant can activate GRK when paired with the 6Q receptor, then phosphorylation of the 6Q receptor might occur, because it is the only substrate. As shown in Fig. 10, coexpression of 11Ala with 6Q-TRH receptors resulted in a significant increase in TRH-stimulated phosphorylation of the 6Q receptor, to approximately 20% of the level observed with WT receptor. It is noteworthy that only a fraction of 6Q receptors would be expected to participate in the formation of 6Q-11Ala, as opposed to 6Q–6Q, dimers.
Fig. 10.
Complementation of TRH-stimulated receptor phosphorylation. HEK293 cells were cotransfected with DNA encoding two different TRH receptors, as shown; when only one receptor was expressed, a control plasmid was included. The 11Ala-TRH receptor was N-terminally tagged with HA, and all other receptors were tagged with V5 epitopes. Cells were incubated with or without 100 nM TRH for 5 min. Phosphorylated receptors were quantified by ELISA using antibody 6959. Signal from mock-transfected cells was subtracted from experimental values. The results are normalized to the phosphorylation of WT-TRH receptor in the same experiment. Coexpression of the 11Ala receptor increased surface expression of the WT and 6Q receptors by 21 and 31%, respectively. The increase in 6Q receptor expression does not account for the >500% increase in 6Q receptor phosphorylation caused by coexpression of 11Ala receptors. Shown are the mean ± S.E.M. values of three independent experiments performed in duplicate. ∗, P < 0.01.
Discussion
The helix 8 region of GPCRs forms an amphipathic helix that lies parallel to the plasma membrane and is anchored by palmitoylated cysteine groups (Palczewski et al., 2000; Shimamura et al., 2008). Helix 8 is believed to move upon receptor activation. For example, disulfide cross-linking approaches show that the aminoterminal part of helix 8 of the M3 muscarinic receptor lies close to the cytoplasmic end of transmembrane helix 1 when the receptor is in an inactive state but moves away when the receptor binds agonist (Li et al., 2007). Spin-labeling studies of rhodopsin indicate movement in the helix 8 region after rhodopsin activation (Altenbach et al., 2008). Crystallographic studies of opsin bound to a peptide from Gα, which is proposed to resemble the activated state of rhodopsin, suggest that a kink between the end of the seventh transmembrane domain and helix 8 is rearranged when the receptor assumes an activated conformation (Scheerer et al., 2008). We have shown here that mutation of positively charged residues in helix 8 produces a TRH receptor that signals strongly in response to agonist, implying that it is essentially normal in its interactions with G protein but is not phosphorylated by GRKs. Disruption of helix 8 by mutation of the normal cysteine acylation sites also produces a TRH receptor that couples effectively to G proteins but is poorly phosphorylated. Likewise, glutamine substitution of positively charged amino acids in the proximal region of the β2-adrenergic receptor impairs GRK-dependent receptor phosphorylation but not signaling, suggesting that helix 8 is important for phosphorylation of multiple class A GPCRs. Although basic clusters in the helix 8 region are required for folding and trafficking of several receptors (Huynh et al., 2009), substitution of charged residues in this segment of the TRH and β2-adrenergic receptors does not appreciably alter surface expression. Deletion of hydrophobic residues in the helix 8 region of the leukotriene B4 type-2 receptor also results in an intracellularly trapped receptor, but no trafficking defect is observed when only the polar residues are mutated (Yasuda et al., 2009). In a few cases, alterations in helix 8 cause constitutive activity and in one, constitutive phosphorylation (Faussner et al., 2005; Suvorova et al., 2009).
Several investigators (Okuno et al., 2005; Swift et al., 2006; Huynh et al., 2009) have surveyed GPCR sequences and noted that a phenylalanine-lysine/arginine motif is often found five or six residues downstream from the tyrosine of the canonical NPXXY at the end of the seventh transmembrane domain. The NPXXY at the cytoplasmic end of transmembrane helix 7 forms ionic and hydrogen bonds with the downstream phenylalanine and in some GPCRs, intracellular loop 1 (Swift et al., 2006). The helix 8 region of the type 1 TRH receptor adheres to this pattern and is highly conserved. Furthermore, the type 1 and type 2 mouse TRH receptors are nearly identical in helix 8 even though the two receptors are only 50% homologous overall. It is interesting that the key positively charged residue for phosphorylation of the TRH receptor, Lys326, is not in the position of the invariant lysine or arginine but rather on the aminoterminal side of the phenylalanine in the NPXXY(X5–6)K/R motif.
Several studies suggest direct contact between helix 8 of GPCRs and G proteins. Peptides from helix 8 of rhodopsin bind directly to transducin and Gβγ (Ernst et al., 2000; Marin et al., 2000), and the helix 8 segments of the β1-adrenergic and angiotensin II receptors interact with G protein subunits (Sano et al., 1997; Delos Santos et al., 2006). Cell-permeant peptides from the helix 8 region of PAR1 disrupt G protein coupling (Swift et al., 2006), and many GPCRs cannot signal effectively after truncation or mutation of helix 8 (Huynh et al., 2009). The discrepancy between the effect of mutations in helix 8 on G protein-dependent signaling and GRK-mediated phosphorylation of the TRH receptor was not predicted, although a similar dissociation has been reported for rhodopsin, in which certain mutations in the first intracellular loop block phosphorylation without preventing transducin binding (Shi et al., 1995). Zidar et al. (2009) reported that one peptide agonist for the CCR7 receptor activates phosphorylation by GRK6 and GRK3, whereas another activates phosphorylation by GRK6 only; both peptides stimulate G protein signaling. Thus, receptor conformations necessary for the activation of G proteins can be distinguished from those required for activation of GRKs by receptor mutation, as shown here, and with selective ligands, as shown for CCR7 receptors.
The WT-TRH receptor is a superb substrate for GRKs, undergoing nearly quantitative GRK-mediated phosphorylation in seconds in response to agonist. The loss of positive charge in helix 8 converts the receptor to a very poor substrate, even though the most important charged residue in helix 8, Lys326, is some distance upstream from the major phosphorylation site at Thr365 (Jones et al., 2007). GRK2, which is primarily responsible for TRH receptor phosphorylation (Jones et al., 2007), is recruited to the plasma membrane in part by Gβγ released upon receptor activation. The defect in 6Q-TRH receptor phosphorylation is unlikely to result from inadequate recruitment of GRK2 by Gβγ, because the mutant receptor activates Gβγ effectively, and phosphorylation is not restored by overexpression of GRK2 in cells or by the addition of purified GRK2 to isolated membranes.
GRKs are believed to make multiple contacts with activated GPCRs. Peptides derived from intracellular loops 2 and 3 and the proximal tail of rhodopsin interact with rhodopsin kinase (GRK1) (Kelleher and Johnson, 1990; Palczewski et al., 1991). Peptides from several domains of the β2-adrenergic receptor inhibit the activity of purified GRK2, with the strongest interaction between intracellular loop 1 and the kinase (Benovic et al., 1990; Winstel et al., 2005). Three different regions of the β2-adrenergic receptor tail show little apparent affinity for GRK2, but the proximal end of helix 8 has not been tested (Benovic et al., 1990). The structures of GRK2 alone and in complexes with Gβγ and Gαq have been solved (Lodowski et al., 2003; Tesmer et al., 2005; Singh et al., 2008; Sterne-Marr et al., 2009), and GRK2 seems to be capable of interacting simultaneously with Gβγ, Gαq, and receptor. Molecular modeling suggests that one molecule of GRK2 may interact with a GPCR dimer (Tesmer et al., 2005; Singh et al., 2008).
Unlike other ATP-binding cassette kinases, GRKs have an open kinase groove, and they depend on interactions with activated receptors to close the kinase region and form an active enzyme. Using a combination of crystallographic, mutagenic, and kinetic analyses, Tesmer and coworkers (Singh et al., 2008; Huang et al., 2009; Sterne-Marr et al., 2009) identified a surface in the aminoterminal region and kinase domains of GRKs 1 and 2 that is critical for kinase interaction with activated GPCRs. The cytoplasmic tail of the receptor sits in an open groove on the kinase while different regions of the receptor interact elsewhere with the enzyme. The idea of multiple contact points is supported by observations showing that the activity of GRKs toward peptide substrates is increased dramatically in the presence of receptor (Palczewski et al., 1991).
Our data suggest that positively charged residues in the helix 8 region are involved in GRK activation. Conversion of these residues to glutamine may disrupt the helical nature of helix 8 or may disrupt the association with negatively charged membrane lipids, thereby altering kinase recognition of the receptor. It is uncertain whether these amino acids contact the kinase directly or are necessary for the agonist-occupied receptor to achieve a conformation capable of activating the enzyme. In the present study, coexpression of the 11Ala-TRH receptor, which has an intact helix 8 but no phosphorylation sites, potentiated the normally weak phosphorylation of 6Q-TRH receptors. This finding suggests that the 11Ala receptor may make the necessary contacts with GRK to cause the kinase domain to close and allow phosphorylation of the 6Q receptor in a dimer pair. Consistent with this, a mutant TRH receptor that cannot be activated or phosphorylated on its own undergoes GRK-dependent phosphorylation when it is expressed with a mutant receptor that signals strongly but lacks phosphorylation sites (Song et al., 2007).
Taken together, the results presented here show that the positively charged face of helix 8 contributes to the phosphorylation of agonist-occupied GPCR by GRKs. For the TRH and β2-adrenergic receptor, the regions of the helix 8 region that are critical for kinase activation are not important for G protein coupling. Additional studies should help clarify the molecular basis for the helix 8-kinase interactions and expand our understanding of the phosphorylation step that initiates desensitization pathways important for physiological GPCR signaling.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK19974].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.059733
- TRH
- thyrotropin-releasing hormone
- GPCR
- G protein-coupled receptor
- GFP
- green fluorescent protein
- GRK
- G protein-coupled receptor kinase
- HEK
- human embryonic kidney
- WT
- wild-type
- ELISA
- enzyme-linked immunosorbent assay
- PBS
- phosphate-buffered saline
- RIPA
- radioimmunoprecipitation assay
- HBSS
- Hanks' balanced salt solution
- HA
- hemagglutinin
- GF109203X
- 3-[1-[3-(dimethylaminopropyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione monohydrochloride.
References
- Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. (2008) High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc Natl Acad Sci U S A 105: 7439–7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, Onorato J, Lohse MJ, Dohlman HG, Staniszewski C, Caron MG, Lefkowitz RJ. (1990) Synthetic peptides of the hamster beta 2-adrenoceptor as substrates and inhibitors of the beta-adrenoceptor kinase. Br J Clin Pharmacol 30 (Suppl 1): 3S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Klein-Seetharaman J, Farrens D, Zhang C, Altenbach C, Hubbell WL, Khorana HG. (1999) Single-cysteine substitution mutants at amino acid positions 306–321 in rhodopsin, the sequence between the cytoplasmic end of helix VII and the palmitoylation sites: sulfhydryl reactivity and transducin activation reveal a tertiary structure. Biochemistry 38: 7925–7930 [DOI] [PubMed] [Google Scholar]
- Cole C, Barber JD, Barton GJ. (2008) The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36: W197–W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LB, Zhu CC, Hinkle PM. (2003) Thyrotropin-releasing hormone receptor processing: role of ubiquitination and proteasomal degradation. Mol Endocrinol 17: 1777–1791 [DOI] [PubMed] [Google Scholar]
- Delos Santos NM, Gardner LA, White SW, Bahouth SW. (2006) Characterization of the residues in helix 8 of the human beta1-adrenergic receptor that are involved in coupling the receptor to G proteins. J Biol Chem 281: 12896–12907 [DOI] [PubMed] [Google Scholar]
- Du D, Raaka BM, Grimberg H, Lupu-Meiri M, Oron Y, Gershengorn MC. (2005) Carboxyl tail cysteine mutants of the thyrotropin-releasing hormone receptor type 1 exhibit constitutive signaling: role of palmitoylation. Mol Pharmacol 68: 204–209 [DOI] [PubMed] [Google Scholar]
- Ernst OP, Meyer CK, Marin EP, Henklein P, Fu WY, Sakmar TP, Hofmann KP. (2000) Mutation of the fourth cytoplasmic loop of rhodopsin affects binding of transducin and peptides derived from the carboxyl-terminal sequences of transducin alpha and gamma subunits. J Biol Chem 275: 1937–1943 [DOI] [PubMed] [Google Scholar]
- Faussner A, Bauer A, Kalatskaya I, Schüssler S, Seidl C, Proud D, Jochum M. (2005) The role of helix 8 and of the cytosolic C-termini in the internalization and signal transduction of B(1) and B(2) bradykinin receptors. FEBS J 272: 129–140 [DOI] [PubMed] [Google Scholar]
- Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, Ernst OP. (2003) Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc Natl Acad Sci U S A 100: 2290–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Pals-Rylaarsdam R, Benovic JL, Hosey MM, Onorato JJ. (1997) Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem 272: 28849–28852 [DOI] [PubMed] [Google Scholar]
- Huang CC, Yoshino-Koh K, Tesmer JJ. (2009) A surface of the kinase domain critical for the allosteric activation of G protein-coupled receptor kinases. J Biol Chem 284: 17206–17215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JB, Spencer M, Niemi GA. (1998) Rhodopsin phosphorylation and its role in photoreceptor function. Vision Res 38: 1341–1352 [DOI] [PubMed] [Google Scholar]
- Huynh J, Thomas WG, Aguilar MI, Pattenden LK. (2009) Role of helix 8 in G protein-coupled receptors based on structure-function studies on the type 1 angiotensin receptor. Mol Cell Endocrinol 302: 118–127 [DOI] [PubMed] [Google Scholar]
- Jiang X, Benovic JL, Wedegaertner PB. (2007) Plasma membrane and nuclear localization of G protein coupled receptor kinase 6A. Mol Biol Cell 18: 2960–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Hinkle PM. (2005) Beta-arrestin mediates desensitization and internalization but does not affect dephosphorylation of the thyrotropin-releasing hormone receptor. J Biol Chem 280: 38346–38354 [DOI] [PubMed] [Google Scholar]
- Jones BW, Hinkle PM. (2008) Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol Pharmacol 74: 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Song GJ, Greuber EK, Hinkle PM. (2007) Phosphorylation of the endogenous thyrotropin-releasing hormone receptor in pituitary GH3 cells and pituitary tissue revealed by phosphosite-specific antibodies. J Biol Chem 282: 12893–12906 [DOI] [PubMed] [Google Scholar]
- Kelleher DJ, Johnson GL. (1990) Characterization of rhodopsin kinase purified from bovine rod outer segments. J Biol Chem 265: 2632–2639 [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38: 289–319 [DOI] [PubMed] [Google Scholar]
- Li JH, Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, Zhang X, Wess J. (2007) Distinct structural changes in a G protein-coupled receptor caused by different classes of agonist ligands. J Biol Chem 282: 26284–26293 [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science 300: 1256–1262 [DOI] [PubMed] [Google Scholar]
- Marin EP, Krishna AG, Zvyaga TA, Isele J, Siebert F, Sakmar TP. (2000) The amino terminus of the fourth cytoplasmic loop of rhodopsin modulates rhodopsin-transducin interaction. J Biol Chem 275: 1930–1936 [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Benkö G, Filipek S, Palczewski K. (2003) Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry 42: 2759–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Yokomizo T, Hori T, Miyano M, Shimizu T. (2005) Leukotriene B4 receptor and the function of its helix 8. J Biol Chem 280: 32049–32052 [DOI] [PubMed] [Google Scholar]
- Palczewski K, Buczyłko J, Kaplan MW, Polans AS, Crabb JW. (1991) Mechanism of rhodopsin kinase activation. J Biol Chem 266: 12949–12955 [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289: 739–745 [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67: 653–692 [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. (2007) Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 69: 511–534 [DOI] [PubMed] [Google Scholar]
- Sano T, Ohyama K, Yamano Y, Nakagomi Y, Nakazawa S, Kikyo M, Shirai H, Blank JS, Exton JH, Inagami T. (1997) A domain for G protein coupling in carboxyl-terminal tail of rat angiotensin II receptor type 1A. J Biol Chem 272: 23631–23636 [DOI] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455: 497–502 [DOI] [PubMed] [Google Scholar]
- Seibold A, Williams B, Huang ZF, Friedman J, Moore RH, Knoll BJ, Clark RB. (2000) Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol Pharmacol 58: 1162–1173 [DOI] [PubMed] [Google Scholar]
- Shi W, Osawa S, Dickerson CD, Weiss ER. (1995) Rhodopsin mutants discriminate sites important for the activation of rhodopsin kinase and Gt. J Biol Chem 270: 2112–2119 [DOI] [PubMed] [Google Scholar]
- Shimamura T, Hiraki K, Takahashi N, Hori T, Ago H, Masuda K, Takio K, Ishiguro M, Miyano M. (2008) Crystal structure of squid rhodopsin with intracellularly extended cytoplasmic region. J Biol Chem 283: 17753–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Wang B, Maeda T, Palczewski K, Tesmer JJ. (2008) Structures of rhodopsin kinase in different ligand states reveal key elements involved in G protein-coupled receptor kinase activation. J Biol Chem 283: 14053–14062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GJ, Jones BW, Hinkle PM. (2007) Dimerization of the thyrotropin-releasing hormone receptor potentiates hormone-dependent receptor phosphorylation. Proc Natl Acad Sci U S A 104: 18303–18308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne-Marr R, Leahey PA, Bresee JE, Dickson HM, Ho W, Ragusa MJ, Donnelly RM, Amie SM, Krywy JA, Brookins-Danz ED, et al. (2009) GRK2 activation by receptors: role of the kinase large lobe and carboxyl-terminal tail. Biochemistry 48: 4285–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova ES, Gripentrog JM, Jesaitis AJ, Miettinen HM. (2009) Agonist-dependent phosphorylation of the formyl peptide receptor is regulated by the membrane proximal region of the cytoplasmic tail. Biochim Biophys Acta 1793: 406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Leger AJ, Talavera J, Zhang L, Bohm A, Kuliopulos A. (2006) Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J Biol Chem 281: 4109–4116 [DOI] [PubMed] [Google Scholar]
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. (2005) Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science 310: 1686–1690 [DOI] [PubMed] [Google Scholar]
- Winstel R, Ihlenfeldt HG, Jung G, Krasel C, Lohse MJ. (2005) Peptide inhibitors of G protein-coupled receptor kinases. Biochem Pharmacol 70: 1001–1008 [DOI] [PubMed] [Google Scholar]
- Yasuda D, Okuno T, Yokomizo T, Hori T, Hirota N, Hashidate T, Miyano M, Shimizu T, Nakamura M. (2009) Helix 8 of leukotriene B4 type-2 receptor is required for the folding to pass the quality control in the endoplasmic reticulum. FASEB J 23: 1470–1481 [DOI] [PubMed] [Google Scholar]
- Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. (2009) Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A 106: 9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]