Abstract
In this study, we investigated the potential of combined treatment with temozolomide (TMZ) chemotherapy and tumor antigen-pulsed dendritic cells (DCs) and the underlying immunological factors of TMZ chemoimmunotherapy with an intracranial GL26 glioma animal model. The combined treatment enhanced the tumor-specific immune responses and prolonged the survival more effectively than either single therapy in GL26 tumor-bearing animals. Apoptosis was induced in the tumors of the animals by the treatment with TMZ. Calreticulin (CRT) surface exposure was detected by immunofluorescence staining of TMZ-treated GL26 cells. TMZ chemotherapy increased tumor antigen cross-priming from tumor cells, leading to cross-priming of tumor antigen-specific CD4+ T cells and CD8+ T cells. This chemotherapy appeared to suppress the frequency of CD4+ CD25+ regulatory T cells (Treg). Moreover, this combined therapy resulted in an increase in the tumor infiltration of CD4+ and CD8+ T cells. Collectively, the findings of this study provide evidence that the combination of TMZ chemotherapy and treatment with DC-based vaccines leads to the enhancement of antitumor immunity through increased tumor-specific immune responses via the cross-priming of apoptotic tumor cell death mediated by CRT exposure and, in part, the suppression of Treg. Therefore, CRT exposure, regulatory T cells, and cross-priming by TMZ chemotherapy may be immunological factors related to the enhancement of the antitumor effects of chemoimmunotherapy in an experimental brain tumor model.
Most tumors express an array of antigens that act as targets for their immune-mediated destruction, and a number of potential therapies have emerged to exploit this (22). The immunotherapeutic strategy used to induce an immune response against tumors is quite attractive because it offers the potential for a high level of tumor-specific cytotoxicity, minimal side effects, and a durable effect.
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) in the induction of primary immune responses (29, 33). Because of their central role in controlling cell-mediated immunity, DCs hold much promise as cellular adjuvants in therapeutic cancer vaccines. DC-based immunotherapy has been reported to induce strong antitumor immune responses in animal experiments and in selected clinical trials involving malignant gliomas (2, 11, 36). However, its clinical effects on patients with malignancies have not been up to the expectations because of immune tolerance, the sheer physical burden of tumor antigens, and the mechanisms of tumor escape from the immune surveillance system, among others (10, 20).
Calreticulin (CRT) acts as a danger signal for DCs, allowing them to phagocytose tumor cells and to prime tumor antigen-specific cytotoxic T cells (CTLs) (12). It was recently reported that CRT exposure on the surfaces of dying tumor cells may determine whether chemotherapy is immunogenic (26). The capacity of chemotherapies to induce immunogenic tumor cell death is associated with the expression of CRT on the tumor cell surface. Furthermore, it was shown with an animal tumor model that the provision of CRT from an exogenous CRT exposure source as enforcement for endogenous CRT exposure could improve the efficacy of chemotherapy by stimulating antitumor immunity (27). Thus, whether chemotherapy triggers such an immunogenic effect depends on the exposure of CRT on the cell surface.
The use of multimodality treatments that combine conventional antitumor therapies with immunotherapy, such as vaccination with DC-based vaccines, has emerged as a potentially plausible approach to the treatment of tumors (3, 5). We previously reported that the use of a multimodality treatment regimen with a DC-based vaccine in combination with the chemotherapeutic agent temozolomide (TMZ) leads to enhanced tumor-specific CTL responses and enhanced antitumor effects, resulting in a cure rate higher than that achieved with either a DC-based vaccine or TMZ alone (17, 28). However, the immunological factors relating to the antitumor effect of TMZ chemoimmunotherapy in a murine glioma model are still unclear.
To explore the association of the immunological factors related to the enhanced antitumor effect by use of the combination of DC immunotherapy and TMZ chemotherapy, we investigated the effect of TMZ on the cross-priming of antigen, regulatory T cells, the in vitro depletion of a T-cell subpopulation, and the surface exposure of CRT, which are thought to be the major factors determining the antitumor immune response.
MATERIALS AND METHODS
Animals and cell lines.
Six- to 8-week-old female C57BL/6 (H-2b) mice were purchased from Shizuoka Laboratory Center (Shizuoka, Japan). The GL26 murine glioma cell line (H-2b) was kindly provided by John S. Yu (Cedars Sinai Medical Center, Los Angeles, CA). The GL26 cells were cultured in Dulbecco modified Eagle medium (Gibco BRL Co., Grand Island, NY) supplemented with 10% heated-inactivated fetal bovine serum (FBS; Gibco), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The CT26 (H-2d), EL4 (H-2b), and YAC-1 (H-2a) cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA). These cells were used as the control target in the CTL assay.
Antibodies.
The bone marrow DC surfaces were characterized by their unique expression of several cell surface-associated markers, as determined with fluorescently labeled monoclonal antibodies (MAbs) and quantified on a FACScan instrument (Becton Dickinson, Franklin Lakes, NJ). The cells were stained with the following antibodies (PharMingen, San Diego, CA): CD11c (HL3), CD80 (16-10A1), CD86 (GL1), and major histocompatibility complex (MHC) class II (2G9).
Proliferation assay.
The GL26 cells were seeded at 1 × 103 cells/well (200 μl) in 96-well flat-bottomed plates, and the plates were incubated overnight at 37°C. After 24 h, the cells were treated with TMZ (Schering-Plough, Kenilworth, NJ) at a variety of concentrations (50 μM, 100 μM, 200 μM, 400 μM, and 800 μM) for 72 h. Cell proliferation was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma Chemical Co., Steinheim, Germany). In brief, 50 μl of MTT (2 mg/ml) solution was added to each well, and then, after incubation for 4 h, the medium containing MTT solution was removed. The precipitate was then resuspended in 100 μl of dimethyl sulfoxide (Sigma Chemical Co.). The medium was used as a blank. The absorbance was measured at 550 nm with background subtraction at 650 nm by using an enzyme-linked immunosorbent assay reader (Molecular Devices, San Francisco, CA).
Induction and detection of tumor apoptosis or necrosis.
For the induction of apoptosis, the GL26 cells were treated with TMZ (100 μM) for 4 h, 12 h, 24 h, 48 h, and 72 h. In addition, the GL26 cells were irradiated at 80 Gy. The irradiated cells were washed twice with phosphate-buffered saline (PBS) and cultured for 4 h, 12 h, 24 h, 48 h, and 72 h. For the induction of necrosis, the GL26 cells underwent one freeze (in liquid nitrogen)-thaw (in a water bath at 37°C) cycle. One freeze-thaw cycle allows the destruction of cells while maintaining the integrity of the cell membrane (8). For the further detection of apoptosis or necrosis, we used an annexin V/7-amino-actinomycin D (7-AAD) apoptosis detection kit (BD PharMingen, San Diego, CA) on a FACScan apparatus (BD Biosciences, Mountain View, CA). In addition, the tumor lysates were prepared as described previously (16). In brief, GL26 cells were resuspended at a density of 1 × 107 cells/ml in serum-free medium. The cell suspension was frozen in liquid nitrogen and was then thawed in a water bath of 37°C. The freeze-thaw cycle was repeated four times in rapid succession. The larger particles were removed by centrifugation at 600 rpm for 10 min. The supernatant was passed through a 0.2-μm-pore-size filter, and an aliquot was stored in a deep freeze.
Generation of bone marrow-derived DCs.
The DCs were prepared from bone marrow, as described previously, with minor modifications (16). In brief, bone marrow cells were harvested from the tibias and femurs of healthy C57BL/6 mice. The cells were washed twice in serum-free RPMI 1640 (Gibco BRL) medium and cultured in six-well culture plates at 5 × 106 cells/well in complete RPMI 1640 medium supplemented with 10% heat-inactivated FBS, recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/ml; R&D Systems, Minneapolis, MN), and recombinant murine interleukin-4 (IL-4; 20 ng/ml; R&D Systems). On day 2, nonadherent granulocytes were gently removed and fresh medium with GM-CSF and IL-4 was added. On day 7, the nonadherent and loosely adherent cells obtained from these cultures were considered to be immature bone marrow-derived DCs. These cells were removed, washed, and placed in 100-mm petri dishes at 106 cells/ml for 48 h in the presence of fresh GM-CSF, IL-4, and 100 ng/ml lipopolysaccharide (LPS) (Sigma, St. Louis, MO) to allow the DCs to fully mature. The tested cells expressed surface marker, such as CD11c, MHC class II, CD80, and CD86 (Fig. 1).
FIG. 1.
Production of mature murine DCs from bone marrow cells. Murine bone marrow cells were stimulated with GM-CSF, IL-4, and LPS, as described in Materials and Methods. Class II, MHC class II.
In vivo antitumor effect of combined treatment.
The brain tumor model, which consisted of the intracerebral (i.c.) inoculation of GL26 cells as described previously (16), was used for this study. The mice were divided into eight groups (n = 7 mice in each group) and were treated intraperitoneally (i.p.) with TMZ (2.5 mg/kg of body weight/day) from days 2 to 6 or subcutaneously with DCs (1 × 106), tumor lysate-pulsed DCs (1 × 106), or apoptotic tumor cell-pulsed DCs (1 × 106) on days 4, 11, 18 after i.c. GL26 cell inoculation. In these experiments, seven mice were used in each treatment group. Representative mice from each treatment group were killed at selected time points to obtain tissues (spleen) for immunological analysis.
In vitro depletion of CD4+ and CD8+ T cells.
Splenocytes were reacted with magnetic beads conjugated to MAbs to CD4 or CD8 (MACS; Miltenyi Biotec GmbH, Germany) for 1 h at 4°C. Following incubation, the cells were washed with PBS and processed through a MACS magnetic separation column. Cell viability after depletion was determined by trypan blue dye exclusion. The cell depletion procedure depleted >98% of the specific T-cell subpopulation, as determined by fluorescent-activated cell sorter (FACS) analysis.
Immunohistochemistry.
Serial 10-μm paraffin sections of tumor tissue were stained with anti-CD4 (rat MAb, 1:50; BD PharMingen) and anti-CD8 (rat MAb, 1:100; BD PharMingen) primary antibodies and isotype control antibodies. The primary antibodies were detected by use of the biotin-peroxidase system (Dako Corp.).
Fluorescence detection of cell surface CRT.
GL26 cells (on a glass slide or in 12-well plates) were first washed with FACS buffer (1× PBS, 5% fetus bovine serum, 0.1% sodium azide) and were then incubated with rabbit anti-mouse CRT antibody (1:100; Stressgen) in FACS buffer at 4°C for 30 min. The cells were allowed to react with anti-rabbit IgG (H+L)-Alexa fluor 488 conjugates (1:500) in FACS buffer at 4°C for 30 min. After the cells were washed three times with FACS buffer, surface CRT was detected by FACS analysis.
Assessment of apoptotic cells in brain tissue.
To detect apoptotic cells in brain tissue sections, the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) method (ApopTag in situ apoptosis detection kit; Oncor, Gaithersburg, MD) was used according to the manufacturer's instructions. The embedded tissues were sectioned (thickness, 4 μm), deparaffinized with xylene, and incubated with 20 μg/ml proteinase K for 15 min at room temperature to digest the proteins. Chemicon proprietary equilibration buffer was applied to the sections at room temperature, and the sections were immediately incubated with DNA strand break labeling buffer containing terminal deoxynucleotidyl transferase in a humidified chamber at 37°C for 1 h. The sections were washed with stop/wash buffer and were then washed with PBS. Finally, the sections were incubated with an antidigoxigenin conjugate at room temperature for 30 min and visualized by using diaminobenzidine (DAB) colorization (DiNonA, Seoul, South Korea).
Cytotoxicity assay.
On day 35 after GL26 cell inoculation, splenocytes were harvested from the mice in the different treatment groups for the preparation of single-cell suspensions. These splenocytes, restimulated in vitro with 4% paraformaldehyde-prefixed GL26 cells in the presence of recombinant murine IL-2 (20 U/ml) for 5 days, were used as effector cells. The GL26, EL4, CT26, and YAC-1 target cells were labeled with 51Cr (100 μCi/1 × 106 cells) for 1 h, washed four times, and then added in triplicate to each well of 96-well V-bottom-well microtiter plates with various numbers of the effector cells. After incubation for 4 h at 37°C, 100 μl of the supernatant of each well was collected, and the radioactivity was counted with a gamma counter. The percent specific lysis was calculated as described previously (16).
ELISPOT assay.
An enzyme-linked immunospot (ELISPOT) assay was performed with a kit purchased from Autoimmun Diagnostika (Strasburg, Germany), according to the manufacturer's instructions. In brief, the restimulated splenocytes were seeded into a 96-well multitest plate (MTP) coated with anti-mouse gamma interferon (IFN-γ) antibody at a concentration of 1 × 105 cells/well in cell culture medium. The plates were incubated for 24 h at 37°C. The cells were removed, and the plates were washed three times with a washing buffer (provided in the kit) and three times with a PBS-Tween buffer (provided in the kit). One hundred microliters of biotinylated anti-mouse IFN-γ MAb (detection antibody; provided in the kit) was then added to the wells. The plates were incubated for 2.5 h at room temperature and washed with PBS-Tween buffer, 100 μl of streptavidin-horseradish peroxidase was then added to each well, and the plates were incubated for 2 h at room temperature. The washing step was repeated, and chromogenic substrate (provided in the kit) and H2O2 were then immediately added to each well. After the spots developed, the reaction was quenched with distilled water and the plates were inverted and allowed to dry overnight in the dark. The number of spots corresponding to the IFN-γ-secreting cells was determined with an automatic AID ELISPOT reader.
Statistical analysis.
The results are expressed as the means ± the standard errors of the means. Statistical analysis was performed by Student's t test for all data with the exception of the survival data, which were analyzed by using the Kaplan-Meier test. The survival data were compared by using a log-rank test. A P value of <0.05 was considered significant.
RESULTS
Inhibition of proliferation of GL26 cells by TMZ.
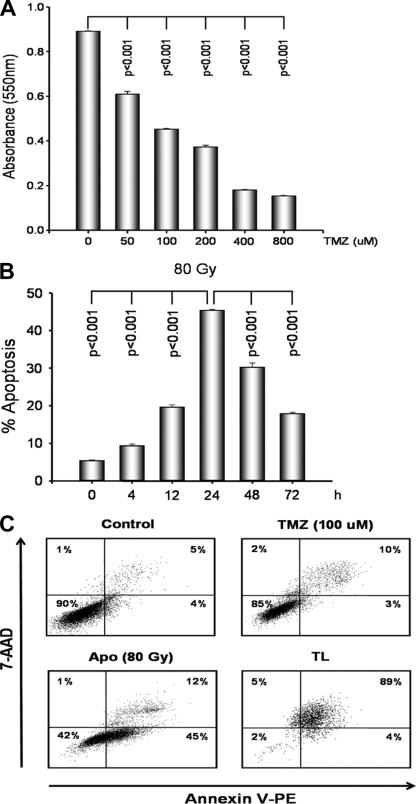
To examine the antiproliferative effect of TMZ on GL26 cells, the cells were treated for 72 h with TMZ at a variety of concentrations (50 to 800 μM), and then an MTT assay was performed. As shown in Fig. 2A, TMZ inhibited the proliferation of GL26 cells in a dose-dependent manner. The 50% inhibitory concentration of TMZ was 100 μM.
FIG. 2.
Effects of TMZ, irradiation, and freeze-thaw treatments on GL26 cells. (A) GL26 cells were seeded at 1 × 103 cells per well in 96-well flat-bottomed plates and incubated overnight. After 24 h, the cells were treated with a variety of concentrations (50, 100, 200, 400, and 800 μM) of TMZ for 72 h, and then cell proliferation was evaluated by the MTT assay. (B) GL26 cells were irradiated with doses of 80 Gy and cultured for various periods of time (4, 12, 24, 48, and 72 h). The GL26 cells were evaluated by FACS analysis for annexin V-phycoerythrin and 7-AAD. Early apoptotic cells were defined as annexin V-phycoerythrin positive and 7-AAD negative. (C) After 24 h, the GL26 cells were treated with TMZ (100 μM), with irradiation (80 Gy), or by freeze-thawing (TL) and were evaluated by FACS analysis. Necrotic cells were defined as annexin V-phycoerythrin positive and 7-AAD positive. Data are representative of those from three independent experiments. The results are given as the means ± standard errors.
Induction of apoptosis or necrosis by GL26 cells.
To determine the optimal conditions for apoptosis, GL26 cells were irradiated with doses of 80 Gy and cultured for different periods of time (4 to 72 h). Apoptosis was assessed with an annexin V/7-AAD apoptosis detection kit. As shown in Fig. 2B, the highest rate of apoptosis induction was evident at 24 h. As shown in Fig. 2C, 24 h after culture, 45% of the GL26 cells were irradiated with 80 Gy and stained annexin V positive and 7-AAD negative (early apoptotic cells). To induce necrosis, the GL26 cells underwent one freeze-thaw cycle and were cultured for 24 h. As shown in Fig. 2C, 89% of the GL26 cells were freeze-thawed and stained annexin V positive and 7-AAD positive (necrotic cells). There were a slight number of late apoptotic cells in the presence of 100 μM TMZ.
In vivo antitumor effect of low-dose TMZ.
In preliminary experiments, treatment with TMZ at higher doses (50 mg/kg and 100 mg/kg i.p.) was associated with a high mortality rate among the animals used for the murine GL26 model (data not shown). We previously evaluated a variety of doses to optimize an effective low dose of TMZ (28). The rate of survival was increased in all mice treated with a variety of doses (2.5 mg/kg/day, 5 mg/kg/day, and 10 mg/kg/day) of TMZ compared with the rate of survival for the control mice. Toxicity, as measured by weight loss and neurological behavior, was not observed in any of the TMZ-treated mice (data not shown). Therefore, we used TMZ at a dose of 2.5 mg/kg/day in the following experiments.
Prolongation of survival of mice by combination treatment with low-dose TMZ and tumor antigen-pulsed DCs.
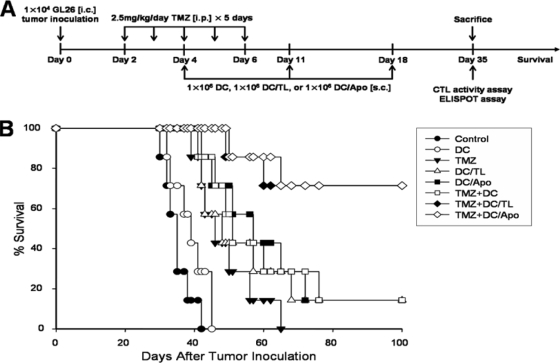
We evaluated the therapeutic efficacy of combination treatment in an established i.c. GL26 glioma model. The treatment schedule is shown schematically in Fig. 3A. As shown in Fig. 3B, treatment with TMZ alone, tumor antigen-pulsed DCs alone (tumor lysate-pulsed DCs [DCs/TL] or apoptotic tumor cell-pulsed DCs [DCs/Apo]), or TMZ and unpulsed DCs (TMZ-DCs) resulted in a length of survival slightly longer than that for mice treated with PBS or DCs alone. Furthermore, combination treatment with TMZ-DCs/TL or TMZ-DCs/Apo significantly prolonged the length of survival compared with that achieved with TMZ alone, TMZ-DCs/TL alone, or TMZ-DCs.
FIG. 3.
Prolongation of survival by combination treatment of the mice comprising the GL26 glioma model with TMZ and tumor antigen-pulsed DCs. (A) Experimental schedule of survival. Five consecutive daily intraperitoneal injections of TMZ (2.5 mg/kg/day) were administered on days 2 to 6 after the inoculation of GL26 cells (1 × 104) intracranially. The mice were treated subcutaneously with DCs (1 × 106), tumor lysate-pulsed DCs (1 × 106), or apoptotic tumor cell-pulsed DCs (1 × 106) on days 4, 11, and 18. (B) Kaplan-Meier survival curve of the mice that were inoculated with GL26 cells intracranially. The significant differences (log-rank test) were as follows: for control versus TMZ treatment, P = 0.0009; for control versus TMZ-DC treatment, P = 0.0004; for control versus TMZ-DC/Apo treatment, P = 0.0002; for TMZ versus TMZ-DC treatment, P = 0.1939; for TMZ versus TMZ-DC/Apo treatment, P = 0.0018; for TMZ-DC versus TMZ-DC/Apo treatment, P = 0.0270. Seven mice were used in each experimental group.
Increased tumor-specific immune response by combination treatment with low-dose TMZ and DCs/TL.
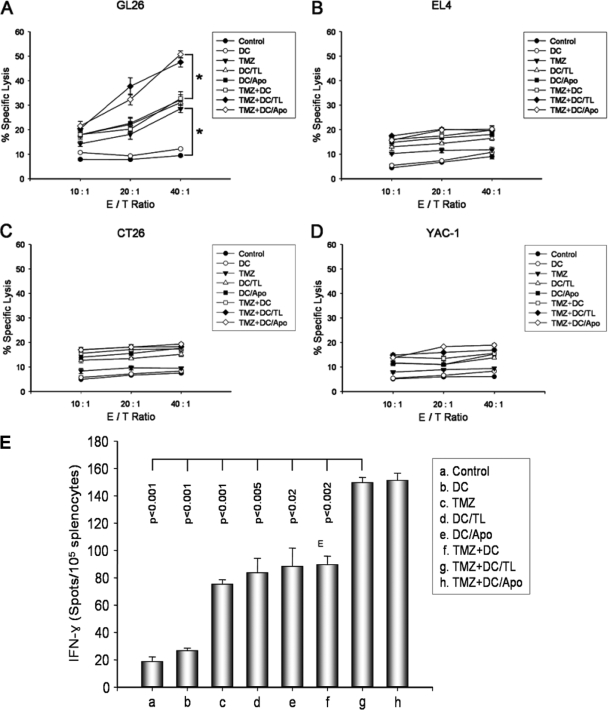
To examine the antitumor immune mechanism associated with combination treatment with TMZ-DCs/TL or TMZ-DCs/Apo, splenocytes were harvested from the mice in the different treatment groups on day 35 after GL26 cell inoculation, and a single-cell suspension was prepared. As shown in Fig. 4A, the specific cytotoxicity of the effector cells from the mice treated with TMZ, DCs/TL, DCs/Apo, and TMZ-DCs induced the killing response of the CTLs against the syngeneic GL26 target cells (28.54% ± 1.52%, 31.06% ± 2.33%, 32.18% ± 2.11%, and 32.73% ± 2.82%, respectively; effector cell /tumor cell [E/T] ratio, 40:1). In particular, the specific cytotoxicity of the effector cells from the mice treated with TMZ-DCs/TL or TMZ-DCs/Apo significantly enhanced the activity of the CTLs against the GL26 target cells (47.54% ± 1.92% and 50.75% ± 1.43%, respectively; E/T ratio, 40:1) but not against the EL4 (H-2b), CT26 (H-2d), and YAC-1 (H-2a) target cells (Fig. 4B, C, and D, respectively). As shown in Fig. 4E, splenocytes from the mice treated with TMZ-DCs/TL or TMZ-DCs/Apo showed significantly higher numbers of IFN-γ-secreting T cells than splenocytes from the mice in the control group treated with other preparations. These results suggest that combination treatment with TMZ chemotherapy and immunotherapy with tumor antigen-pulsed DCs enhances the tumor-specific immune response against GL26 glioma cells.
FIG. 4.
The efficient induction of CTL activity and the tumor-specific immune response were enhanced by combination treatment with TMZ and tumor antigen-pulsed DCs in the GL26 glioma model. (A to D) On day 35 after the inoculation of GL26 (1 × 104) cells, splenocytes from each group were harvested in vitro and restimulated with 4% paraformaldehyde and prefixed with GL26 cells as effector cells for 5 days. These effector cells were then assessed for their cytolytic activity against target cells (GL26, EL4, CT26, and YAC-1 cells) labeled with 51Cr. The 51Cr release was measured after 4 h of incubation at a variety of E/T ratios. (E) The numbers of IFN-γ-secreting splenocytes from treated mice, as described above, were measured by the ELISPOT assay after restimulation in vitro with 4% paraformaldehyde and prefixation with GL26 cells for 5 days. The results represent the mean number of IFN-γ spots per 105 splenocytes from individually tested mice. *, statistically significant (P < 0.01) by Student's t test compared with the results for all other groups. The results are given as the means ± standard errors and are representative of those from two independent experiments.
Cross-priming effect by TMZ.
To further determine whether the enhanced antitumor effect of the combination therapy was due to the direct effect of TMZ, in which it increased the cross-priming of APCs, tumor-specific CD4+ cells and CD8+ T cells were evaluated by the ELISPOT assay with tumor-bearing mice treated with TMZ and/or DCs/TL. Tumor-free control mice were also examined.
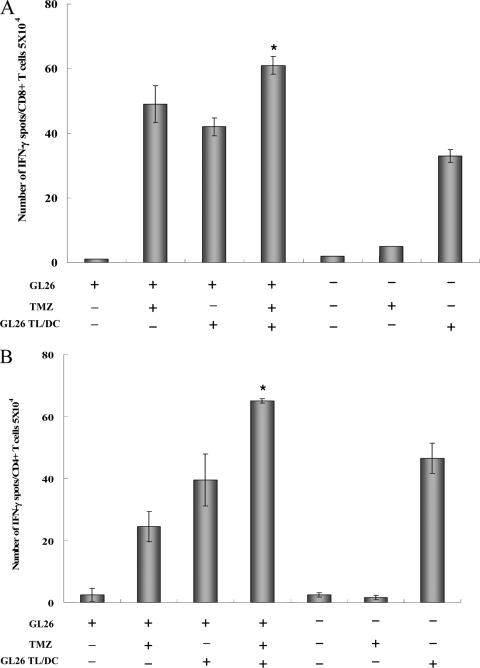
In the tumor-bearing mice, monotherapy with the DC vaccine or TMZ induced significant tumor-specific CD4+ T-cell and CD8+ T-cell immune responses (Fig. 5A and B). However, combination treatment with TMZ-DCs/TL resulted in a robust increase in the number of IFN-γ-secreting tumor-specific CD4+ T cells and CD8+ T-cell precursors compared to the numbers obtained by the use of monotherapy with TMZ alone or DCs/TL alone. Thus, our data demonstrate that TMZ chemotherapy increases the level of tumor antigen cross-priming from tumor cell death, leading to the cross-priming of tumor-specific T cells.
FIG. 5.
Effect of cross-priming by TMZ. Tumor-bearing mice or tumor-free mice were treated i.p. with TMZ on five consecutive days (2.5 mg/kg/day); and GL26 tumor lysate-pulsed DCs (1 × 106 cells/mouse in 200 μl of PBS) were injected on days 13, 20, and 27. One week after the last injection, the mice were killed and the spleen cells were pooled. Splenocytes were stimulated in vitro with tumor lysate-pulsed DCs for 18 h. The numbers of IFN-γ-secreting CD8+ T cells (A) and CD4+ T cells (B) specific for GL26 tumor cells were determined by the ELISPOT assay with splenocytes from individual mice. *, statistically significant (P < 0.01) by Student's t test compared with the results for all other groups. The results are given as the means ± standard errors and are representative of those from two independent experiments.
Increased tumor infiltration by lymphocytes by combination treatment with low-dose TMZ and tumor antigen-pulsed DCs.
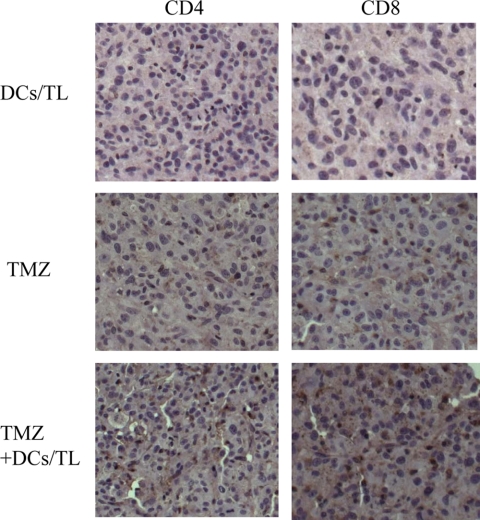
Because effective tumor immunity has been associated with increased lymphocytic infiltration of tumors, we evaluated the tumor infiltration of CD4+ and CD8+ T cells from brain tumor tissue. An increased level of infiltration of CD4+ and CD8+ T cells was observed in brain tumors from mice vaccinated with TMZ-DCs/TL compared with the level of infiltration achieved by vaccination of TMZ alone or DCs/TL alone (Fig. 6). This result suggests that the observed tumor lymphocyte infiltration involves CD4+ and CD8+ cytotoxic T cells.
FIG. 6.
Immunohistochemical characterization of CD4+ and CD8+ T-cell infiltration in a brain tumor section. Representative images of the tumor infiltration of CD4+ and CD8+ T cells were obtained by fluorescence microscopy. Magnifications, ×200.
Surface exposure of CRT and induction of apoptosis in brain tumor tissue by TMZ.
To investigate the effect of the surface exposure of CRT by TMZ on immunogenic cell death, GL26 cells were treated with TMZ (100 to 400 μM) for 24 to 48 h, followed by immunofluorescence staining with a CRT-specific antibody and FACS analysis. As shown in Fig. 7A, the treatment of cells with TMZ for 48 h induces the surface exposure of CRT, whereas the treatment of cells for 24 h did not (data not shown). In addition, the rate of apoptosis in brain tumor tissue was significantly higher in mice that were treated with TMZ than in control mice (Fig. 7B).
FIG. 7.
Surface exposure of CRT on TMZ-treated GL26 cells and histological examination of apoptosis of TMZ-treated GL26 brain tumor cells. (A) GL26 cells were treated with TMZ at the indicated concentration, followed by immunofluorescence staining with a CRT-specific antibody and cytofluorometric analysis. FITC, fluorescein isothiocyanate. (B) Photomicrographs of stained tumor tissue from control mice (left panel) and TMZ (2.5 mg/kg)-treated mice (right panel) used in the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling assay Magnifications, ×200.
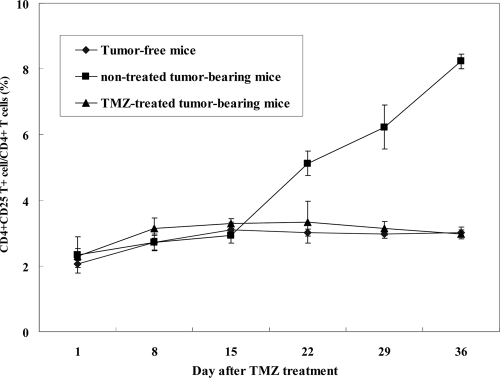
Effect of TMZ on Treg.
To address the effect of TMZ on CD4+ CD25+ regulatory T cells (Treg), C57BL/6 mice were treated with TMZ 2 days after tumor cell inoculation or were not treated. Splenocytes were prepared on days 1, 8, 15, 22, 29, and 36 after TMZ treatment. The cells were double labeled with anti-CD4 and anti-CD25 MAbs. As shown in Fig. 8, the frequency of CD4+ CD25+ Treg on day 22 increased more slowly in untreated GL26 tumor-bearing mice. However, on days 22, 29, and 36 after TMZ treatment, the frequency of CD4+ CD25+ Treg was suppressed in GL26 tumor-bearing mice. This result indicates that treatment with TMZ induces the suppressive effect of CD4+ CD25+ Treg in GL26 tumor-bearing mice.
FIG. 8.
Effect of TMZ on CD4+ CD25+ regulatory T cells from GL26 tumor-bearing mice. C57BL/6 mice were treated with TMZ or were not treated 2 days after GL26 tumor cell inoculation. Splenocytes were prepared on days 1, 8, 15, 22, 29, and 36 after TMZ treatment on five consecutive days (2.5 mg/kg/day). The cells were double-labeled with anti-CD4 and anti-CD25 MAbs.
DISCUSSION
Chemotherapy and immunotherapy are expected to be among the important strategies that will be used in the future as an improved means of treatment of malignant gliomas. Chemotherapy alone frequently fails due to the development of drug resistance or serious toxicity; immunotherapy has been unable to maintain effective antitumor immune responses in the presence of a bulky tumor burden because of the effects of tumor-derived immunosuppressive factors such as transforming growth factor β and IL-10 (6, 7). Many studies have reported that treatment with a combination of chemotherapy and immunotherapy may be more effective than treatment with either modality in the combination alone. Recent preclinical and clinical data have shown that many chemotherapeutic agents, such as cyclophosphamide and gemcitabine, enhance the efficacy of immunotherapy (15, 19, 25). Explainable mechanisms of action are the inhibition of Treg by chemotherapeutic agents, the enhancement of antigen presentation by tumors, alteration of the tumor microenvironment, or some yet unknown mechanisms, as well as the generally accepted lessening of the inhibitory immune effect by tumors and improvement of the effector T-cell/tumor cell ratio after chemotherapy (18). Previously, we reported that TMZ in combination with vaccination with DCs had enhanced antitumor effects in an animal model (17, 28). The present study examined the underlying immunological factors for this and showed that TMZ chemotherapy and DC-based vaccination in combination elicited the enhancement of antitumor immunity through the increased tumor-specific immune response via the increased cross-priming of antigens from apoptotic tumor cells mediated by CRT exposure and, in part, the suppression of Treg.
Concerning the in vitro activity of TMZ against glioma cells, Roos et al. (30) reported that TMZ (100 μM) inhibits cell proliferation, induces G2/M arrest, and induces apoptosis in U87 human glioma cells. The in vitro effect of TMZ on GL26 murine glioma cells is not well known. In the present study, TMZ inhibited the proliferation of GL26 cells in a dose-dependent manner at a variety of concentrations (50 to 800 μM) (Fig. 2A). Apoptosis was induced in TMZ-treated tumors (Fig. 7B), and these apoptotic GL26 cells were efficiently phagocytosed by immature DCs (data not shown). This result indicates that phagocytosed TMZ-treated GL26 apoptotic cells may be related to the increased antigen presentation of DCs.
Combination treatment with low-dose TMZ and tumor antigen-pulsed DCs significantly enhanced the tumor-specific immune responses and prolonged the survival of tumor-bearing animals (Fig. 3B and 4A and E). Chemotherapy increases the rate of antigen cross-presentation, and DC-based immunotherapy appears to promote efficient cross-priming and can drive the activation and expansion of tumor antigen-specific T cells (9, 24). Our data are consistent with the notion that tumor-bearing mice treated with TMZ plus tumor lysate-pulsed DCs display a significant increase in the number of tumor-specific IFN-γ-secreting CD4+ T cells and CD8+ T cells compared to the number in tumor-free mice (Fig. 5A and B). Moreover, combination treatment with low-dose TMZ and tumor antigen-pulsed DCs resulted in the robust infiltration of CD4+ T cells and CD8+ T cells into the brain tumor (Fig. 6). Taken together, these results indicate that CD4+ T cells and CD8+ T cells are responsible for the enhanced cell-mediated immunity achieved with the combined treatment. However, a direct assay, such as an in vivo depletion study, should be performed to determine the cell population responsible for the antitumor effects of TMZ plus tumor lysate-pulsed DCs observed in the present study.
The exposure of CRT on the cell surface is known to be a major factor in determining the antitumor immune response (26). CRT exposure occurs upstream of apoptosis or necrosis as part of a specific danger-signaling system. As shown in Fig. 7A and B, CRT surface exposure was detectable by immunofluorescence staining of TMZ-treated glioma cells, and the rate of apoptosis in the brain tumor tissue was significantly higher in mice that were treated with TMZ than in control mice. TMZ might injure the tumor cells, which in turn increases the level of release of danger signals, such as uric acid and heat shock protein (4, 31). The tumors in which apoptosis is massively induced release the danger signals, which are thought to stimulate DC maturation, which in turn can present antigens and stimulate T lymphocytes for a strong Th1 response (32). Together, these results provide indirect evidence that the increased number of apoptotic tumor cells after chemotherapy leads to more tumor antigen-pulsed DCs in vaccinated brain tumor models, resulting in increased numbers of tumor-specific CTLs through cross-priming. Further experiments are needed to investigate the direct evidence of cross-priming in mice deficient in transporters associated with antigen processing, in which host cells cannot cross-prime antigens. Recently, another report showed that tumor cell death triggered by chemotherapy initiates an immunoadjuvant pathway that contributes to the success of cytotoxic treatments. For example, the release of high mobility group box 1 protein (HMGB1) from dying tumor cells promotes the cross-presentation of tumor antigens and tumor-specific cytotoxic T-cell responses (1).
Machiels et al. (21) and Ghiringhelli et al. (13) reported that cyclophosphamide chemotherapy following DC-based immunotherapy increases the antitumor immune responses by suppressing regulatory T cells and increasing the Th1-type immune response. In our experimental setting, the frequency of CD4+ CD25+ regulatory T cells was not changed at an early stage (up to day 15) after the GL26 tumor inoculation in animals either not treated with TMZ or treated with TMZ. At a late stage, on days 22, 29, and 36, the frequency of CD4+ CD25+ regulatory T cells was increased in the group not treated TMZ but not increased in the group treated with TMZ (Fig. 8). This result indicates that treatment with TMZ induces the suppressive effect of CD4+ CD25+ Treg in GL26 tumor cell-bearing mice. Recently, a time-dependent increase in the frequency and the number of CD4+ regulatory T cells was observed within the brain tumor during the growth of the glioma in a syngeneic mouse glioma model (14). The suppression of Treg by TMZ at an early stage of tumor development might be one explanation for the observed enhancement of antitumor immunity in this study. Further studies will be needed to confirm the effect of TMZ on the regulatory T cells in advanced brain tumors.
Regarding the doses of TMZ chemotherapy, Tentori et al. (34) reported that TMZ (100 mg/kg/day i.p. for 3 days) displays activity against malignant melanomas, gliomas, and lymphomas growing in murine brain tumor models. In our mouse model of a GL26 brain tumor, the administration of TMZ at a low dose (2.5 mg/kg/day/i.p. for 5 days) prolonged survival without causing toxicity, and TMZ at higher doses (50 mg/kg and 100 mg/kg) was associated with a high rate of mortality (data not shown). The significant in vivo antitumor effects of the combined treatment demonstrated here may be related to the early treatment of animals with a minimal tumor burden. In addition, these findings also support the contention that the optimal timing and the dose of chemotherapy are important factors in the clinical implementation of this therapeutic strategy.
Although the splenocytes are exclusively reactive against glioma proteins, further experiments are needed to investigate whether these splenocytes are reactivated by an irrelevant control protein. Furthermore, a major barrier to the use of total tumor-derived antigen is the possibility that it will induce autoimmunity to peptides that are shared by healthy tissues. In clinical trials with DCs pulsed with total tumor lysate or total tumor RNA as the source of the antigen, no clinically relevant autoimmune responses were detected (23, 35). However, we need to investigate further whether the tumor-specific CTLs stimulated by tumor lysate-pulsed DCs can recognize antigens expressed by healthy control tissues as well as tumor antigens in this study.
In conclusion, the combination of low-dose TMZ chemotherapy and tumor antigen-pulsed DC immunotherapy may have the potential to be a safe and effective strategy for the treatment of malignant gliomas with minimal tumor burden, and immunological factors relating to the enhancement of the antitumor effect of TMZ chemoimmunotherapy against an experimental brain tumor may involve cross-priming mediated by increased CRT exposure and, in part, the suppression of Treg. Maximizing the effects of the combined protocols, treatment doses, treatment numbers, treatment timing, and administration routes and optimizing the treatment schedules for TMZ chemotherapy are goals that will be pursued.
Acknowledgments
This study was supported by a grant (grant 200700401) from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
Footnotes
Published ahead of print on 4 November 2009.
REFERENCES
- 1.Apetoh, L., A. Tesniere, F. Ghiringhelli, G. Kroemer, and L. Zitvogel. 2008. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 68:4026-4030. [DOI] [PubMed] [Google Scholar]
- 2.Ardavin, C., S. Amigorena, and C. Reis e Sousa. 2004. Dendritic cells: immunobiology and cancer immunotherapy. Immunity 20:17-23. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, C., F. Bauernfeind, A. Sterzik, M. Orban, M. Schnurr, H. A. Lehr, S. Endres, A. Eigler, and M. Dauer. 2007. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 56:1275-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder, R. J., and P. K. Srivastava. 2005. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 6:593-599. [DOI] [PubMed] [Google Scholar]
- 5.Chandy, A. G., M. Nurkkala, A. Josefsson, and K. Eriksson. 2007. Therapeutic dendritic cell vaccination with Ag coupled to cholera toxin in combination with intratumoural CpG injection leads to complete tumour eradication in mice bearing HPV 16 expressing tumours. Vaccine 25:6037-6046. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Q., V. Daniel, D. W. Maher, and P. Hersey. 1994. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int. J. Cancer 56:755-760. [DOI] [PubMed] [Google Scholar]
- 7.Constam, D. B., J. Philipp, U. V. Malipiero, P. ten Dijke, M. Schachner, and A. Fontana. 1992. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J. Immunol. 148:1404-1410. [PubMed] [Google Scholar]
- 8.Driessens, G., M. Hamdane, V. Cool, T. Velu, and C. Bruyns. 2004. Highly successful therapeutic vaccinations combining dendritic cells and tumor cells secreting granulocyte macrophage colony-stimulating factor. Cancer Res. 64:8435-8442. [DOI] [PubMed] [Google Scholar]
- 9.Dudley, M. E., J. R. Wunderlich, P. F. Robbins, J. C. Yang, P. Hwu, D. J. Schwartzentruber, S. L. Topalian, R. Sherry, N. P. Restifo, A. M. Hubicki, M. R. Robinson, M. Raffeld, P. Duray, C. A. Seipp, L. Rogers-Freezer, K. E. Morton, S. A. Mavroukakis, D. E. White, and S. A. Rosenberg. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298:850-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, G. P., A. T. Bruce, H. Ikeda, L. J. Old, and R. D. Schreiber. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991-998. [DOI] [PubMed] [Google Scholar]
- 11.Fong, L., and E. G. Engleman. 2000. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 18:245-273. [DOI] [PubMed] [Google Scholar]
- 12.Gardai, S. J., K. A. McPhillips, S. C. Frasch, W. J. Janssen, A. Starefeldt, J. E. Murphy-Ullrich, D. L. Bratton, P. A. Oldenborg, M. Michalak, and P. M. Henson. 2005. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321-334. [DOI] [PubMed] [Google Scholar]
- 13.Ghiringhelli, F., N. Larmonier, E. Schmitt, A. Parcellier, D. Cathelin, C. Garrido, B. Chauffert, E. Solary, B. Bonnotte, and F. Martin. 2004. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 34:336-344. [DOI] [PubMed] [Google Scholar]
- 14.Grauer, O. M., S. Nierkens, E. Bennink, L. W. Toonen, L. Boon, P. Wesseling, R. P. Sutmuller, and G. J. Adema. 2007. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int. J. Cancer 121:95-105. [DOI] [PubMed] [Google Scholar]
- 15.Höltl, L., R. Ramoner, C. Zelle-Rieser, H. Gander, T. Putz, C. Papesh, W. Nussbaumer, C. Falkensammer, G. Bartsch, and M. Thurnher. 2005. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol. Immunother. 54:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, C. H., M. J. Hong, S. D. Park, C. K. Kim, M. Y. Park, H. J. Sohn, H. I. Cho, T. G. Kim, and Y. K. Hong. 2006. Enhancement of anti-tumor immunity specific to murine glioma by vaccination with tumor cell lysate-pulsed dendritic cells engineered to produce interleukin-12. Cancer Immunol. Immunother. 55:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, C. H., S. J. Woo, J. S. Park, H. S. Kim, M. Y. Park, S. D. Park, Y. K. Hong, and T. G. Kim. 2007. Enhanced antitumour immunity by combined use of temozolomide and TAT-surviving pulsed dendritic cells in a murine glioma. Immunology 122:615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lake, R. A., and B. W. Robinson. 2005. Immunotherapy and chemotherapy—a practical partnership. Nat. Rev. Cancer 5:397-405. [DOI] [PubMed] [Google Scholar]
- 19.Liu, J. Y., Y. Wu, X. S. Zhang, J. L. Yang, H. L. Li, Y. Q. Mao, Y. Wang, X. Cheng, Y. Q. Li, J. C. Xia, M. Masucci, and Y. X. Zeng. 2007. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol. Immunother. 56:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu, B., and O. J. Finn. 2008. T-cell death and cancer immune tolerance. Cell Death Differ. 15:70-79. [DOI] [PubMed] [Google Scholar]
- 21.Machiels, J. P., R. T. Reilly, L. A. Emens, A. M. Ercolini, R. Y. Lei, D. Weintraub, F. I. Okoye, and E. M. Jaffee. 2001. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 61:3689-3697. [PubMed] [Google Scholar]
- 22.Melief, C. J., R. E. Toes, J. P. Medema, S. H. van der Burg, F. Ossendorp, and R. Offringa. 2000. Strategies for immunotherapy of cancer. Adv. Immunol. 75:235-282. [DOI] [PubMed] [Google Scholar]
- 23.Nair, S. K., M. Morse, D. Boczkowski, R. I. Cumming, L. Vasovic, E. Gilboa, and H. K. Lyerly. 2002. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann. Surg. 235:540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowak, A. K., R. A. Lake, A. L. Marzo, B. Scott, W. R. Heath, E. J. Collins, J. A. Frelinger, and B. W. Robinson. 2003. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 170:4905-4913. [DOI] [PubMed] [Google Scholar]
- 25.Nowak, A. K., B. W. Robinson, and R. A. Lake. 2002. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 62:2353-2358. [PubMed] [Google Scholar]
- 26.Obeid, M., A. Tesniere, F. Ghiringhelli, G. M. Fimia, L. Apetoh, J. L. Perfettini, M. Castedo, G. Mignot, T. Panaretakis, N. Casares, D. Métivier, N. Larochette, P. van Endert, F. Ciccosanti, M. Piacentini, L. Zitvogel, and G. Kroemer. 2007. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13:54-61. [DOI] [PubMed] [Google Scholar]
- 27.Obeid, M., A. Tesniere, T. Panaretakis, R. Tufi, N. Joza, P. van Endert, F. Ghiringhelli, L. Apetoh, N. Chaput, C. Flament, E. Ullrich, S. de Botton, L. Zitvogel, and G. Kroemer. 2007. Ecto-calreticulin in immunogenic chemotherapy. Immunol. Rev. 220:22-34. [DOI] [PubMed] [Google Scholar]
- 28.Park, S. D., C. H. Kim, C. K. Kim, J. A. Park, H. J. Sohn, Y. K. Hong, and T. G. Kim. 2007. Cross-priming by temozolomide enhances antitumor immunity of dendritic cell vaccination in murine brain tumor model. Vaccine 25:3485-3491. [DOI] [PubMed] [Google Scholar]
- 29.Porgador, A., D. Snyder, and E. Gilboa. 1996. Induction of antitumor immunity using bone marrow-generated dendritic cells. J. Immunol. 156:2918-2926. [PubMed] [Google Scholar]
- 30.Roos, W. P., L. F. Batista, S. C. Naumann, W. Wick, M. Weller, C. F. Menck, and B. Kaina. 2006. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O(6)-methylguanine. Oncogene 118:2790-2795. [DOI] [PubMed] [Google Scholar]
- 31.Shi, Y., J. E. Evans, and K. L. Rock. 2003. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516-521. [DOI] [PubMed] [Google Scholar]
- 32.Skoberne, M., A. S. Beignon, and N. Bhardwaj. 2004. Danger signals: a time and space continuum. Trends Mol. Med. 10:251-257. [DOI] [PubMed] [Google Scholar]
- 33.Steinman, R. M., and M. C. Nussenzweig. 1980. Dendritic cells: features and functions. Immunol. Rev. 53:127-147. [DOI] [PubMed] [Google Scholar]
- 34.Tentori, L., C. Leonetti, M. Scarsella, G. D'Amati, M. Vergati, I. Portarena, W. Xu, V. Kalish, G. Zupi, J. Zhang, and G. Graziani. 2003. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin. Cancer Res. 9:5370-5379. [PubMed] [Google Scholar]
- 35.Yamanaka, R., J. Homma, N. Yajima, N. Tsuchiya, M. Sano, T. Kobayashi, S. Yoshida, T. Abe, M. Narita, M. Takahashi, and R. Tanaka. 2005. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin. Cancer Res. 11:4160-4167. [DOI] [PubMed] [Google Scholar]
- 36.Yu, J. S., C. J. Wheeler, P. M. Zeltzer, H. Ying, D. N. Finger, P. K. Lee, W. H. Yong, F. Incardona, R. C. Thompson, M. S. Riedinger, W. Zhang, R. M. Prins, and K. L. Black. 2001. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 61:842-847. [PubMed] [Google Scholar]