Abstract
In our 14-valent Luminex assay for pneumococcal antibodies, we identified two groups of sera that caused false-positive results. The first group bound nonspecifically to the Luminex microspheres. The second group reacted specifically with bovine serum albumin (BSA). We describe here methods that eliminated the false-positive reactivity of both groups.
We previously described a multiplexed pneumococcal antibody assay based on the Luminex xMAP technology (11). Several other laboratories subsequently also described multiplexed Luminex assays for detecting these antibodies (1, 6, 15). In addition, the Luminex xMAP technology has been used for multiplexed assays to measure antibody responses to vaccines for other infectious diseases, including those caused by Neisseria meningitidis, Haemophilus influenza type b, Bordetella pertussis, Clostridium tetani, Corynebacterium diphtheriae, Bacillus anthracis, and papilloma virus (3, 4, 10, 12, 13, 17). Waterboer et al. (18) documented an intrinsic problem with the use of the Luminex technology for serological assays. They found that some human sera bind directly to the carboxylated MicroPlex (formally MultiAnalyte) microspheres, causing a very high level of nonspecific background. These workers also found that SeroMAP microspheres, introduced by Luminex Corporation specifically for use in serological assays, reduced but did not eliminate the nonspecific-binding problem.
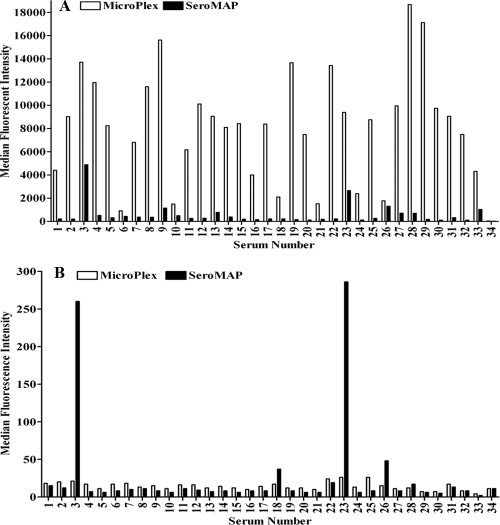
Using our original protocol (11) for the 14-valent pneumococcal antibody assay with lot B MicroPlex microspheres, we encountered serum samples with very high-level false-positive results that were near or above the analytical measurement range (AMR) of the assay (Table 1). Approximately 15 of every 1,000 sera tested for pneumococcal antibodies exhibited this behavior. We termed these samples “polyspecific,” although they did not react specifically to pneumococcal polysaccharides (PnPs). We tested a panel of 33 of these polyspecific sera and 1 control serum sample not showing polyspecific reactivity against an unconjugated MicroPlex microsphere and an unconjugated SeroMAP microsphere. The serum samples used in this study were submitted to ARUP Laboratories for pneumococcal antibody testing. All samples were deidentified according to protocols approved by the University of Utah Institutional Review Board (no. 7275). Serum samples were diluted 1:25 in phosphate-buffered saline (PBS), pH 7.2, with 5 μg/ml pneumococcal C-polysaccharide (C-Ps) (Staten Serum Institut, Copenhagen, Denmark), 5 μg/ml pneumococcal polysaccharide 22F (American Type Culture Collection, Manassas, VA), and 0.0015% bromcresol purple (BCP) (Sigma-Aldrich, St. Louis, MO). A MicroPlex (region 7) (Luminex Corporation, Austin, TX) microsphere and a SeroMAP (region 8) (Luminex Corporation, Austin, TX) microsphere were pelleted by centrifugation and resuspended in blocking/storage (B/S) buffer consisting of PBS with 0.1% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) or in BSA-free StabliGuard immunoassay stabilizer (SG01) (SurModics, Inc., Eden Prairie, MN). Serum dilutions were incubated with the uncoupled microspheres for 20 min at room temperature with shaking, washed once with PBS by filtration, incubated for 20 min at room temperature with shaking with phycoerythrin (PE)-labeled affinity-purified anti-human IgG (γ) (Southern Biotech, Birmingham, AL) in B/S buffer, and washed once with PBS. Microspheres were counted with a Luminex 100 analyzer. The MicroPlex and SeroMAP microspheres were compared in the two diluents in the same assay run, with the same sample dilutions and PE conjugate, to eliminate run-to-run variation. As shown in Fig. 1A, all 33 of the polyspecific sera tested reacted strongly to the unconjugated MicroPlex microsphere suspended in B/S buffer, with median fluorescence intensity (MFI) values that ranged from 905 to 18,674. In contrast, the MFI of the control serum sample was 38. Compared to those for the MicroPlex microsphere, the MFI values for the SeroMAP microsphere suspended in B/S buffer were low. All 33 of the polyspecific sera, however, had background MFI values above 110, compared to the control serum sample, which had an MFI of 28. Twenty-four of the 33 sera (72.7%) had MFI values above the cutoff value of 200. A background MFI value of 200 could falsely elevate the pneumococcal antibody assay results by 0.1 μg/ml or more for 5 of the 14 serotypes. If the long-term protective level after pneumococcal vaccine immunization is considered to be 1 μg/ml, a background MFI level of 200 could lead to misinterpretation of protective status. In addition, 10 of the polyspecific sera had background MFI levels above 500 with the SeroMAP microsphere, and 5 of these sera had MFI levels above 1,000. Two of the polyspecific sera, no. 3 and 23, had very high levels of nonspecific reactivity to the SeroMAP microspheres, with MFI values of 4,877 and 2,666, respectively.
TABLE 1.
IgG concentrations in serum before (protocol 1) and after (protocol 2) removal of nonspecific binding to microspheres
| Serum | Protocol | IgG concn (μg/ml) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PnPs1 | PnPs3 | PnPs4 | PnPs5 | PnPs6B | PnPs7F | PnPs8 | PnPs9N | PnPs9V | PnPs12F | PnPs14 | PnPs18C | PnPs19F | PnPs23F | ||
| 3 | 1 | 11.44 | 14.40 | >11.61 | >80.03 | 5.94 | 37.37 | >32.83 | 4.56 | 25.25 | >18.03 | 17.41 | 19.89 | >27.77 | 19.90 |
| 2 | 1.42 | 7.03 | 0.28 | 2.21 | 0.22 | 1.31 | 0.33 | 0.35 | 0.61 | 0.46 | 1.78 | 0.88 | 1.78 | 0.12 | |
| 9 | 1 | 19.52 | 15.89 | >11.61 | >80.09 | 10.62 | >37.00 | >32.83 | 11.45 | >25.25 | >18.03 | 38.63 | 39.44 | >27.77 | >28.78 |
| 2 | 1.16 | 0.36 | 0.29 | 2.06 | 0.31 | 1.16 | 1.00 | 0.53 | 0.61 | 0.84 | 8.37 | 2.91 | 0.35 | 0.76 | |
| 23 | 1 | 11.44 | 14.46 | >11.61 | >80.09 | 5.94 | >37.00 | >32.83 | 7.56 | >25.25 | >18.03 | 19.41 | 19.89 | >27.77 | 19.90 |
| 3 | 2.00 | 4.83 | 0.31 | 2.78 | 0.22 | 1.56 | 0.38 | 0.57 | 1.02 | 0.67 | 2.05 | 1.23 | 0.17 | 0.32 | |
| 28 | 1 | >20.40 | >24.74 | >11.61 | >80.09 | >48.48 | >37.00 | >32.83 | >20.71 | 25.25 | >18.03 | 17.88 | 16.85 | >27.77 | 23.02 |
| 2 | 0.33 | 0.32 | 0.14 | 1.69 | 0.16 | 0.81 | 0.14 | 0.16 | 0.48 | 0.24 | 0.26 | 4.49 | 3.02 | 4.57 | |
| 34 | 1 | 0.90 | 3.01 | 0.17 | 1.74 | 0.32 | 1.83 | 2.20 | 0.18 | 0.76 | 0.44 | 2.80 | 0.41 | 0.07 | 0.57 |
| 2 | 0.97 | 1.83 | 0.53 | 1.45 | 0.45 | 1.27 | 3.17 | 0.20 | 1.01 | 0.35 | 6.12 | 0.32 | 0.13 | 0.30 | |
FIG. 1.
Nonspecific reactivity of human sera to Luminex microspheres. Shown are median fluorescence intensities for 33 polyspecific sera and a control serum sample reacted against unconjugated MicroPlex (clear bars) and SeroMAP (solid bars) microspheres. (A) Microspheres suspended in B/S buffer. (B) Microspheres suspended in StabliGuard.
Nonspecific binding to uncoupled MicroPlex microspheres was completely eliminated by resuspending the uncoupled microspheres in StabliGuard (Fig. 1B). Compared to those for B/S buffer, the MFI values for the MicroPlex microspheres suspended in StabliGuard were reduced by an average of 99.7%. The MFI values for the 33 polyspecific sera against the uncoupled MicroPlex microsphere in StabliGuard ranged from 8 to 26. Except for the two sera (no. 3 and 23) whose MFI values were above 250, StabliGuard also eliminated the nonspecific binding of the 33 polyspecific sera to the SeroMAP microsphere.
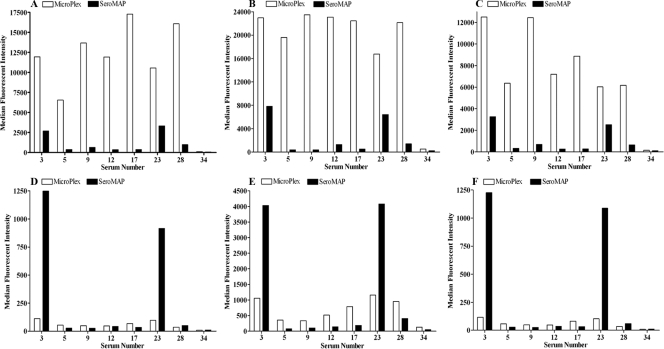
We evaluated immunoglobulin-inhibiting reagent (IIR) (Bioreclamation, Inc., Westbury, NY), a reagent for removing heterophile antibodies, and polysorbate 20 (Tween 20; Sigma-Aldrich, St. Louis, MO) for their effects on nonspecific binding to MicroPlex and SeroMAP microspheres. IIR and Tween 20 were added separately to the sample diluent described above at concentrations of 400 μg/ml and 0.05%, respectively. The sample diluents with the two additives were compared to a sample diluent without an additive. Eight of the sera with high levels of nonspecific binding and the control serum sample, with no nonspecific binding (Fig. 1), were diluted 1:25 in each of the three sample diluents and tested in a single assay run against an uncoupled MicroPlex microsphere and an uncoupled SeroMAP microsphere suspended in either B/S buffer or StabliGuard. The results are shown in Fig. 2. As in Fig. 1A, the 7 polyspecific sera showed very high levels of nonspecific binding to the MicroPlex microsphere suspended in B/S buffer, with MFI values from 6,534 to 17,285 (Fig. 2A), and no nonspecific binding to the same microsphere suspended in StabliGuard (Fig. 2D). The MFI values for the 7 polyspecific sera with the SeroMAP microsphere in B/S buffer were 353 to 3,331 (Fig. 2A). Unlike those observed for the MicroPlex microsphere, however, the levels of nonspecific binding of the two high-binding-level sera (no. 3 and 23) to the SeroMAP microsphere were not completely blocked with StabliGuard and remained high, with MFI values of 1,249 and 916, respectively (Fig. 2D). Addition of Tween 20 to the sample diluent increased the nonspecific binding of the 7 polyspecific sera to both the MicroPlex and the SeroMAP microspheres suspended in B/S buffer by approximately 40% (Fig. 2B). Tween 20 also interfered with the blocking effect of StabliGuard. The levels of nonspecific binding of all the sera to both microspheres suspended in StabliGuard were higher with Tween 20 in the sample diluent than without Tween 20 (Fig. 2E). Addition of IIR to the sample diluent reduced the nonspecific binding of the polyspecific sera to the MicroPlex and SeroMAP microspheres suspended in B/S buffer by an average of 25% (Fig. 2C). IIR had no effect on the microspheres suspended in StabliGuard.
FIG. 2.
Median fluorescence intensities for 7 polyspecific sera and a control serum sample reacted against unconjugated MicroPlex (clear bars) and SeroMAP (solid bars) microspheres. (A, B, and C) Microspheres suspended in B/S buffer. (D, E, and F) Microspheres suspended in StabliGuard. (A and D) Sample diluent with no additives. (B and E) Sample diluent with Tween 20. (C and F) Sample diluent with IIR.
On the basis of the results of the studies described above, we modified our original 14-valent pneumococcal antibody assay to eliminate the false-positive assay results caused by the nonspecific reactivity of some sera to the microspheres. In our original protocol, which we refer to as protocol 1, pneumococcal polysaccharides (PnPs) were coupled with carboxylated MicroPlex microspheres, washed in B/S buffer to block any remaining activated carboxylated groups, and resuspended in B/S buffer (11). In the revised protocol, which we refer to as protocol 2, the MicroPlex microspheres were coupled with PnPs, washed with B/S buffer, and resuspended in StabliGuard rather than in B/S buffer. We also removed Tween 20 from the sample diluent and the PBS wash buffer and added BCP dye to the sample diluent. Both sample diluents contained 5 μg/ml each of C-Ps and 22F. Serum samples were diluted 1:25 in protocol 2 and 1:101 in protocol 1. For both protocol 1 and protocol 2, the assay was performed as described above.
Table 1 shows the Luminex pneumococcal IgG assay results for four of the polyspecific sera and the control serum sample (Fig. 1 and 2). The results obtained using protocol 1 are compared to those obtained using protocol 2. The four sera with very high levels of nonspecific reactivity to the MicroPlex microspheres produce high-level false-positive assay results for all 14 PnPs serotypes when protocol 1 with microspheres suspended in B/S buffer is used. The polyspecific reactivity is eliminated in protocol 2 by using StabliGuard as the microsphere diluent. The control serum sample (no. 34) that did not react nonspecifically with the microspheres produced comparable results with both protocols.
Using protocol 2, we encountered a subset of polyspecific sera that did not react nonspecifically to the uncoupled microspheres, and their false-positive reactivity was not affected by StabliGuard. These sera appeared to react specifically to BSA. These sera also reacted strongly to microspheres coated with BSA. Protocol 2 was further modified to eliminate the B/S wash step after the microspheres were coupled with PnPs, which was found to be unnecessary with StabliGuard. In protocol 3, after PnPs were coupled with MicroPlex microspheres, microspheres were washed in StabliGuard and resuspended in StabliGuard. Otherwise, protocol 3 was identical to protocol 2.
Table 2 shows the polyspecific reactivities of two sera against all 14 PnPs serotypes observed after MicroPlex microspheres were blocked in B/S buffer prior to being resuspended in StabliGuard (protocol 2). The polyspecific reactivity was eliminated by eliminating the B/S blocking step in protocol 3.
TABLE 2.
IgG concentrations in serum for 14 PnPs serotypes before (protocol 2) and after (protocol 3) removal of a BSA blocking step
| Serum | Protocol | IgG concn (μg/ml) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PnPs1 | PnPs3 | PnPs4 | PnPs5 | PnPs6B | PnPs7F | PnPs8 | PnPs9N | PnPs9V | PnPs12F | PnPs14 | PnPs18C | PnPs19F | PnPs23F | ||
| 1 | 2 | >20.40 | >24.74 | >11.61 | >80.09 | >48.48 | >37.00 | >32.83 | >20.71 | >25.25 | >18.03 | >96.46 | >42.95 | >27.77 | >28.78 |
| 3 | 0.70 | 0.51 | 3.32 | 2.89 | 4.62 | 1.55 | 0.87 | 0.98 | 4.37 | 0.65 | 6.21 | 4.80 | 9.33 | 2.61 | |
| 2 | 2 | >20.40 | >24.74 | >11.61 | >80.09 | >48.48 | >37.00 | 10.19 | >20.40 | >24.74 | >11.61 | >80.09 | >48.48 | >37.00 | >32.83 |
| 3 | 1.14 | 0.40 | 0.34 | 7.02 | 8.34 | 1.09 | 0.88 | 0.50 | 1.38 | 1.32 | 0.65 | 0.92 | 12.57 | 3.43 | |
The SeroMAP microspheres seem to us to be less desirable than the MicroPlex microspheres for use in Luminex-based serological assays. The SeroMAP microspheres also bind nonspecifically to some sera, although to a lesser degree than the MicroPlex microspheres (8, 18). Unlike what was observed for the MicroPlex microspheres, however, the nonspecific binding to the SeroMAP microspheres by two of the sera in this study could not be completely blocked with StabliGuard. Also, the SeroMAP microspheres have lower capacities for binding antigens, so the specific signals are lower than those observed with MicroPlex microspheres (18).
Waterboer et al. (18) approached the nonspecific-binding problem by adding additives to their serum preincubation buffer. They were able to suppress nonspecific binding to SeroMAP and MicroPlex microspheres by adding a combination of polyvinyl alcohol, polyvinylpyrrolidone, and Superchemiblock heterophile blocking agent (Millipore, Billerica, MA). The addition of additives to the preincubation buffer, however, reduced the specific signals by one-third (18). Super Chemiblock contains mouse immunoglobulins and is used to remove human anti-mouse antibodies (HAMA), heterophile antibodies, and rheumatoid factor. We evaluated a similar reagent, IIR. IIR contains purified immunoglobulins with high levels of affinity for anti-animal antibodies (5). In our hands, IIR reduced the nonspecific binding to unconjugated MicroPlex and SeroMAP microspheres by only about 25% (Fig. 1C). In addition, preabsorption of serum with heterophile blocking tubes (Scantibodies Laboratory, Inc., Santee, CA) had no effect. These reagents are effective in removing heterophile antibodies from other assay systems (5). Heterophile antibody interference in Luminex-based immunoassays for cytokines has been reported (2, 7). However, the reactivity of some human sera to the unconjugated Luminex MicroPlex and SeroMAP microspheres appears to us to be nonspecific binding of human IgG to the microspheres rather than a specific reaction of heterophile antibodies or other antibodies to antigenic determinants on the microspheres. The nonspecific binding was completely blocked with StabliGuard, a passive blocking agent. The above-mentioned additives to the sample diluent appear to function as competitive inhibitors of binding of IgG to the microspheres rather than as absorbents of specific antibodies.
Antibodies to BSA in serum from a 22-month-old child were reported to cause false-positive results in serological assays for human immunodeficiency virus type 1 and organisms causing other infectious diseases (19). Newell and coworkers also reported false-positive results in a commercial Luminex assay for antibodies to human leukocyte antigen (HLA) class I and class II antigens caused by antibodies to BSA used to block the microspheres (9). Anti-BSA antibodies have been reported to occur in 70% of children and 25% of adults (14). About 90% of the sera in this subset that showed false-positive reactivity in our pneumococcal antibody assay were from children 10 years of age or younger. These anti-BSA antibodies could possibly be absorbed out by addition of BSA to the sample diluent or microsphere diluents. However, the levels for the specific MFI signals were higher with protocol 3 after BSA was removed from the sample and microsphere diluents than with protocol 1. Also, StabliCoat Plus microarray stabilizer, a similar product from SurModics, Inc., but containing BSA, was less effective in eliminating nonspecific binding to the MicroPlex microspheres. Considering the high prevalence of antibodies to BSA in the population, we chose to eliminate the BSA blocking step and use BSA-free StabliGuard. The absence of BSA in the sample diluent allowed us to add an indicator dye (BCP) to the sample diluent to verify when a serum sample was added to the sample diluent (16). StabliGuard also allowed us to use a more concentrated serum dilution (1:25 dilution) for the assay. This improved the resolution of the assay at the low end of the AMR without incurring higher background levels. In conclusion, blocking of the Luminex microspheres with StabliGuard and elimination of a BSA/PBS wash step after antigen coupling were simple, inexpensive, and effective methods for removing nonspecific reactivity in a Luminex-based serological assay for pneumococcal antibodies. Although we have not tested these procedures with other Luminex assays, the protocols described here might be applicable to other serological assays based on the Luminex xMAP technology.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Biagini, R. E., S. A. Schlottmann, D. L. Sammons, J. P. Smith, J. C. Snawder, C. A. Striley, B. A. MacKenzie, and D. N. Weissman. 2003. Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin. Diagn. Lab. Immunol. 10:744-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jager, W., B. J. Prakken, J. W. Bijlsma, W. Kuis, and G. T. Rijkers. 2005. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J. Immunol. Methods 300:124-135. [DOI] [PubMed] [Google Scholar]
- 3.de Voer, R. M., R. M. Schepp, F. G. Versteegh, F. R. van der Klis, and G. A. Berbers. 2009. Simultaneous detection of Haemophilus influenzae type b polysaccharide-specific antibodies and Neisseria meningitidis serogroup A, C, Y, and W-135 polysaccharide-specific antibodies in a fluorescent-bead-based multiplex immunoassay. Clin. Vaccine Immunol. 16:433-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias, D., J. Van Doren, S. Schlottmann, S. Kelly, D. Puchalski, W. Ruiz, P. Boerckel, J. Kessler, J. M. Antonello, T. Green, M. Brown, J. Smith, N. Chirmule, E. Barr, K. U. Jansen, and M. T. Esser. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab. Immunol. 12:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kricka, L. J. 1999. Human anti-animal antibody interferences in immunological assays. Clin. Chem. 45:942-956. [PubMed] [Google Scholar]
- 6.Lal, G., P. Balmer, E. Stanford, S. Martin, R. Warrington, and R. Borrow. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296:135-147. [DOI] [PubMed] [Google Scholar]
- 7.Martins, T. B., B. M. Pasi, C. M. Litwin, and H. R. Hill. 2004. Heterophile antibody interference in a multiplexed fluorescent microsphere immunoassay for quantitation of cytokines in human serum. Clin. Diagn. Lab. Immunol. 11:325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins, T. B., N. H. Augustine, and H. R. Hill. 2006. Development of a multiplexed fluorescent immunoassay for the quantitation of antibody responses to group A streptococci. J. Immunol. Methods 316:97-106. [DOI] [PubMed] [Google Scholar]
- 9.Newell, H., J. D. Smith, P. Rogers, E. Birks, A. J. Danskine, R. E. Fawson, and M. L. Rose. 2006. Sensitization following LVAD implantation using leucodepleted blood is not due to HLA antibodies. Am. J. Transplant. 6:1712-1717. [DOI] [PubMed] [Google Scholar]
- 10.Opalka, D., C. E. Lachman, S. A. MacMullen, K. U. Jansen, J. F. Smith, N. Chirmule, and M. T. Esser. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab. Immunol. 10:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 12.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin. Diagn. Lab. Immunol. 9:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince, H. E., M. Lape-Nixon, and J. Matud. 2006. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin. Vaccine Immunol. 13:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothberg, R. M., and R. S. Farr. 1965. Anti-bovine serum albumin and anti-alpha lactalbumin in the serum of children and adults. Pediatrics 35:571-588. [PubMed] [Google Scholar]
- 15.Schlottmann, S. A., N. Jain, N. Chirmule, and M. T. Esser. 2006. A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. J. Immunol. Methods 309:75-85. [DOI] [PubMed] [Google Scholar]
- 16.Tel, R. M., J. de Jong, and G. T. Berends. 1979. Bromocresol purple, a non-specific colour reagent for the determination of serum albumin. J. Clin. Chem. Clin. Biochem. 17:627-631. [DOI] [PubMed] [Google Scholar]
- 17.van Gageldonk, P. G., F. G. van Schaijk, F. R. van der Klis, and G. A. Berbers. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 335:79-89. [DOI] [PubMed] [Google Scholar]
- 18.Waterboer, T., P. Sehr, and M. Pawlita. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309:200-204. [DOI] [PubMed] [Google Scholar]
- 19.Willman, J. H., T. B. Martins, T. D. Jaskowski, H. R. Hill, and C. M. Litwin. 1999. Heterophile antibodies to bovine and caprine proteins causing false-positive human immunodeficiency virus type 1 and other enzyme-linked immunosorbent assay results. Clin. Diagn. Lab. Immunol. 6:615-616. [DOI] [PMC free article] [PubMed] [Google Scholar]