Abstract
Bovine respiratory syncytial virus (BRSV) infects cells of the respiratory mucosa, so it is desirable to develop a vaccination strategy that induces mucosal immunity. To achieve this, various delivery routes were compared for formalin-inactivated (FI) BRSV formulated with CpG oligodeoxynucleotide (ODN) and polyphosphazene (PP). Intranasal delivery of the FI-BRSV formulation was superior to subcutaneous delivery in terms of antibody, cell-mediated, and mucosal immune responses, as well as reduction in virus replication after BRSV challenge. Although intranasal delivery of FI-BRSV also induced higher serum and lung antibody titers and gamma interferon (IFN-γ) production in the lungs than intranasal-subcutaneous and/or subcutaneous-intranasal prime-boost strategies, no significant differences were observed in cell-mediated immune responses or virus replication in the lungs of challenged mice. Interleukin 5 (IL-5), eotaxin, and eosinophilia were enhanced after BRSV challenge in the lungs of subcutaneously immunized mice compared to unvaccinated mice, but not in the lungs of mice immunized intranasally or through combinations of the intranasal and subcutaneous routes. These results suggest that two intranasal immunizations with FI-BRSV formulated with CpG ODN and PP are effective and safe as an approach to induce systemic and mucosal responses, as well to reduce virus replication after BRSV challenge. Furthermore, intranasal-subcutaneous and subcutaneous-intranasal prime-boost strategies were also safe and almost as efficacious. In addition to the implications for the development of a protective BRSV vaccine for cattle, formulation with CpG ODN and PP could also prove important in the development of a mucosal vaccine that induces protective immunity against human RSV.
Human respiratory syncytial virus (HRSV) is the most important cause of lower respiratory tract infection in infants and young children worldwide (47) and is responsible for significant mortality. Like HRSV, bovine respiratory syncytial virus (BRSV) is an enveloped, nonsegmented, single-stranded RNA Pneumovirus of the family Paramyxoviridae and order Mononegavirales. BRSV is responsible for significant economic loss to the cattle industry (49) and is one of the four known viral components of bovine shipping fever. It has been estimated that BRSV is responsible for more than 60% of epizootic respiratory disease in dairy herds and up to 70% in beef herds, with typical mortality levels ranging from 2 to 3% but reaching 20% in some cases (38). HRSV and BRSV have similar clinical outcomes in their respective host species, ranging from asymptomatic infection to bronchiolitis and pneumonia, and sometimes death (46), and the pathogeneses of both viruses are directly related to the host immune response (19).
While several commercial BRSV vaccines are currently available for use in cattle, vaccines that are more efficacious are desirable. Also, there currently is no approved HRSV vaccine for use in humans. In the 1960s, studies using parenterally delivered formalin-inactivated (FI) HRSV vaccines were carried out with children (6, 17, 28, 54). Not only did these vaccines fail to protect the children from natural infection, in most cases disease was enhanced. In one study, 80% of vaccinated children were hospitalized, and two of them died (28). Disease enhancement has also been documented in a few studies with FI-BRSV in calves, with a potential role for the vaccine dose in the induction of immunopathology (3, 20, 27, 33, 51, 55).
Once FI-RSV started to be investigated in mouse models, it became generally accepted that the disease enhancement caused by FI-RSV vaccines is due to the induction of a Th2-biased immune response (9, 10, 52, 53). In a recent report, lack of antibody affinity for protective epitopes after poor toll-like receptor (TLR) stimulation was found to be responsible for lack of protection from RSV (14). An adjuvant that promotes Th1-type or balanced immune responses, including high-affinity antibodies, would therefore be a desirable component of vaccine formulations. CpG oligodeoxynucleotides (ODN) are short stretches of DNA that feature unmethylated CG dinucleotides flanked by two 5′ purines and two 3′ pyrimidines. CpG ODN are ligands of TLR9 and induce production of interleukin 1 (IL-1), IL-6, IL-12, and tumor necrosis factor alpha (TNF-α) by dendritic cells and macrophages, as well as production of gamma interferon (IFN-γ), IL-6, and IL-10 by natural killer cells (5, 23, 24). CpG ODN generally induce an overall Th1-type immune response in mice (8, 13, 25, 26). We have previously used CpG ODN to shift the immune response induced by immunization with FI-BRSV from a Th2-biased response to a more balanced response in mice (32, 42) and calves (33). TLR stimulation by CpG ODN is also expected to contribute to affinity maturation (14).
Since RSV infects cells of the respiratory mucosa, it is desirable to develop a vaccination strategy that induces mucosal immunity using alternate routes of immunization. This approach may also avoid enhancement of disease while maintaining immunity. For example, while scarification of mice with live recombinant vaccinia virus expressing the HRSV G protein (rVV-G) induced enhanced lung immunopathology upon subsequent challenge (41), intranasal (i.n.) and intraperitoneal (i.p.) immunization with rVV-G resulted in protection and no lung lesions (48). Similarly, i.n. immunization with rVV expressing the HRSV F protein resulted in mild lung inflammation upon challenge in comparison to that induced by intradermal immunization (34).
One of the challenges of i.n. immunization is delivering the vaccine components in such a way that they are not degraded before an immune response can be initiated. Polyphosphazenes (PP) are synthetic polymers that feature a backbone of alternating phosphorus and nitrogen atoms with organic side groups attached at each phosphorus atom (43). PP form noncovalent complexes when formulated with antigens and/or other adjuvants, enhancing their stability and allowing multimeric presentation. Immunization with antigens formulated with PP has been shown to induce robust immune responses against rotavirus (37), cholera virus (56), influenza virus (40, 44, 45), hepatitis B virus (39), and BRSV (32).
We previously demonstrated the adjuvant effects of CpG ODN on subcutaneously (s.c.) delivered FI-BRSV vaccines in mice (42) and calves (33), as well as an i.n. delivered FI-BRSV vaccine in mice (32). Since there is evidence that combinations of mucosal and parenteral administration lead to immune responses that were as strong or stronger than those resulting from either mucosal or parenteral immunization alone (21, 22, 29, 30), the purpose of this study was to determine the effects of the route of delivery on antibody, mucosal, and cell-mediated immune responses, as well as protection against BRSV. We compared i.n. prime-s.c. boost (i.n./s.c.) and s.c. prime-i.n. boost (s.c./i.n.) immunization protocols for FI-BRSV, formulated with CpG ODN and PP, to two i.n. or s.c. administrations. The results of this study demonstrated that FI-BRSV formulated with CpG ODN and PP is more effective after two i.n. than after two s.c. administrations in terms of antibody, cell-mediated, and mucosal immune responses, as well as safety and reduction in virus replication after BRSV challenge, while i.n./s.c. and s.c./i.n. prime-boost strategies proved to be as safe and almost as efficacious as i.n./i.n. delivery.
MATERIALS AND METHODS
Cells and virus.
The 375 strain of BRSV (American Type Culture Collection) was propagated in bovine turbinate (BT) cells (American Type Culture Collection), and virus titers were determined as previously described (32). FI-BRSV was prepared as previously described (28) with modifications (32). The final vaccine protein concentration was 120 μg/ml, and 1.5 μg was given per immunization. Challenge virus was prepared as previously described (32).
Immunization and challenge.
Six- to 8-week-old female BALB/c mice (Charles River) were randomly allocated into groups of 10 animals each and immunized with a total volume of 25 μl (for i.n. immunizations) or 50 μl (for s.c. immunizations), as indicated in Tables 1 and 2. The first trial consisted of five groups of 10 mice each (Table 1) and the second trial of six groups of 10 mice each (Table 2). FI-BRSV was given at 1.5 μg per immunization. CpG ODN 1826 (TCC ATG ACG TTC CTG ACG TT) (CpG motifs are underlined), provided by Merial Limited (Deluth, GA), was phosphorothioate modified during synthesis to enhance nuclease resistance and was given at 4 μg per immunization. PP, a 90% substituted poly(di-p-dicarboxylatophenoxy)-phosphazene (PCPP)/10% hydroxylate (90:10 PCPP/OH), was synthesized by John Klaehn (Idaho National Laboratory) according to a previously published method (2) and was given at 25 μg per immunization. The components of each vaccine were mixed prior to immunization and given as a single administration. Two weeks after the second immunization, all groups were challenged with BRSV as previously described (32). Half of the mice were humanely euthanized 4 days after challenge for detection of lung viral RNA and the other half 6 days after challenge for bronchoalveolar lavage fluids, lung fragment cultures, lung homogenate supernatants, and isolation of splenocytes for enzyme-linked immunospot (ELISPOT) assays. All procedures involving animals were performed in accordance with the guidelines of the Canadian Council for Animal Care.
TABLE 1.
Formulation of FI-BRSV with CpG ODN and PP, individually and as coadjuvants
| Groupa | 1st immunization (day 0) | 2nd immunization (day 21) | Challenge (day 35) |
|---|---|---|---|
| FI-BRSV-CpG | Intranasal | Intranasal | BRSV |
| FI-BRSV-PP | Intranasal | Intranasal | BRSV |
| FI-BRSV-CpG-PP | Intranasal | Intranasal | BRSV |
| Saline-BRSV challenge | Intranasal | Intranasal | BRSV |
| Saline-saline | Intranasal | Intranasal | Saline |
FI-BRSV was formulated with 4 μg CpG ODN and/or 25 μg PP.
TABLE 2.
Immunization protocols for FI-BRSV coadjuvanted with CpG ODN and PP
| Groupa | 1st immunization (day 0) | 2nd immunization (day 21) | Challenge (day 35) |
|---|---|---|---|
| FI-BRSV (i.n./i.n.) | Intranasal | Intranasal | BRSV |
| FI-BRSV (i.n./s.c.) | Intranasal | Subcutaneous | BRSV |
| FI-BRSV (s.c./i.n.) | Subcutaneous | Intranasal | BRSV |
| FI-BRSV (s.c./s.c.) | Subcutaneous | Subcutaneous | BRSV |
| Saline-BRSV challenge | Intranasal | Intranasal | BRSV |
| Saline-saline | Intranasal | Intranasal | Saline |
FI-BRSV was formulated with 4 μg CpG ODN and 25 μg PP.
Lung fragment culture supernatants.
Lung fragment cultures were prepared as previously described (16, 31) with modifications (32). Briefly, 6 days after challenge, the lungs of euthanized mice were lavaged and moved to tubes containing RPMI 1640 medium (Gibco-Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Gibco-Invitrogen), 10 mM HEPES buffer, 0.1 mM nonessential amino acids (Gibco-Invitrogen), 1 mM sodium pyruvate (Gibco-Invitrogen), 50 μg/ml gentamicin (Gibco-Invitrogen), and 1× antibiotic/antimycotic (Gibco-Invitrogen) on ice. Subsequently, the lungs were cut into pieces and deposited into 48-well plates containing 500 μl per well of supplemented RPMI 1640 medium. Following 5 days of incubation at 37°C, the supernatants were collected and stored at −80°C.
ELISA and virus neutralization assay.
Sera and lung fragment culture supernatants were assayed for BRSV-specific IgG and IgA and BRSV neutralizing antibodies as previously described (32). For the enzyme-linked immunosorbent assay (ELISA), ninety-six-well polystyrene Immulon 2 microtiter plates (Thermo Electron, OY, Finland) were coated overnight at 37°C with Nonidet-P40 (Sigma-Aldrich, St. Louis, MO)-treated BRSV-infected BT cells or mock-infected BT cells. After being washed, the plates were incubated overnight at 4°C with serially diluted samples, beginning at 1:10 or 1:40 and continuing in 4-fold dilutions. Alkaline phosphatase (AP)-labeled goat anti-mouse IgG or IgA (Kirkegaard & Perry Laboratories, Gaithersburg, MD) at dilutions of 1:5,000 and 1:2,500 were used to detect bound IgG and IgA, respectively. BRSV-specific serum IgG1 and IgG2a titers were measured after challenge using biotinylated goat anti-mouse IgG1 and IgG2a antibodies (Caltag Laboratories, Burlingame, CA) and diluted 1:5,000, followed by streptavidin-AP (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at a dilution of 1:20,000. Reactions were visualized with p-nitrophenyl phosphate (Sigma-Aldrich). For the neutralization assay, sera and lung fragment culture supernatants were heat inactivated at 56°C for 30 min and serially diluted twofold in 96-well round-bottom culture plates (Corning Incorporated). Five hundred PFU/well of BRSV strain 375 was added to each sample dilution, and the plates were incubated for 1 h at 37°C. Subsequently, sample-virus mixtures were added to duplicate BT cell cultures and incubated at 37°C for 6 days. BRSV plaques were visualized by immunostaining, and virus neutralizing titers are expressed as the highest dilution of sample that resulted in less than 50% of cells displaying cytopathic effects.
IFN-γ and IL-5 ELISPOT assays.
Splenocytes were isolated as previously described (4), with modifications (32). Ninety-six-well Multiscreen-HA ELISPOT plates (Millipore, Bedford, MA) were coated overnight at 4°C with murine IFN-γ- or IL-5-specific monoclonal antibodies (BD PharMingen) at a concentration of 2 μg/ml. The plates were washed with phosphate-buffered saline (PBS) (pH 7.4; Gibco-Invitrogen) and blocked with minimal essential medium (MEM) for 1 to 2 h at 37°C. Splenocytes were resuspended in AIM-V medium (Gibco-Invitrogen) supplemented with 0.1 mM nonessential amino acids (Gibco-Invitrogen), 10 mM HEPES buffer, 1 mM sodium pyruvate (Gibco-Invitrogen), and 50 μM 2-mercaptoethanol (Sigma-Aldrich) and cultured at 106 cells per well in triplicate wells in the presence of 25 μg/ml BRSV-infected or mock-infected cell lysate. The plates were incubated for 20 h at 37°C and then washed with double-distilled H2O (ddH2O) and PBS with 0.05% Tween 20 (Sigma-Aldrich). This was followed by incubations with biotinylated anti-mouse IFN-γ or IL-4 (BD PharMingen) at 2 μg/ml in PBS with 1% bovine serum albumin (BSA) for 1 to 2 h at room temperature (RT) and then AP-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc.) diluted 1:1,000 in PBS with 1% bovine serum albumin (Sigma-Aldrich) for 1 to 2 h at RT. Bound antibodies were visualized with 5-bromo-4-chloro-3-indolylphosphate and nitroblue substrate tablets (Sigma-Aldrich). The plates were washed with ddH2O and air dried. Spots were counted in a blinded manner with the aid of an inverted microscope. The results are expressed as the difference between the number of cytokine-secreting cells per 106 cells in BRSV-infected lysate-stimulated wells and the number of cytokine-secreting cells per 106 cells in mock-infected lysate-stimulated wells.
IFN-γ, IL-5, and eotaxin ELISAs of lung homogenate supernatants.
Four days after challenge, mice were humanely euthanized and their lungs were removed to 2-ml screw-cap tubes (VWR International) containing 2.4-mm zirconia microbeads (Biospec Products) and 1 ml of Dulbecco's modified Eagle's medium (DMEM) (Gibco-Invitrogen) supplemented with 10 mM HEPES buffer, 0.1 mM nonessential amino acids (Gibco-Invitrogen), 1 mM sodium pyruvate (Gibco-Invitrogen), 50 μg/ml gentamicin (Gibco-Invitrogen), 10 μg/ml aprotinin (Sigma-Aldrich), 10 μg/ml leupeptin (Sigma-Aldrich), 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich), and 1× antibiotic-antimycotic (Gibco-Invitrogen). Lungs were homogenized in a minibeadbeater (BioSpec Products) for 10 s, clarified by centrifugation for 1 min at 10,000 × g, and stored at −80°C. The lung homogenate supernatants were assayed for the presence of IFN-γ, IL-5, and eotaxin using Quantikine Mouse Immunoassay Kits (Research and Diagnostic Systems) according to the manufacturer's instructions.
Eosinophils in bronchoalveolar lavage fluids.
Six days after challenge, mice were euthanized and bronchoalveolar lavage (BAL) fluids were collected and pooled from each vaccine group. Cytospin slides were prepared, using 1 × 105 and 5 × 104 cells, and stained with Wright-Giemsa stain (Bayer HealthCare). The number of eosinophils for each vaccine group was determined by examination of at least 200 cells.
Detection of viral RNA.
Four days after challenge, mice were humanely euthanized. The lungs were removed to 2-ml screw-cap tubes (VWR International) containing 2.4-mm zirconia microbeads (Biospec Products) and 1 ml of Trizol reagent (Invitrogen) and homogenized in a minibeadbeater (BioSpec Products) for 10 s. RNA was isolated from lung homogenates using Trizol according to the manufacturer's instructions. DNA removal and cDNA synthesis were performed using the Quantitect Reverse Transcription Kit (Qiagen) according to the manufacturer's instructions. Real-time quantitative PCRs (qPCRs) were prepared using Platinum SYBR Green qPCR Supermix-UDG (Invitrogen) according to the manufacturer's instructions in ICycler IQ PCR plates (Bio-Rad) sealed with ICycler IQ optical tape (Bio-Rad). Primers amplifying a 168-bp fragment of the BRSV F gene (primer A, 5′-AACCGGCCTCCTTCAGTAGA-3′; primer B, 5′-TGGACACTGCTACACCACT-3′) were designed using primer design software for personal computers (Clone Manager version 6.00) from a consensus sequence generated from 27 different BRSV F gene sequences using the MultAlin multiple-sequence alignment tool available online at http://bioinfo.genopole-toulouse.prd.fr/multalin/11). qPCR was performed on an ICycler IQ Multicolor Real-Time PCR Detection System (Bio-Rad) as previously described (32). Serial dilutions of a plasmid that contains a truncated version of the BRSV F gene used in qPCRs carried out under the same conditions outlined above allowed construction of a standard curve that enabled the determination of gene copy numbers. The results are expressed as viral RNA copies per ml of lung homogenate.
Statistical analysis.
All data were analyzed using statistical software (GraphPad PRISM version 3.00). As sample sizes were small (n = 5 or 10), outcome variables were assumed not to be normally distributed. Therefore, differences among all groups were examined using the Kruskal-Wallis test. If a significant difference was found among the groups, median ranks between pairs of groups were compared using the Mann-Whitney U test. Differences were considered significant if P < 0.05.
RESULTS
FI-BRSV formulated with both CpG ODN and PP induces strong humoral and cell-mediated immune responses after two intranasal vaccinations.
CpG ODN and PP were selected as adjuvants for formulation of an FI-BRSV vaccine, because CpG ODN promotes Th1-biased or balanced immune responses while PP forms noncovalent complexes with the antigens in the formulation, which may stabilize the vaccine components. Previously, three i.n. immunizations of FI-BRSV formulated with both CpG ODN and PP were shown to induce stronger immune responses and protection from viral challenge than FI-BSRV formulated with either CpG ODN or PP (32). In order to evaluate combinations of intranasal and subcutaneous prime-boost strategies, we first examined whether this formulation could also be used as a two-dose vaccine, as well as with a reduced amount of CpG ODN.
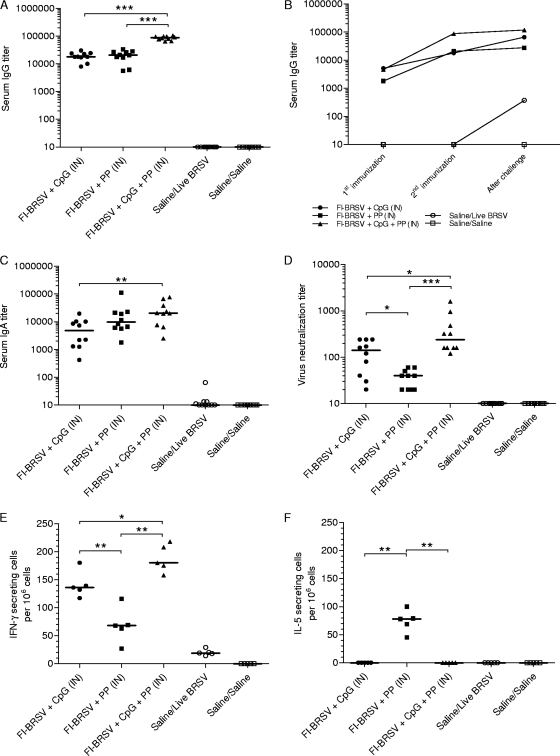
The humoral immune responses induced by the various vaccine formulations were examined by measuring BRSV-specific serum antibody responses. All vaccinated groups developed increased IgG levels compared to the control groups. After the second immunization, the IgG titers induced by FI-BRSV-CpG-PP were higher than those elicited by the other vaccine formulations (Fig. 1A). When the kinetics of the immune responses prior to and after challenge were evaluated, only the FI-BRSV-CpG and placebo groups developed significantly increased serum IgG after challenge with BRSV (Fig. 1B). Regardless of the vaccine formulation used, both IgG1 and IgG2a subtypes were detected, with some predominance of IgG2a (Table 3).
FIG. 1.
Systemic responses to BRSV. (A) BRSV-specific IgG 2 weeks after the second immunization. (B) Kinetics of the BRSV-specific IgG response. (C) BRSV-specific IgA after challenge. (D) Virus neutralizing antibodies after challenge. (E and F) Numbers of IFN-γ-secreting (E) and numbers of IL-5-secreting (F) splenocytes in response to in vitro restimulation with BRSV-infected cell lysates. Mice were immunized i.n. with FI-BRSV-CpG, FI-BRSV-PP, FI-BRSV-CpG-PP, or saline. CpG ODN was given at 4 μg per immunization, and PP was given at 25 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). Five of the mice were euthanized 4 days later and the other half 6 days later. Cytokine production was measured 6 days after challenge. Virus neutralization titers are expressed as the highest dilution of serum that resulted in less than 50% of cells displaying cytopathic effects. Cytokine-secreting cells are shown as the difference between the number of cytokine-secreting cells per 106 cells in BRSV-infected lysate-stimulated wells and the number of cytokine-secreting cells per 106 cells in mock-infected lysate-stimulated wells. In panels A, C, D, E, and F, each data point represents an individual animal, and median values are indicated by horizontal bars. In panel B, median values are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 3.
BRSV-specific IgG1 and IgG2a titers for different formulations
| Group | Titera |
|
|---|---|---|
| IgG1 | IgG2a | |
| FI-BRSV-CpG | 3.22 ± 0.20 | 3.59 ± 0.13 |
| FI-BRSV-PP | 3.35 ± 0.14 | 4.18 ± 0.14 |
| FI-BRSV-CpG-PP | 4.12 ± 0.21 | 4.24 ± 0.17 |
| Saline-BRSV challenge | 1.90 ± 0.30 | 1.55 ± 0.44 |
| Saline-saline | 1.28 ± 0.23 | 0.32 ± 0.21 |
Titers were determined after BRSV challenge and are expressed as the mean ± standard error of the mean of log10 values of the reciprocal of the highest dilution resulting in a value of 2 standard deviations above the negative control serum value.
BRSV-specific serum IgA was measured after challenge. I.n. immunization with all three FI-BRSV formulations resulted in IgA production, although FI-BRSV-CpG-PP induced higher IgA titers than FI-BRSV-CpG (Fig. 1C). To evaluate the biological activity of the BRSV-specific serum antibodies, virus neutralizing titers were determined after challenge. Mice immunized with FI-BRSV-CpG-PP developed higher neutralizing antibody titers than animals immunized with either FI-BRSV-CpG or FI-BRSV-PP, while FI-BRSV-CpG-immunized mice had higher neutralizing antibody levels than FI-BRSV-PP-immunized animals. These results demonstrate that, in terms of BRSV-specific serum antibody production, the FI-BRSV-CpG-PP formulation resulted in higher antibody levels than the other formulations.
As a measurement of cell-mediated immunity, the BRSV-induced IFN-γ and IL-5 production by in vitro-restimulated splenocytes was measured 6 days after challenge (Fig. 1E). FI-BRSV-CpG-PP elicited higher levels of IFN-γ secretion than FI-BRSV-CpG or FI-BRSV-PP, while FI-BRSV-CpG induced higher levels of IFN-γ secretion than FI-BRSV-PP. In contrast to IFN-γ production, immunization with FI-BRSV-PP resulted in the largest amounts of IL-5 (Fig. 1F). These data suggest that FI-BRSV formulated with CpG ODN or both CpG ODN and PP elicited a Th1-biased immune response, while FI-BRSV-PP induced a balanced response.
Intranasal administration of FI-BRSV formulated with CpG ODN and PP induces strong mucosal immune responses.
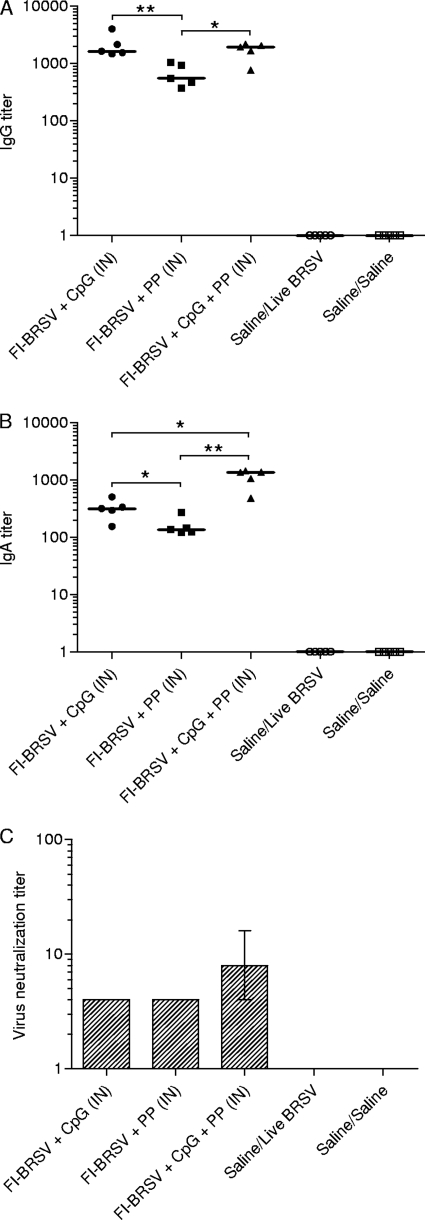
To evaluate the mucosal immune responses induced by the various vaccine formulations, secretion of BRSV-specific IgG and IgA in lung fragment culture supernatants was measured. FI-BRSV-CpG and FI-BRSV-CpG-PP induced higher IgG production than FI-BRSV-PP (Fig. 2A), while FI-BRSV-CpG-PP induced higher IgA titers than FI-BRSV-CpG or FI-BRSV-PP, and FI-BRSV-CpG elicited higher IgA levels than FI-BRSV-PP (Fig. 2B). Although there were no significant differences between groups, FI-BRSV-CpG-PP tended to induce higher virus neutralizing antibody levels than FI-BRSV-CpG or FI-BRSV-PP (Fig. 2C). Thus, in terms of BRSV-specific antibody production in the lungs, CpG ODN-containing formulations, and in particular the combination of CpG ODN and PP, outperformed FI-BRSV formulated with PP alone.
FIG. 2.
Mucosal responses to BRSV. BRSV-specific IgG (A), IgA (B), and virus neutralizing antibodies (C) in lung fragment culture supernatants. Mice were immunized with FI-BRSV-CpG, FI-BRSV-PP, FI-BRSV-CpG-PP, or saline. CpG ODN was given at 4 μg per immunization, and PP was given at 25 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). The IgG, IgA, and virus neutralizing antibody titers were measured on day 6 after challenge. Virus neutralization titers are expressed as the highest dilution of lung fragment culture supernatant that resulted in less than 50% of cells displaying cytopathic effects. In panels A and B, each data point represents an individual animal, and median values are indicated by horizontal bars. In panel C, median values and interquartile ranges are shown. *, P < 0.05; **, P < 0.01.
Reduction in virus replication after BRSV challenge is more significant in mice immunized intranasally with FI-BRSV formulated with CpG ODN and PP.
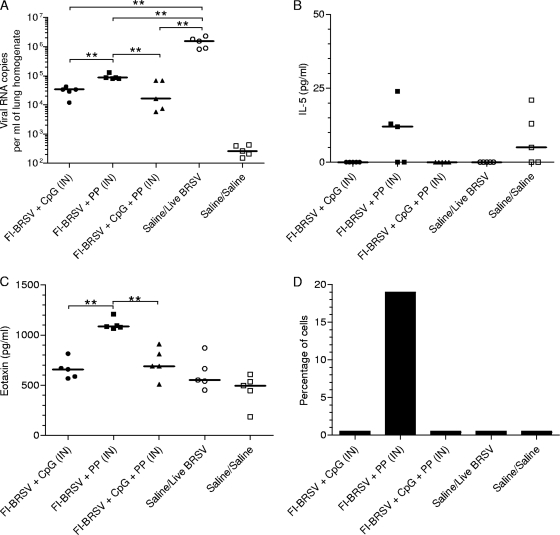
To assess the abilities of the three vaccine formulations to protect from infection, mice were challenged with BRSV. Virus replication in the lungs was examined by detection of viral RNA. All vaccinated groups experienced decreases in viral replication compared to the mock-vaccinated, virus-challenged control group (Fig. 3A), and vaccination with FI-BRSV-CpG or FI-BRSV-CpG-PP led to lower viral replication than immunization with FI-BRSV-PP. The low viral-RNA copy numbers observed in the mock-vaccinated, mock-challenged mice were due to the formation of nonspecific PCR products synthesized in the absence of viral RNA. Of the three formulations tested, vaccination with FI-BRSV-CpG-PP resulted in the lowest median viral-RNA copy number.
FIG. 3.
Viral RNA, cytokines, and cell populations in the lungs. (A) Detection of viral RNA in lung tissue. (B and C) IL-5 production (B) and eotaxin production (C) in lung homogenate supernatants. (D) Percentages of eosinophils in bronchoalveolar lavage fluids. Mice were immunized with FI-BRSV-CpG, FI-BRSV-PP, FI-BRSV-CpG-PP, or saline. CpG ODN were given at 4 μg per immunization, and PP was given at 25 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). Viral RNA was measured on day 4, and cytokines and eosinophils were measured on day 6 after challenge. The results are expressed as viral-RNA copies per ml of lung homogenate. In panels A, B, and C, each data point represents an individual animal, and median values are indicated by horizontal bars. In panel D, the bars represent the percentages of eosinophils in pooled bronchoalveolar lavage fluids per minimum of 200 cells. **, P < 0.01.
In addition to virus replication, the potential for immunopathological consequences after vaccination and viral challenge is a critical issue that needs to be addressed. To examine the mice for evidence of immunopathology, the amounts of IL-5 and eotaxin in the lungs were measured. FI-BRSV formulated with CpG ODN or CpG ODN and PP tended to induce lower IL-5 levels (Fig. 3A) and produced significantly smaller eotaxin amounts (Fig. 3C) than FI-BRSV formulated with PP alone. The presence or absence of pulmonary eosinophilia was also determined 6 days after challenge. Eosinophils were present only in the group that received FI-BRSV-PP. These data show that i.n. delivered FI-BRSV vaccines that contain CpG ODN do not result in the induction of pulmonary eosinophilia upon subsequent challenge with BRSV. As these analyses were performed on BAL fluids pooled from each vaccine group, no statistical analyses were possible.
The delivery route is critical for induction of serum IgG and IgA by FI-BRSV, with intranasal delivery being optimal in comparison to subcutaneous delivery or combinations of intranasal and subcutaneous prime-boost strategies.
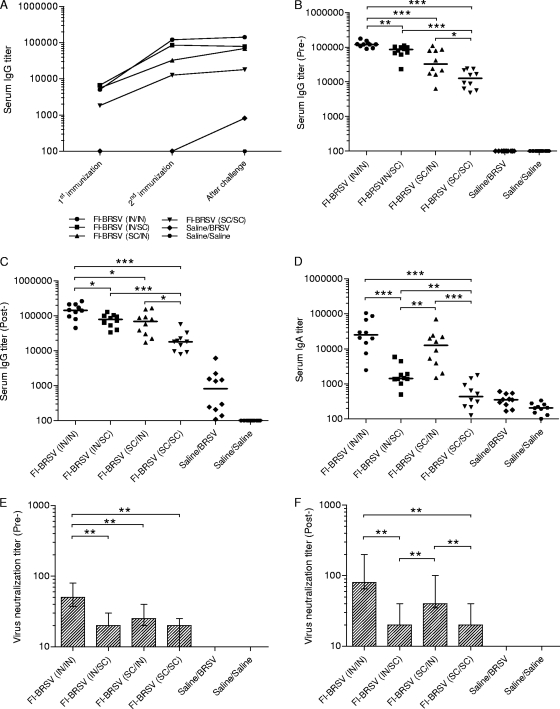
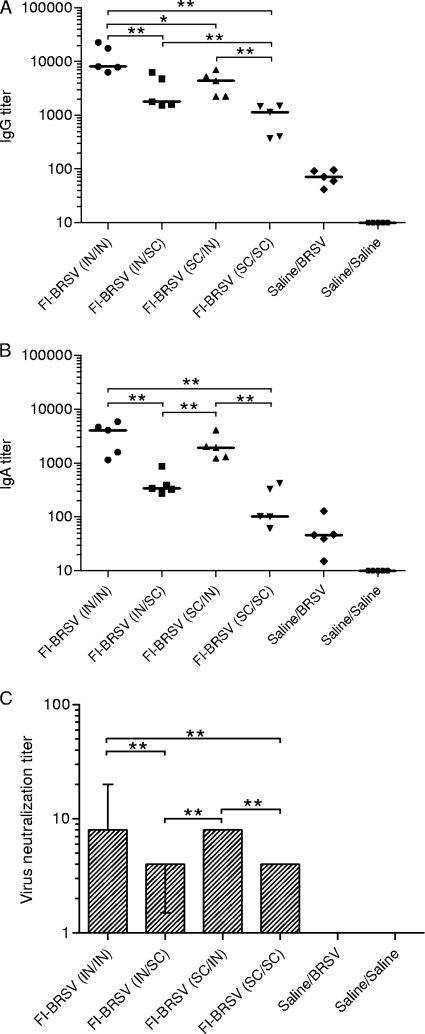
The results of the above-described experiment showed that the FI-BRSV-CpG-PP formulation was most effective compared to FI-BRSV-CpG and FI-BRSV-PP after two vaccinations. There is evidence that combinations of mucosal and parenteral administration result in immune responses that are as strong as or stronger than those resulting from either mucosal or parenteral immunization alone (21, 22, 29, 30), so to determine the optimal route of delivery in terms of induction of immunity and protection from BRSV, mice were immunized with FI-BRSV, formulated with both CpG ODN and PP, using different strategies. Increased IgG titers were observed in all vaccinated groups compared to the mock-vaccinated control groups (Fig. 4A). After the second immunization, the IgG levels induced by i.n./i.n. administration of FI-BRSV were higher than the amounts induced by any other delivery route, while both i.n./s.c. and s.c./i.n. delivery of the FI-BRSV formulation resulted in stronger antibody responses than s.c./s.c. administration (Fig. 4B). No increases in IgG production were observed after challenge; the immunized groups displayed a trend that was very similar to that seen after the second immunization (Fig. 4C). These results suggest that in terms of anti-BRSV serum IgG production, the route of delivery is critical for FI-BRSV vaccines, with i.n./i.n. performing better and s.c./s.c. performing worse than any of the other routes. Both IgG1 and IgG2a, with some predominance of IgG2a, were induced in all mice, whether immunized i.n./i.n., i.n./s.c., s.c./i.n., or s.c./s.c. (Table 4).
FIG. 4.
Serum antibody responses to BRSV. (A) Kinetics of the BRSV-specific IgG response. (B) BRSV-specific IgG 2 weeks after the second immunization. (C) BRSV-specific IgG after challenge. (D) BRSV-specific IgA after challenge. (E) Virus neutralizing antibodies after two immunizations. (F) Virus neutralizing antibodies after challenge. Mice were immunized with FI-BRSV (i.n./i.n.), FI-BRSV (i.n./s.c.), FI-BRSV (s.c./i.n.), or FI-BRSV (s.c./s.c.) formulated with 4 μg CpG ODN and 25 μg PP per immunization or with saline. FI-BRSV was given at 1.5 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). Five of the mice were euthanized 4 days later and the other half 6 days later. Virus neutralization titers are expressed as the highest dilution of serum that resulted in less than 50% of cells displaying cytopathic effects. In panel A, median values are shown. In panels B, C, and D, each data point represents an individual animal, and median values are indicated by horizontal bars. In panels E and F, median values and interquartile ranges are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 4.
BRSV-specific IgG1 and IgG2a titers for different delivery routes
| Group | Titera |
|
|---|---|---|
| IgG1 | IgG2a | |
| FI-BRSV (i.n./i.n.) | 3.92 ± 0.31 | 4.10 ± 0.14 |
| FI-BRSV (i.n./s.c.) | 3.27 ± 0.37 | 4.29 ± 0.09 |
| FI-BRSV (s.c./i.n.) | 3.45 ± 0.32 | 3.89 ± 0.21 |
| FI-BRSV (s.c./s.c.) | 3.14 ± 0.12 | 4.09 ± 0.19 |
| Saline-BRSV challenge | 0.80 ± 0.27 | 1.71 ± 0.57 |
| Saline-saline | 0.64 ± 0.26 | 0.76 ± 0.32 |
Titers were determined after BRSV challenge and are expressed as the mean ± standard error of the mean of log10 values of the reciprocal of the highest dilution resulting in a value of 2 standard deviations above the negative control serum value.
In addition, BRSV-specific serum IgA was measured after challenge. I.n./i.n. administration of FI-BRSV resulted in higher IgA levels than i.n./s.c. or s.c./s.c. delivery (Fig. 4D). However, there was no difference with FI-BRSV (s.c./i.n.). Both i.n./s.c. and s.c./i.n. delivery of FI-BRSV resulted in IgA levels higher than those induced by s.c./s.c. delivery, while the s.c./i.n. delivered vaccine performed better than the i.n./s.c. delivered vaccine. Overall, the differences between i.n./i.n. or heterologous delivery and s.c./s.c. administration were more prominent for IgA than for IgG. The route of delivery, therefore, appears to be a more critical determinant for serum IgA induction by FI-BRSV.
The biological activities of the antibodies produced in the sera were evaluated by determining virus neutralizing titers. After the second immunization, FI-BRSV (i.n./i.n.) induced higher neutralizing antibody titers than the corresponding vaccines delivered via different routes (Fig. 4E). Both i.n./i.n. and s.c./i.n. delivery of FI-BRSV resulted in higher neutralizing antibody levels than i.n./s.c. or s.c./s.c. delivery after challenge (Fig. 4F). Overall, in terms of virus neutralizing antibody production in the serum, i.n./i.n. administration was more efficacious.
Intranasal delivery of FI-BRSV results in increased IFN-γ and decreased IL-5 production by splenocytes in comparison to subcutaneous delivery or combinations of intranasal and subcutaneous prime-boost strategies.
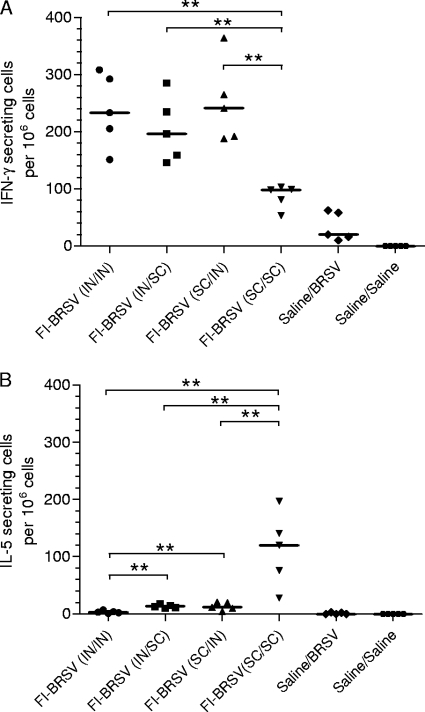
Secretion of IFN-γ and IL-5 by in vitro-restimulated splenocytes was determined 6 days after challenge as an indicator of cell-mediated immunity. I.n./i.n., i.n./s.c, and s.c./i.n. administration of FI-BRSV resulted in higher numbers of IFN-γ-secreting cells than s.c./s.c. delivery (Fig. 5A). In contrast, the group immunized s.c./s.c. with FI-BRSV exhibited the highest numbers of IL-5-secreting cells compared to the other delivery routes (Fig. 5B). Furthermore, i.n./s.c. and s.c./i.n. delivery of FI-BRSV resulted in higher IL-5 production than i.n./i.n. delivery. These results indicate that, based on the balance between IFN-γ and IL-5 secretion by in vitro-restimulated splenocytes, i.n./i.n., i.n./s.c., and s.c./i.n. administration induce a Th1-biased response, while s.c./s.c. delivery results in a more balanced response.
FIG. 5.
Numbers of IFN-γ-secreting (A) and IL-5-secreting (B) splenocytes in response to in vitro restimulation with BRSV-infected cell lysates. Mice were immunized with FI-BRSV (i.n./i.n.), FI-BRSV (i.n./s.c.), FI-BRSV (s.c./i.n.), or FI-BRSV (s.c./s.c.) formulated with 4 μg CpG ODN and 25 μg PP per immunization or with saline. FI-BRSV was given at 1.5 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). The cytokine production was measured on day 6 after challenge. The results are the difference between the number of cytokine-secreting cells per 106 cells in BRSV-infected lysate-stimulated wells and the number of cytokine-secreting cells per 106 cells in mock-infected lysate-stimulated wells. Each data point represents an individual animal, and median values are indicated by horizontal bars. **, P < 0.01.
Lung antibody levels induced by FI-BRSV depend on the route of delivery, with intranasal immunization and subcutaneous prime followed by intranasal boost being optimal.
The secretion of BRSV-specific IgG and IgA in lung fragment cultures was examined as an indicator of mucosal immunity. In terms of IgG production, i.n./i.n. administration of FI-BRSV gave rise to stronger responses than i.n./s.c., s.c./i.n., or s.c./s.c. delivery (Fig. 6A), while i.n./s.c. and s.c./i.n. administration of FI-BRSV led to higher IgG titers than s.c./s.c. delivery. The determination of IgA production in lung fragment culture supernatants revealed a pattern similar to that observed for IgG production. I.n./i.n. and s.c./i.n. administration of FI-BRSV gave rise to higher IgA titers than i.n./s.c. and s.c./s.c. delivery (Fig. 6B).
FIG. 6.
BRSV-specific antibodies in the lung. BRSV-specific IgG (A), IgA (B), and virus neutralizing antibodies (C) in lung fragment culture supernatants. Mice were immunized with FI-BRSV (i.n./i.n.), FI-BRSV (i.n./s.c.), FI-BRSV (s.c./i.n.), or FI-BRSV (s.c./s.c.) formulated with 4 μg CpG ODN and 25 μg PP per immunization or with saline. FI-BRSV was given at 1.5 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). The IgG, IgA, and virus neutralizing antibody titers were measured on day 6 after challenge. Virus neutralization titers are expressed as the highest dilution of lung fragment culture supernatant that resulted in less than 50% of cells displaying cytopathic effects. In panels A and B, each data point represents an individual animal, and median values are indicated by horizontal bars. In panel C, the bars represent the medians, and interquartile ranges are shown. *, P < 0.05; **, P < 0.01.
The biological activity of the lung antibodies was evaluated in virus neutralization assays. Both i.n./i.n. and s.c./i.n. delivery of FI-BRSV resulted in higher neutralizing antibody levels than i.n./s.c. or s.c./s.c. delivery (Fig. 6C). These results demonstrate that overall, FI-BRSV induced the highest antibody production in the lungs when given i.n./i.n. and the lowest mucosal antibody levels when given s.c./s.c.
Intranasal immunization with FI-BRSV reduces viral replication after BRSV challenge compared to alternative delivery routes.
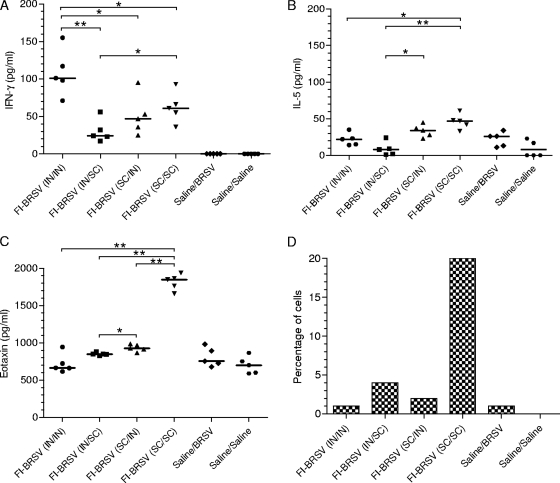
To further examine the effect of the delivery route on the local immune response bias, IFN-γ and IL-5 levels in the lungs were measured. I.n./i.n. delivery of the FI-BRSV vaccine resulted in larger amounts of IFN-γ than any of the other routes. In addition, there was a significant difference between the i.n./s.c. and s.c./s.c. routes (Fig. 7A). However, s.c./s.c. administration of FI-BRSV induced higher lung IL-5 levels than i.n./i.n. or i.n./s.c. delivery, while s.c./i.n. administration gave rise to higher IL-5 levels than i.n./s.c. delivery (Fig. 7B). Thus, at the level of local immunity in the lung, i.n./i.n. administration of FI-BRSV is preferable to the other delivery routes with regard to the balance between IFN-γ and IL-5 secretion.
FIG. 7.
IFN-γ (A), IL-5 (B), and eotaxin (C) production in lung homogenate supernatants and percentages of eosinophils in bronchoalveolar lavage fluids (D). Mice were immunized with FI-BRSV (i.n./i.n.), FI-BRSV (i.n./s.c.), FI-BRSV (s.c./i.n.), or FI-BRSV (s.c./s.c.) formulated with 4 μg CpG ODN and 25 μg PP per immunization or with saline. FI-BRSV was given at 1.5 μg per immunization. All animals were challenged with BRSV two weeks after the second immunization (except for the saline/saline group). The cytokines and eosinophils were measured on day 6 after challenge. In panels A, B, and C, each data point represents an individual animal, and median values are indicated by horizontal bars. In panel D, the bars represent the percentages of eosinophils in pooled bronchoalveolar lavage fluids per minimum of 200 cells. *, P < 0.05; **, P < 0.01.
Furthermore, to ensure that there was no induction of immunopathology, the amount of eotaxin in the lungs was determined. With the exception of FI-BRSV (s.c./s.c.), which induced significantly higher eotaxin production, all vaccine strategies resulted in eotaxin levels comparable to those in the control groups (Fig. 7C). Also, s.c./i.n. delivery of FI-BRSV elicited higher eotaxin levels than i.n./s.c. delivery. The lungs were also evaluated for eosinophilia 6 days after challenge. The only group with elevated eosinophil numbers was the one that received FI-BRSV (s.c./s.c.); none of the other delivery strategies resulted in eosinophilia (Fig. 7D). As these determinations were performed on BAL fluids pooled from each group, no statistical analyses were possible. These data suggest that the route of delivery is critical in determining whether FI-BRSV induces lung IL-5 and eotaxin production, as well as eosinophilia.
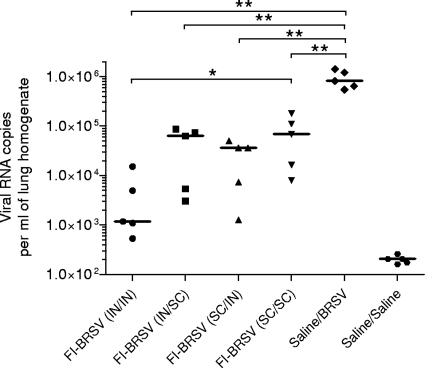
The amount of virus in the lungs was determined to assess the abilities of the various vaccine formulations and delivery routes to protect from BRSV infection. All vaccinated groups experienced lower levels of viral replication than the mock-vaccinated, virus-challenged control group. In terms of viral replication, FI-BRSV (i.n./i.n.) performed better than FI-BRSV (s.c./s.c.) and equivalent to FI-BRSV (i.n./s.c.) and FI-BRSV (s.c./i.n.) (Fig. 8). These data suggest that, in terms of reducing viral replication, i.n./i.n. delivery of FI-BRSV is superior to s.c./s.c. delivery and tended to be superior to the i.n./s.c. and s.c./i.n. routes.
FIG. 8.
Detection of viral RNA in lung tissue. Mice were immunized with FI-BRSV (i.n./i.n.), FI-BRSV (i.n./s.c.), FI-BRSV (s.c./i.n.), or FI-BRSV (s.c./s.c.) formulated with 4 μg CpG ODN and 25 μg PP per immunization or with saline. FI-BRSV was given at 1.5 μg per immunization. All animals were challenged with BRSV 2 weeks after the second immunization (except for the saline/saline group). The RNA was measured on day 4 after challenge. The results are expressed as viral-RNA copies per ml of lung homogenate. Each data point represents an individual animal, and median values are indicated by horizontal bars. *, P < 0.05; **, P < 0.01.
DISCUSSION
Mucosal delivery of a BRSV vaccine has several potential advantages. The first encounter between BRSV and a prospective host occurs in the lung mucosa, so in addition to systemic immunity, mucosal immunity is expected to be important for long-term protection from BRSV. Second, mucosal vaccination should more readily circumvent inactivation by circulating maternal antibodies in newborn calves. Here, we confirmed that coformulation of i.n. delivered FI-BRSV with both CpG ODN and PP outperforms i.n. delivered FI-BRSV formulated with CpG ODN or PP alone in terms of immune responses and protection, agreeing with our previously published study (32). This is encouraging, as we administered two instead of three vaccinations, as well as a smaller amount of CpG ODN per dose, in the present study, which is important from the viewpoint of compliance, vaccine cost, and the use of intranasal and subcutaneous prime-boost strategies. In the subsequent study, we defined the optimal route of delivery by comparing several vaccination protocols for FI-BRSV in terms of induction of systemic and mucosal immunity, as well as reduction in viral replication in BRSV-challenged mice.
Although it is not the only component of a protective immune response, humoral immunity is a classical indicator of how well a vaccine has performed. Here, we observed that i.n./i.n. delivery of FI-BRSV was generally superior to i.n./s.c., s.c./i.n., or s.c./s.c. delivery for induction of antibody responses. A successful response against BRSV, however, requires more than humoral immunity alone; cell-mediated immunity is required, as well. In terms of the balance between IFN-γ and IL-5 secretion by in vitro-restimulated splenocytes, i.n./i.n. delivery of FI-BRSV coformulated with CpG ODN and PP was superior to s.c./s.c. delivery, but i.n./s.c. or s.c./i.n. delivery was efficacious, as well. This high-IFN-γ, low-IL-5 cell-mediated immunity profile is characteristic of a Th1-type immune response, which is necessary to avoid RSV vaccine-enhanced pathology. Our observation that i.n./i.n. immunization induced systemic cell-mediated immunity agrees with our previous report (32) and several additional studies. Mucosal immunization with a ricin toxin B subunit-rotavirus NSP4 fusion protein vaccine resulted in antigen-induced IFN-γ secretion by in vitro-restimulated splenocytes (7), and both sublingual and i.n. administration of cholera toxin-adjuvanted ovalbumin promoted the production of IFN-secreting T cells (12).
While the induction of both humoral and cell-mediated immune responses is important for protection against BRSV, the first line of defense is found in the lung mucosa, where the host encounters the virus. IgG, IgA, and virus neutralizing antibodies in lung fragment cultures were assayed as indicators of mucosal immunity. Secretion of mucosal IgA, in particular, has been observed following i.n. immunization with CpG ODN-adjuvanted vaccines against hepatitis B virus (35), Streptococcus pyogenes (50), and BRSV (32). I.n./i.n. delivery of FI-BRSV was generally better than any other delivery route, but s.c./i.n. delivery resulted in IgA and virus neutralizing antibody levels comparable to those elicited following i.n./i.n. delivery. Lung IFN-γ, ΙL-5, eotaxin, and eosinophilia levels were also measured as indicators of the quality of the immune responses. Subcutaneous vaccine delivery resulted in eotaxin and eosinophilia levels that were above normal, whereas the i.n./i.n., i.n./s.c., and s.c./i.n. delivery protocols did not lead to increased eotaxin and eosinophilia. While scarification of mice with live rVV expressing the HRSV G protein (rVV-G) resulted in enhanced lung immunopathology upon subsequent challenge (41), i.n. and intraperitoneal immunization with rVV-G elicited protection and no lung lesions (48). Similarly, i.n. immunization with rVV expressing the HRSV F protein resulted in mild lung inflammation upon challenge in comparison to that induced by intradermal immunization (34), which is in agreement with our results and suggests that mucosal immunization is less likely to trigger RSV-induced immunopathology than immunization by other routes. Alternatively, the s.c. route can be used to induce protection from BRSV challenge without induction of immunopathology, provided the FI-BRSV vaccine is coformulated with high enough doses of CpG ODN, as was demonstrated in a study by Oumouna et al. (42), in which doses 5-fold-higher than the amounts used in the experiment reported here were used.
The most important hallmark of a successful vaccine is the demonstrable ability to protect from a live experimental challenge. Mucosally delivered vaccines, when formulated with CpG ODN, have been demonstrated to confer protection; i.n. delivery of herpes simplex virus (HSV) recombinant glycoprotein B (18) or HIV immunogen (15) resulted in protection of mice from intravaginal challenge with HSV or live recombinant vaccinia virus expressing HIV gag, respectively. Because of BRSV's lability and limited growth in mice, reverse transcription-PCR assays have been developed to detect viral RNA as an alternative to live virus isolation (1). Using quantitative-PCR reagents and detection systems, we have increased the sensitivity of these assays, allowing us to quantify the amount of viral RNA in the lungs of mice experimentally infected with BRSV (32). Generally, mice treated with any of the immunization protocols tested experienced significant reductions in viral replication compared to a mock-immunized, virus-challenged control group. That being said, i.n./i.n. delivery of FI-BRSV coformulated with CpG ODN and PP more efficiently prevented viral replication than s.c./s.c. delivery and tended to be more effective than the other two delivery routes.
Our observations suggest that i.n./i.n. administration of FI-BRSV formulated with CpG ODN and PP is optimal, though i.n./s.c. and s.c./i.n. delivery generally resulted in responses identical or close to those induced following i.n./i.n. delivery. S.c./i.n. delivery led to IgA production after challenge identical to that induced by i.n./i.n. delivery in both the serum and lung fragment cultures. Furthermore, both i.n./s.c. and s.c./i.n. delivery resulted in cell-mediated immunity profiles virtually identical to those induced by i.n./i.n. delivery, as well as low levels of lung eotaxin and eosinophilia and reduction in viral replication, again similar to what was observed following i.n./i.n. delivery. Mucosal and parenteral prime-boost strategies have been observed in a few studies to result in immune responses that are as strong as or stronger than those resulting from either mucosal or parenteral immunization alone (21, 22, 29, 30). Whereas Layton and Smithyman (30) evaluated combinations of oral and intramuscular (i.m.) immunization, the studies by Glynn et al. (21), Lauterslager et al. (29), and McCluskie et al. (36), along with our study, are some of the few examining the delivery route combinations that include i.n. immunization. When Lauterschlager (29) investigated oral, i.n., s.c., or i.p. prime in combination with an oral boost, oral immunization appeared to be more effective in i.n., s.c., or i.p. primed mice than in orally primed mice. In the case of Yersinia pestis antigen, two i.n. and two s.c. immunizations elicited equivalent serum and bronchioalveolar IgG1 responses, while i.n. priming followed by s.c. or transcutaneous boost induced the highest anti-F1 and anti-V total IgG responses (21). McCluskie et al. reported that, when hepatitis B virus surface antigen was adjuvanted with CpG ODN, both i.n. prime-i.m. boost and i.m. prime-i.n. boost resulted in robust systemic and mucosal immune responses that were similar to those resulting from i.n. immunization (36).
In conclusion, i.n./i.n. delivery of FI-BRSV formulated with CpG ODN and PP proved to be the most effective strategy in terms of induction of humoral, cell-mediated, and mucosal immunity, as well as reduction of viral replication after BRSV challenge, while the i.n./s.c. and s.c./i.n. delivery strategies were safe and almost as efficacious. These results are promising because (i) mucosal vaccination will more likely circumvent maternal antibody interference and (ii) the use of fewer doses and/or smaller CpG ODN amounts than those reported previously to be effective with FI-BRSV would make the vaccine more affordable. While results obtained in mice are not necessarily predictive of what will occur in other species, the fact that these vaccine formulations, combined with i.n./i.n. delivery, induce protective immunity in this model might have implications for the development of a protective BRSV vaccine for cattle. In addition, due to the similarities of human and bovine RSV, formulation with CpG ODN and PP could prove important in the development of a mucosal vaccine that induces safe and protective immunity against HRSV in humans.
Acknowledgments
We thank Zoe Lawman, Marlene Snider, and Shirley Hauta for technical assistance, as well as VIDO Animal Care for the handling and care of the animals. We also thank Merial (Duluth, GA) for the CpG ODN and John Klaehn of Idaho National Laboratory, LLC, in Idaho Falls, ID, for the synthesis of polyphosphazene polymer 6.
This work was supported by grants from the Canadian Institute of Health Research and the Krembil Foundation. L.A.B. is the recipient of a Canadian Research Chair in Vaccinology.
Footnotes
Published ahead of print on 28 October 2009.
VIDO Journal Series number 521.
REFERENCES
- 1.Almeida, R. S., H. G. Domingues, L. T. Coswig, R. C. D'Arce, R. F. de Carvalho, and C. W. Arns. 2004. Detection of bovine respiratory syncytial virus in experimentally infected balb/c mice. Vet. Res. 35:189-197. [DOI] [PubMed] [Google Scholar]
- 2.Andrianov, A. K., Y. Y. Svirkin, and M. P. LeGolvan. 2004. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromolecules 5:1999-2006. [DOI] [PubMed] [Google Scholar]
- 3.Antonis, A. F., R. S. Schrijver, F. Daus, P. J. Steverink, N. Stockhofe, E. J. Hensen, J. P. Langedijk, and R. G. Van Der Most. 2003. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J. Virol. 77:12067-12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baca-Estrada, M. E., M. Snider, S. K. Tikoo, R. Harland, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 1996. Immunogenicity of bovine herpesvirus 1 glycoprotein D in mice: effect of antigen form on the induction of cellular and humoral immune responses. Viral Immunol. 9:11-22. [DOI] [PubMed] [Google Scholar]
- 5.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 6.Chin, J., R. L. Magoffin, L. A. Shearer, J. H. Schieble, and E. H. Lennette. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89:449-463. [DOI] [PubMed] [Google Scholar]
- 7.Choi, N. W., M. K. Estes, and W. H. Langridge. 2006. Mucosal immunization with a ricin toxin B subunit-rotavirus NSP4 fusion protein stimulates a Th1 lymphocyte response. J. Biotechnol. 121:272-283. [DOI] [PubMed] [Google Scholar]
- 8.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors, M., N. A. Giese, A. B. Kulkarni, C. Y. Firestone, H. C. Morse III, and B. R. Murphy. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 68:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors, M., A. B. Kulkarni, C. Y. Firestone, K. L. Holmes, H. C. Morse III, A. V. Sotnikov, and B. R. Murphy. 1992. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J. Virol. 66:7444-7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuburu, N., M. N. Kweon, J. H. Song, C. Hervouet, C. Luci, J. B. Sun, P. Hofman, J. Holmgren, F. Anjuere, and C. Czerkinsky. 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25:8598-8610. [DOI] [PubMed] [Google Scholar]
- 13.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 14.Delgado, M. F., S. Coviello, A. C. Monsalvo, G. A. Melendi, J. Z. Hernandez, J. P. Batalle, L. Diaz, A. Trento, H. Y. Chang, W. Mitzner, J. Ravetch, J. A. Melero, P. M. Irusta, and F. P. Polack. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumais, N., A. Patrick, R. B. Moss, H. L. Davis, and K. L. Rosenthal. 2002. Mucosal immunization with inactivated human immunodeficiency virus plus CpG oligodeoxynucleotides induces genital immune responses and protection against intravaginal challenge. J. Infect. Dis. 186:1098-1105. [DOI] [PubMed] [Google Scholar]
- 16.Etchart, N., B. Baaten, S. R. Andersen, L. Hyland, S. Y. Wong, and S. Hou. 2006. Intranasal immunisation with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur. J. Immunol. 36:1136-1144.16619288 [Google Scholar]
- 17.Fulginiti, V. A., J. J. Eller, O. F. Sieber, J. W. Joyner, M. Minamitani, and G. Meiklejohn. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 89:435-448. [DOI] [PubMed] [Google Scholar]
- 18.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 19.Gershwin, L. J. 2007. Bovine respiratory syncytial virus infection: immunopathogenic mechanisms. Anim. Health Res. Rev. 8:207-213. [DOI] [PubMed] [Google Scholar]
- 20.Gershwin, L. J., E. S. Schelegle, R. A. Gunther, M. L. Anderson, A. R. Woolums, D. R. Larochelle, G. A. Boyle, K. E. Friebertshauser, and R. S. Singer. 1998. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 16:1225-1236. [DOI] [PubMed] [Google Scholar]
- 21.Glynn, A., L. C. Freytag, and J. D. Clements. 2005. Effect of homologous and heterologous prime-boost on the immune response to recombinant plague antigens. Vaccine 23:1957-1965. [DOI] [PubMed] [Google Scholar]
- 22.Glynn, A., C. J. Roy, B. S. Powell, J. J. Adamovicz, L. C. Freytag, and J. D. Clements. 2005. Protection against aerosolized Yersinia pestis challenge following homologous and heterologous prime-boost with recombinant plague antigens. Infect. Immun. 73:5256-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann, G., and A. M. Krieg. 2000. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164:944-953. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 25.Ioannou, X. P., S. M. Gomis, B. Karvonen, R. Hecker, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2002. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine 21:127-137. [DOI] [PubMed] [Google Scholar]
- 26.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 27.Kalina, W. V., A. R. Woolums, R. D. Berghaus, and L. J. Gershwin. 2004. Formalin-inactivated bovine RSV vaccine enhances a Th2 mediated immune response in infected cattle. Vaccine 22:1465-1474. [DOI] [PubMed] [Google Scholar]
- 28.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 29.Lauterslager, T. G., W. Stok, and L. A. Hilgers. 2003. Improvement of the systemic prime/oral boost strategy for systemic and local responses. Vaccine 21:1391-1399. [DOI] [PubMed] [Google Scholar]
- 30.Layton, G. T., and A. M. Smithyman. 1983. The effects of oral and combined parenteral/oral immunization against an experimental Escherichia coli urinary tract infection in mice. Clin. Exp. Immunol. 54:305-312. [PMC free article] [PubMed] [Google Scholar]
- 31.Logan, A. C., K. P. Chow, A. George, P. D. Weinstein, and J. J. Cebra. 1991. Use of Peyer's patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect. Immun. 59:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mapletoft, J. W., M. Oumouna, J. Kovacs-Nolan, L. Latimer, G. Mutwiri, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2008. Intranasal immunization of mice with a formalin-inactivated bovine respiratory syncytial virus vaccine co-formulated with CpG oligodeoxynucleotides and polyphosphazenes results in enhanced protection. J. Gen. Virol. 89:250-260. [DOI] [PubMed] [Google Scholar]
- 33.Mapletoft, J. W., M. Oumouna, H. G. Townsend, S. Gomis, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2006. Formulation with CpG oligodeoxynucleotides increases cellular immunity and protection induced by vaccination of calves with formalin-inactivated bovine respiratory syncytial virus. Virology 353:316-323. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka, T., Y. Okamoto, Z. Matsuzaki, S. Endo, E. Ito, H. Tsutsumi, R. A. Williamson, H. Sakurai, D. R. Burton, and I. Saito. 2002. Characteristics of immunity induced by viral antigen or conferred by antibody via different administration routes. Clin. Exp. Immunol. 130:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCluskie, M. J., and H. L. Davis. 1998. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161:4463-4466. [PubMed] [Google Scholar]
- 36.McCluskie, M. J., R. D. Weeratna, P. J. Payette, and H. L. Davis. 2002. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol. Med. Microbiol. 32:179-185. [DOI] [PubMed] [Google Scholar]
- 37.McNeal, M. M., M. N. Rae, and R. L. Ward. 1999. Effects of different adjuvants on rotavirus antibody responses and protection in mice following intramuscular immunization with inactivated rotavirus. Vaccine 17:1573-1580. [DOI] [PubMed] [Google Scholar]
- 38.Meyer, G., M. Deplanche, and F. Schelcher. 2008. Human and bovine respiratory syncytial virus vaccine research and development. Comp. Immunol. Microbiol. Infect. Dis. 31:191-225. [DOI] [PubMed] [Google Scholar]
- 39.Mutwiri, G., P. Benjamin, H. Soita, and L. A. Babiuk. 2008. Co-administration of polyphosphazenes with CpG oligodeoxynucleotides strongly enhances immune responses in mice immunized with hepatitis B virus surface antigen. Vaccine 26:2680-2688. [DOI] [PubMed] [Google Scholar]
- 40.Mutwiri, G., P. Benjamin, H. Soita, H. Townsend, R. Yost, B. Roberts, A. K. Andrianov, and L. A. Babiuk. 2007. Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) is a potent enhancer of mixed Th1/Th2 immune responses in mice immunized with influenza virus antigens. Vaccine 25:1204-1213. [DOI] [PubMed] [Google Scholar]
- 41.Openshaw, P. J., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 4:493-500. [DOI] [PubMed] [Google Scholar]
- 42.Oumouna, M., J. W. Mapletoft, B. C. Karvonen, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2005. Formulation with CpG oligodeoxynucleotides prevents induction of pulmonary immunopathology following priming with formalin-inactivated or commercial killed bovine respiratory syncytial virus vaccine. J. Virol. 79:2024-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne, L. G., and A. K. Andrianov. 1998. Protein release from polyphosphazene matrices. Adv. Drug Deliv. Rev. 31:185-196. [DOI] [PubMed] [Google Scholar]
- 44.Payne, L. G., S. A. Jenkins, A. Andrianov, and B. E. Roberts. 1995. Water-soluble phosphazene polymers for parenteral and mucosal vaccine delivery. Pharm. Biotechnol. 6:473-493. [DOI] [PubMed] [Google Scholar]
- 45.Payne, L. G., S. A. Jenkins, A. L. Woods, E. M. Grund, W. E. Geribo, J. R. Loebelenz, A. K. Andrianov, and B. E. Roberts. 1998. Poly[di(carboxylatophenoxy)phosphazene] (PCPP) is a potent immunoadjuvant for an influenza vaccine. Vaccine 16:92-98. [DOI] [PubMed] [Google Scholar]
- 46.Philippou, S., P. Otto, P. Reinhold, M. Elschner, and H. J. Streckert. 2000. Respiratory syncytial virus-induced chronic bronchiolitis in experimentally infected calves. Virchows Arch. 436:617-621. [DOI] [PubMed] [Google Scholar]
- 47.Smyth, R. L., and P. J. Openshaw. 2006. Bronchiolitis. Lancet 368:312-322. [DOI] [PubMed] [Google Scholar]
- 48.Stott, E. J., L. A. Ball, K. K. Young, J. Furze, and G. W. Wertz. 1986. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J. Virol. 60:607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stott, E. J., and G. Taylor. 1985. Respiratory syncytial virus. Brief review. Arch. Virol. 84:1-52. [DOI] [PubMed] [Google Scholar]
- 50.Teloni, R., C. von Hunolstein, S. Mariotti, S. Donati, G. Orefici, and R. Nisini. 2004. Antibody classes and subclasses induced by mucosal immunization of mice with Streptococcus pyogenes M6 protein and oligodeoxynucleotides containing CpG motifs. Indian J. Med. Res. 119(Suppl.):126-130. [PubMed] [Google Scholar]
- 51.van Drunen Littel-van den Hurk, S., J. W. Mapletoft, N. Arsic, and J. Kovacs-Nolan. 2007. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev. Med. Virol. 17:5-34. [DOI] [PubMed] [Google Scholar]
- 52.Waris, M. E., C. Tsou, D. D. Erdman, D. B. Day, and L. J. Anderson. 1997. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J. Virol. 71:6935-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 70:2852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weibel, R. E., J. Stokes, Jr., M. B. Leagus, C. C. Mascoli, and M. R. Hilleman. 1966. Respiratory virus vaccines. V. Field evaluation for efficacy of heptavalent vaccine. Am. Rev. Respir. Dis. 94:362-379. [DOI] [PubMed] [Google Scholar]
- 55.West, K., L. Petrie, D. M. Haines, C. Konoby, E. G. Clark, K. Martin, and J. A. Ellis. 1999. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine 17:809-820. [DOI] [PubMed] [Google Scholar]
- 56.Wu, J. Y., W. F. Wade, and R. K. Taylor. 2001. Evaluation of cholera vaccines formulated with toxin-coregulated pilin peptide plus polymer adjuvant in mice. Infect. Immun. 69:7695-7702. [DOI] [PMC free article] [PubMed] [Google Scholar]