Abstract
Vaccine delivery systems using lactic acid bacteria are under development, but their efficiency is insufficient. Autologous cytokines, such as interleukin-1β (IL-1β), are potential adjuvants for mucosal vaccines and can be provided by recombinant lactic acid bacteria. The aim of this study was the construction and evaluation of recombinant Lactobacillus casei producing IL-1β as an adjuvant delivery agent. The recombinant strain was constructed using an expression/secretion vector plasmid, including a mature IL-1β gene from mouse. The biological activity of the cytokine was confirmed by IL-8 production from Caco-2 cells. In response to the recombinant L. casei secreting IL-1β, expression of IL-6 was detected in vivo using a ligated-intestinal-loop assay. The release of IL-6 from Peyer's patch cells was also detected in vitro. Intragastric immunization with heat-killed Salmonella enterica serovar Enteritidis (SE) in combination with IL-1β-secreting lactobacilli resulted in relatively high SE-specific antibody production. In this study, it was demonstrated that recombinant L. casei secreting bioactive murine IL-1β provided adjuvant effects for intragastric immunization.
At present, a number of vaccines based on lactic acid bacteria (LAB vaccines) are under development (31). These commensals, including Lactococcus lactis and Lactobacillus strains, have been used as vaccine delivery vectors. Several LAB vaccines, such as tetanus toxin fragment C (TTFC)-producing LAB, exhibit high immunogenicity and can induce both mucosal and systemic immune responses, as well as protective immunity (11, 22). However, most LAB vaccines administered via the mucosal route, especially the oral or intragastric (i.g.) route, exhibit relatively low efficiency. As expected, a high dosage is required to elicit effective immunity. It is usually required that mice receive more than 109 CFU of bacteria on three or more consecutive days with two or more boosts (4, 5, 16). In several cases, LAB vaccines were administered in combination with a supplemental adjuvant to induce sufficient immune responses (3, 32). In order to improve the efficiency of vaccination, several kinds of additional factors that assist LAB vaccines have been investigated. Steidler et al. carried out a pioneering study that demonstrated the adjuvant effect provided by L. lactis expressing TTFC intracellularly and also secreting either murine interleukin-2 (mIL-2) or mIL-6 (28). In another report, a single-chain murine IL-12 was produced by L. lactis and enhanced antigen-specific Th1 cytokine production (2). Recently, a unique strategy to accelerate bacterial uptake by dendritic cells (DCs) was developed by Mohamadzadeh et al. (18). They achieved effective oral vaccination using recombinant Lactobacillus acidophilus secreting a protective antigen of Bacillus anthracis in combination with a DC-targeted peptide. Although all these adjuvant molecules are promising, there are still only a few options for their use as mucosal adjuvants at present. Hence, exploration of other mucosal adjuvants that are applicable to LAB vaccines is important.
The present study investigated IL-1β as a mucosal vaccine. IL-1β is produced by activated monocytes and macrophages, etc., as a precursor that is proteolytically processed into a mature form by IL-1β-converting enzyme (ICE), also known as caspase 1 (6). This proinflammatory cytokine and other IL-1 family cytokines play important roles in modulating the adaptive immune response (7). In fact, a deficiency of IL-1β impairs T-cell-mediated cellular immune responses (26, 29). It was reported previously that IL-1β exhibited adjuvant effects in both mucosal and systemic immunization (27). In addition, Nakae et al. demonstrated that IL-1 enhanced T-cell-dependent antibody production (19). This evidence suggests that IL-1β may be a promising adjuvant for mucosal immunization if it is produced by recombinant lactic acid bacteria. Unlike other mucosal adjuvants, such as cholera toxin, IL-1β is an autologous protein and therefore nonimmunogenic. This may be a preferable feature, because adaptive immunity against adjuvant proteins induced by repeated doses may reduce the adjuvant effect in further immunizations. The aim of this study was to construct an IL-1β-secreting Lactobacillus and to evaluate its adjuvant effect.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Lactobacillus casei IGM393 and a nonexpressing control strain carrying pLPEmpty (LCN) were grown in de Mann-Rogosa-Sharpe (MRS) medium (Difco) at 37°C (14). Erythromycin (5 μg/ml) was used as a supplement for the culture of recombinant lactobacilli. Bicarbonate buffer (50 mM; pH 7.0 to 8.0) was also added to the bacterial culture in some experiments to control the pH of the media. Salmonella enterica serovar Enteritidis (SE) no. 40 was grown in Luria-Bertani (LB) broth (Difco) at 37°C under aerobic conditions (1).
Plasmid construction and transformation.
For expression of murine IL-1β in L. casei, a plasmid was constructed from pIGM2, which was established in our previous study (13). Although the plasmid already included a promoter from Lactobacillus brevis slpA (slpAp) and a secretion signal of L. casei prtP (SSprtP) for heterologous expression and secretion, minor modification of the sequence was required in order to express the proper size of IL-1β. DNA fragments consisting of slpAp, SSprtP, and the IL-1β gene were generated by an overlap PCR technique. Total RNA was prepared from the spleen of a BALB/c mouse, followed by reverse transcription (RT) using Retroscript (Ambion). The IL-1β fragment was amplified from the total cDNA using primers IGM617 (GCG AAA TCC AAG CAA AGG CGG TTC CCA TTA GAC AAC TGC A) and IGM603 (CCC CCT CGA GCC GGC TTA GGA AGA CAC GGA TTC C). A DNA segment from slpAp to SSprtP was prepared by PCR with pIGM2 as the template and with primers IGM289 (CCC AAG CTT AGA TCT GAT TAC AAA GGC TTT AAG CAG G) and IGM618 (TGC AGT TGT CTA ATG GGA ACC GCC TTT GCT TGG ATT TCG C). The two resulting fragments were then connected by PCR with IGM289 and IGM603. The expression cassette of IL-1β was digested with BglII and XhoI and inserted into the same restriction site of pIGM2. Transformation of L. casei was performed by the method described previously (21).
Immunoblotting.
Overnight cultures of recombinant lactobacilli were separated into bacterial cells and culture supernatants by centrifugation. The bacterial cells were washed with 50 mM Tris (pH 8.0) and treated with 5 mg/ml lysozyme and 20 U/ml of mutanolysin for 30 min. The spheroplasts were washed with 0.3 M sucrose in 50 mM Tris buffer, dissolved in Laemmli sample buffer, and boiled for 10 min. The culture supernatants were concentrated 50-fold using trichloroacetic acid, dissolved in Laemmli sample buffer, and boiled for 5 min. These samples were applied for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto a polyvinylidene fluoride filter. The blot was conjugated with anti-mouse IL-1β (Peprotech Inc.) and horseradish peroxidase (HRP)-labeled anti-rabbit IgG (Sigma). IL-1β-specific bands were visualized with ECL-plus (GE Healthcare) using a ChemiDoc system (Bio-Rad).
Quantitative determination of mIL-1β.
Prewarmed MRS medium supplemented with erythromycin and bicarbonate buffer (MRSCE) (final pH, 7.0, 7.5, or 8.0) was inoculated with washed cells from 1/10 volume of overnight culture of recombinant lactobacilli. After 5 h of incubation, the culture supernatants were collected by centrifugation and sterilized using a 0.45-μm-pore filter (Millipore). The concentration of IL-1β in each culture supernatant was determined using an mIL-1β enzyme-linked immunosorbent assay (ELISA) kit (Biosource) in accordance with the manufacturer's instructions.
Caco-2 cell culture and stimulation with IL-1β.
Caco-2 cells, purchased from the American Type Culture Collection, were maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10% fetal calf serum (FCS), 1% nonessential amino acids, 100 IU/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in a 5% CO2 incubator. A single-cell suspension was prepared by trypsin-EDTA treatment and seeded in a 98-well culture plate (2 × 104 cells/well). After overnight incubation, the culture supernatant of recombinant lactobacilli or purified recombinant murine IL-1β (Peprotech) was added to the Caco-2 culture, followed by an additional 16 h of incubation. The culture supernatants were then collected, and the amount of human IL-8 was determined using an OptEIA ELISA kit (BD Biosciences).
Mice.
Female BALB/c mice and C3H/HeJ mice (8 to 10 weeks old) were purchased from Japan SLC (Shizuoka, Japan). The care and use of experimental animals complied with the Principal Law on Animal Experimentation of the National Institute of Health Sciences of Japan.
Ligated-intestinal-loop assay.
BALB/c mice starved overnight were injected intraperitoneally (i.p.) with 0.1 ml of pentobarbital (80 mg/kg of body weight). The abdomen was incised, and the ileum was ligated with surgical thread approximately 0.5 cm each side of one Peyer's patch (PP). Recombinant lactobacilli (1 × 109 CFU/ml) suspended in MRSCE medium (pH 7.0) were injected into the ligated intestinal loop containing a single PP. The abdomen was then closed by sewing, and the mice were kept for 4 h before sacrifice. The loop was collected and washed extensively with phosphate-buffered saline (PBS). The single PP and a small piece of non-PP lamina propria (LP) in the loop were isolated and transferred to lysis buffer for RNA preparation. Total RNA was isolated using an SV Total RNA isolation system (Promega) in accordance with the manufacturer's instructions. The concentration of RNA was measured using a NanoDrop ND-1000.
RT-PCR.
Total cDNA was prepared from 1 μg of RNA using Retroscript. PCR was then performed to detect IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p35, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), macrophage inflammatory protein 2 (MIP-2), and β-actin. The sequences of primers are shown in Table 1. The reaction conditions were 30 cycles of 94°C for 25 s, 50°C for 15 s, and 72°C for 40 s. The PCR products were applied to 2% agarose gel electrophoresis and visualized with ethidium bromide using a ChemiDoc gel documentation system.
TABLE 1.
Cytokine-specific primer pair sequences used in PCR
| Gene | Sense primer | Antisense primer | Sizea | Reference |
|---|---|---|---|---|
| IL-1β | TCATGGGATGATGATGATAACCTGCT | CCCATACTTTAGGAAGACACGGATT | 502 | 30 |
| IL-2 | TGATGGACCTACAGGAGCTCCTGAG | GAGTCAAATCCAGAACATGCCGCAG | 167 | 30 |
| IL-4 | CGAAGAACACCACAGAGAGTGAGCT | GACTCATTCATGGTGCAGCTTATCG | 180 | 30 |
| IL-5 | GTGAAAGAGACCTTGACACAGCTG | CACACCAAGGAACTCTTGCAGGTA | 290 | 10 |
| IL-6 | CTGGTGACAACCACGGCCTTCCCTA | ATGCTTAGGCATAACGCACTAGGTT | 600 | 30 |
| IL-10 | ACCTGGTAGAAGTGATGCCCCAGGCA | CTATGCAGTTGATGAAGATGTCAAA | 237 | 30 |
| IL-12 p35 | GCAAGAGACACAGTCCTGGG | TGCATCAGCTCATCGATGGC | 618 | 30 |
| IFN-γ | AGCGGCTGACTGAACTCAGATTGTAG | GTCACAGTTTTCAGCTGTATAGGG | 243 | 30 |
| TNF-α | GGCAGGTCTACTTTGGAGTCATTGC | ACATTCGAGGCTCCAGTGAATTCGG | 307 | 30 |
| TGF-β | TGGACCGCAACAACGCCATCTATGAG | TGGAGCTGAAGCAATAGTTGGTATCCAG | 502 | 23 |
| MIP-2 | GCCAGTGAGCTGCGCTGTCAGTGC | GTTAGCCTTGCCTTTGTTCAGTATG | 221 | 33 |
| β-Actin | TGGAATCCTGTGGCATCCATGAAAC | TAAAACGCAGCTCAGTAACAGTCCG | 348 | 30 |
Predicted size of the PCR product (base).
Preparation and stimulation of PP cells.
A single-cell suspension of PP was prepared from BALB/c mice in accordance with a protocol described previously (24). PP cells were prepared using collagenase type 1 (Sigma-Aldrich). The PP cells were then separated into CD11c+ and CD11c− cells using a Magnetic Cell Separation (MACS) system (Miltenyi Biotec) in accordance with the manufacturer's instructions. The CD11c+ and CD11c− cells were suspended in RPMI 1640 medium (Sigma) supplemented with 10% FCS and seeded in a 96-well microplate. The stimulants, which were recombinant L. casei cells and cell-free MRS culture supernatants, were added to the wells and incubated for 24 h. The culture supernatants of the PP cell cultures were collected and analyzed by ELISA. The quantity of IL-6 was measured using a mouse IL-6 ELISA kit (Biosource).
Immunization of mice.
C3H/HeJ mice were previously optimized for immunization against SE (14). The mice were immunized i.g. with a mixture of 5 × 109 CFU/mouse of heat-killed SE (HKSE) and 5 × 109 CFU/mouse of viable recombinant L. casei. HKSE was prepared from an overnight culture. SE cells were collected, washed gently, and suspended in distilled water. The suspension was incubated at 60°C for 30 min and then freeze-dried. Complete inactivation of SE was confirmed by LB plate culture for a week. Recombinant L. casei for administration was prepared from overnight cultures. Prior to immunization, the lactobacilli were incubated in MRSCE (pH 8.0) for 1 h to enhance mIL-1β expression and then resuspended in fresh MRS supplemented with bicarbonate buffer (MRSC) (pH 8.0). A mixture of HKSE and recombinant lactobacilli was prepared by simple mixing of both suspensions in MRSC (2.5 × 1010 CFU/ml each; 0.2 ml/dose). Three consecutive daily doses were performed 3 times at 2-week intervals. The mice were sacrificed 2 weeks after the last immunization, and blood and feces were collected. Sera were prepared from blood samples by centrifugation. Fecal extracts were prepared as cleared supernatants of suspended feces in PBS (1:10). Both samples were stored at −20°C until they were used.
Detection of SE-specific antibodies.
SE-specific IgG in serum and IgA in fecal extracts were detected by ELISA. HKSE cells were homogenized in PBS using a FastPrep bead beater (Bio 101), and the cleared lysate was collected by centrifugation. The concentration of protein in the HKSE lysate was determined by a Bradford protein assay (Bio-Rad). A microtiter plate was coated overnight with 5 μg/ml (as a protein solution) of HKSE lysate. Properly diluted samples were then added to each well and incubated for 2 h. HRP-conjugated anti-mouse IgG (or IgA) was added and incubated for 1 h. A TMB Substrate Reagent Set (BD Biosciences) was used for color development, and 2 N H2SO4 was added to stop the reaction. The optical density at 450 nm was measured using a microplate reader.
Statistical analysis.
Statistical significance (P < 0.05) was determined by Tukey's multiple-analysis test.
RESULTS
Secretion of IL-1β from recombinant L. casei.
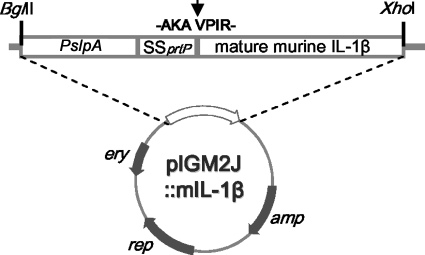
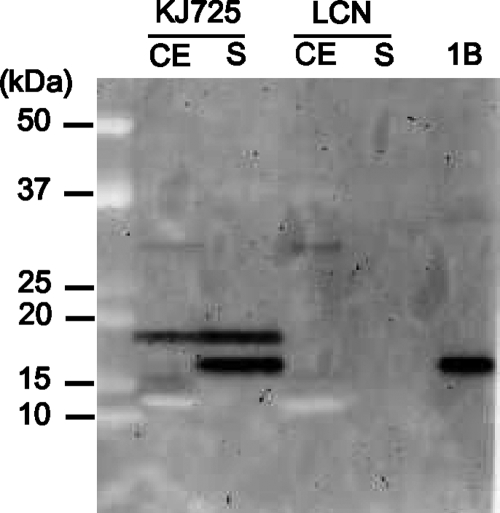
The ligated plasmid pIGM2J::m IL-1β, shown in Fig. 1, was introduced directly into L. casei by electroporation. The production of murine IL-1β by recombinant L. casei was confirmed by immunoblotting. As shown in Fig. 2, IL-1β-specific bands were detected in both cell extracts and the culture supernatants of recombinant L. casei (KJ725). A single band corresponding to the size of IL-1β fused to a signal peptide appeared in the lane of the cell extract of KJ725, while two bands were observed in the lane of the culture supernatant. The smaller band seemed to be the same size as purified IL-1β. No signal was detected from the control strain. The influence of the pH on IL-1β secretion was also determined. Approximately 1 μg/ml of IL-1β was secreted if the initial pH of the medium was between 7.0 and 8.0 (Table 2). However, only one-third of the IL-1β was detected without pH control.
FIG. 1.
Feature map of pIGM2J::mIL-1β. The structure, expression cassette, restriction sites, and cleavage point of the fusion protein (arrow) are shown. Genes: amp, ampicillin resistance; rep, replication protein; ery, erythromycin resistance.
FIG. 2.
Detection of IL-1β-specific bands by immunoblotting. Lysozyme-treated cell extracts (CE), concentrated culture supernatants (S), and 10 ng of purified IL-1β (1B) were applied to SDS-PAGE and immunoblotting. Anti-IL-1β antibody was used at 1:5,000, and HRP-conjugated anti-rabbit IgG was used at 1:50,000. Molecular masses are shown on the left.
TABLE 2.
Yield of IL-1β in KJ725 culture with and without buffer
| Culture condition | IL-1β yield (μg/ml)a |
|---|---|
| No buffer (pH 6.3) | 0.326 |
| Buffer (pH 7.0) | 1.032 |
| Buffer (pH 7.5) | 0.966 |
| Buffer (pH 8.0) | 1.116 |
Mean value of triplicate cultures.
Biological activity of the recombinant IL-1β.
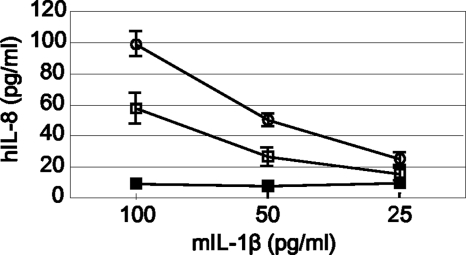
The biological activity of the recombinant IL-1β produced by KJ725 was determined by stimulation of the Caco-2 cells. Dose-dependent IL-8 release induced by IL-1β was observed in a Caco-2 cell culture stimulated with the supernatant of KJ725 (Fig. 3). Despite the same amount of IL-1β being used, the release of IL-8 by stimulation with KJ725 was less than that with purified IL-1β. No IL-8-inducing activity was detected by stimulation of Caco-2 cells with the culture supernatant of LCN.
FIG. 3.
Biological activity of recombinant murine IL-1β produced by KJ725. Human IL-8 released from Caco-2 cells was measured after 24 h of stimulation with culture supernatant of KJ725 (empty squares) or LCN (filled squares) or with purified murine IL-1β standard (empty circles). The concentration of total murine IL-1β in the culture supernatant of KJ725 (in MRSEC medium) was determined by ELISA and then adjusted to 100, 50, and 25 pg/ml by dilution in complete RPMI medium. The corresponding volume of culture supernatant of LCN was added to the Caco-2 cell culture as a negative control. The result shown is representative of three separate experiments; the values are the means ± standard deviations (SD) of duplicate samples.
Detection of cytokine expression by ligated-intestinal-loop assay.
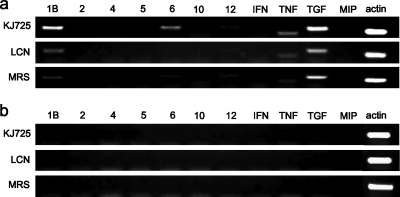
In order to evaluate the immunological impact of KJ725 in vivo, a ligated-intestinal-loop assay was performed. Preliminary experiments indicated that over 109 CFU/ml of bacterial suspension induced cytokine expression stably, and thus, it was applied in this study. The same experiment was repeated to normalize the results. Eleven kinds of cytokine expression in PP and LP were analyzed by RT-PCR. Specific bands of IL-1β, IL-6, IL-12, TNF-α, and TGF-β, with β-actin as an internal standard, were constantly detected in PP cells, while no bands, except for the band of β-actin, were detected in LP cells (Fig. 4).
FIG. 4.
Cytokine expression induced by ligated-intestinal-loop assay using RT-PCR. PCR products were applied to 2% agarose gels. Eleven kinds of cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, and IL-12p35 [lanes 1B to 12]; IFN-γ [IFN]; TNF-α [TNF]; TGF-β [TGF]; and MIP-2 [MIP]) expressed in PP cells (a) and LP cells (b) were detected by RT-PCR using specific primer pairs. As an internal control, a DNA fragment of β-actin (actin) was also amplified. The data represent at least three separate experiments.
Production of IL-6 by PP CD11c+ cells.
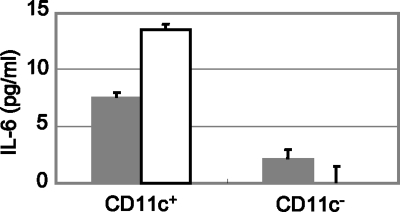
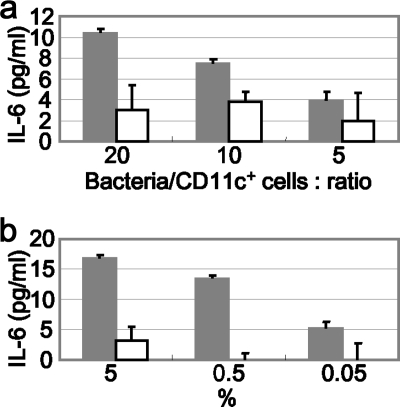
PP cells were separated into CD11c+ cells and CD11c− cells using MACS. Each cell type was stimulated with the KJ725 cell or culture supernatant. As shown in Fig. 5, IL-6 was efficiently produced by CD11c+ cells in response to both KJ725 cells and culture supernatant. Meanwhile, a low level of IL-6 was induced by stimulation with LCN cells and culture supernatant compared with KJ725 cultures (Fig. 6).
FIG. 5.
IL-6 release by PP CD11c+ or CD11c− cells. MACS-separated CD11c+ or CD11c− cells (5 × 104 cells/well) were stimulated with KJ725 cells (solid bars; 5 × 105 CFU/well) or culture supernatant (open bars; 0.5%). After 24 h of incubation, samples were collected and released IL-6 was detected by ELISA. The results shown are representative of three separate experiments; the values are means and SD.
FIG. 6.
IL-6 released from PP CD11c+ cells. MACS-separated CD11c+ cells (5 × 104 cells/well) were stimulated with recombinant L. casei cells (bacteria/CD11c+ cell ratios, 20, 10, and 5) (a) or culture supernatant (5%, 0.5%, and 0.05% diluted in RPMI 1640 medium) (b). After 24 h of incubation, samples were collected and released IL-6 was detected by ELISA. The results are representative of three separate experiments; the values are means and SD. Solid bars, KJ725 cells/supernatants; open bars, LCN cells/supernatants.
Enhancement of antibody production by IL-1β-producing L. casei.
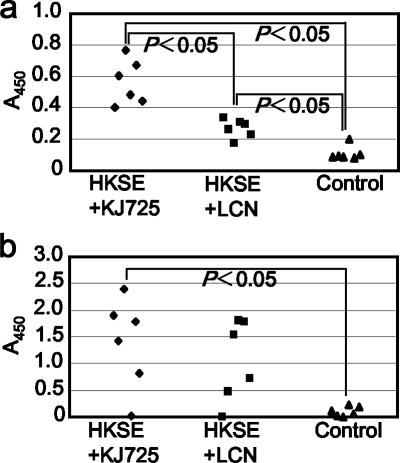
To evaluate the adjuvant effect of KJ725, i.g. immunization of mice was performed. HKSE was used as an immunogen and administered in combination with either KJ725 or LCN. As shown in Fig. 7, SE-specific antibodies were induced by i.g. immunization with HKSE. In the group that received HKSE and KJ725, the highest level of SE-specific IgG (P < 0.05 compared with the HKSE and LCN group or the MRS group) and IgA (P < 0.05 compared with the MRS group) was detected in their sera and feces.
FIG. 7.
SE-specific antibody production induced by HKSE with either KJ725 or LCN. Anti-SE IgG in 100-fold-diluted serum (a) and anti-SE IgA in 50-fold-diluted cecum lavage fluid (b) were detected by ELISA. Each value represents the absorbance at 450 nm (A450). The immunization groups are shown at the bottom. Statistical significance was accepted at P < 0.05. The control was a group that received MRSC medium alone.
DISCUSSION
The delivery of vaccine antigens by live bacterial carriers, both pathogenic and commensal bacteria, has been studied (17). Compared with vaccine delivery systems using attenuated pathogens, vaccines based on lactic acid bacteria require high doses, a high frequency of immunization, and highly immunogenic antigens for successful induction of protective immunity. For instance, Lactobacillus plantarum expressing Borrelia burgdorferi OspA was administered at 4 × 1010 cells in four consecutive daily doses with additional priming and two boosts (total, 16 days; 6.4 × 1011 cells), while an attenuated Salmonella enterica serovar Typhimurium producing OspA was inoculated at 108 organisms with a total of five weekly doses (total, 5 days; 5 × 108 cells) (5, 8). In this context, it is important to provide supplemental adjuvant effects for the development of vaccine using lactic acid bacteria. For this purpose, recombinant L. casei secreting mature murine IL-1β was constructed. Using the secretion system developed in our previous study, recombinant IL-1β conjugated with signal peptide was produced intracellularly, and a mature form of IL-1β was then secreted extracellularly. The results of immunoblotting indicated that the secretion system worked properly. At the same time, a considerable part of the extracellular IL-1β remained as a fusion protein. This protein seemed to be leaked or secreted without the involvement of signal peptidase. Further investigations are required to explain the phenomenon. The amounts of IL-1β production were measured under different pH conditions, because IL-10 production using the same system was found to be pH dependent in the previous study (13). The results showed that an approximately 3-fold-larger amount of the protein was produced when carbonate buffer was added to the culture. Considering this characteristic, MRS medium was supplemented with the buffer when the recombinant strains were applied to the in vivo assay. The biological activity of IL-1β was determined by the stimulation of Caco-2 cells as described previously (9, 25). In response to IL-1β produced by the recombinant L. casei, IL-8 was released from the cell culture. The activity of IL-1β from recombinant bacteria was approximately 60% of that of purified IL-1β. Because the amino acid sequence of the N terminus of IL-1β is critical for its biological activity, extra peptides at the N terminus drastically impaired the activity (12). In fact, the biological activity was lost by adding only 5 additional amino acids (DTNSD) at the N terminus of IL-1β (data not shown). As described above, part of IL-1β secreted by recombinant L. casei was conjugated with the signal peptide. Hence, contamination by inactive IL-1β resulted in lower biological activity.
The immunological properties of the recombinant L. casei producing IL-1β were analyzed in vivo using a ligated-intestinal-loop assay. In this assay, the expression of cytokine genes was detected only in the PP. The result of cytokine profiling by RT-PCR showed that the expression of IL-6 seemed to be upregulated. In order to confirm the induction of IL-6, PP cells were prepared and stimulated with IL-1β-secreting lactobacilli. The result showed that IL-6 was efficiently released from CD11c+ cells by stimulation with both IL-1β-secreting bacteria and the culture supernatants in a dose-dependent manner. IL-6 is produced by monocytes, macrophages, dendritic cells, etc., and enhances antibody production from B cells (15, 20). Moreover, it was reported that CD11c+/B220−/CD11b+ Peyer's patch DCs secrete IL-6 and induce antibody production from naïve B cells (24). These findings suggested that IL-1β-secreting lactobacilli could be applied in vaccination as an adjuvant that increases antibody production. In order to evaluate this effect, the immunization of mice was performed using HKSE in combination with the recombinant L. casei strains. The result showed that comparatively large amounts of SE-specific antibodies, both IgG and IgA, were achieved by immunization with HKSE plus IL-1β-secreting lactobacilli. This result suggested that the recombinant strain had adjuvant effects in vivo. If purified IL-1β was administered orally or intragastrically, it could be degraded by the digestive process in the gastrointestinal (GI) tract. Meanwhile, IL-1β released by the recombinant L. casei could be delivered directly to the GI tract with less exposure to gastric acid, bile, and digestive enzymes. This is an advantageous feature of adjuvant delivery using recombinant lactobacilli. Because IL-1β is a proinflammatory cytokine, there may be a risk of side effects. In this study, however, apparent side effects, such as weight loss and diarrhea, were not observed.
Several kinds of cytokines have been produced by lactic acid bacteria to complement vaccine efficacy (2, 28). The present study demonstrated adjuvant effects of recombinant L. casei secreting biologically active IL-1β. Although it is known that IL-1β can be used as a mucosal adjuvant, it has not been reported previously that intragastrically administered recombinant lactobacilli secreting cytokines exhibited an adjuvant effect. The recombinant strain developed in this study could be a powerful tool to improve the efficacy of vaccines based on lactic acid bacteria or other delivery agents that have relatively weak immunogenicity.
Acknowledgments
This study was supported by a grant from the Ministry of Health, Labor, and Welfare (Research on Food Safety) and partly by a grant from the Food Safety Commission of Japan.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Amano, F., H. Karahashi, Y. Ishii, and S. Igimi. 2000. Different susceptibility between Salmonella Enteritidis in logarithmic and stationary phases to an anti-Salmonella neutralizing antibody that blocks binding and infection to a human colon epithelial cell line, T-84. Biosci. Microflora 14:71-75. [Google Scholar]
- 2.Bermúdez-Humarán, L. G., N. G. Cortes-Perez, F. Lefèvre, V. Guimarães, S. Rabot, J. M. Alcocer-Gonzalez, J. J. Gratadoux, C. Rodriguez-Padilla, R. S. Tamez-Guerra, G. Corthier, A. Gruss, and P. Langella. 2005. A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 175:7297-7302. [DOI] [PubMed] [Google Scholar]
- 3.Cheun, H. I., K. Kawamoto, M. Hiramatsu, H. Tamaoki, T. Shirahata, S. Igimi, and S. I. Makino. 2004. Protective immunity of SpaA-antigen producing Lactococcus lactis against Erysipelothrix rhusiopathiae infection. J. Appl. Microbiol. 96:1347-1353. [DOI] [PubMed] [Google Scholar]
- 4.Corthésy. B., S. Boris, P. Isler, C. Grangette, and A. Mercenier. 2005. Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori urease B subunit partially protects against challenge with Helicobacter felis. J. Infect. Dis. 192:1441-1449. [DOI] [PubMed] [Google Scholar]
- 5.del Rio, B., R. J. Dattwyler, M. Aroso, V. Neves, L. Meirelles, J. F. Seegers, and M. Gomes-Solecki. 2008. Oral immunization with recombinant Lactobacillus plantarum induces a protective immune response in mice with Lyme disease. Clin. Vaccine Immunol. 15:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello, C. A. 1998. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. N. Y. Acad. Sci. 856:1-11. [DOI] [PubMed] [Google Scholar]
- 7.Dunne, A., and L. A. O'Neill. 2003. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE 2003:re3. [DOI] [PubMed] [Google Scholar]
- 8.Dunne, M., B. K. al-Ramadi, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Oral vaccination with an attenuated Salmonella typhimurium strain expressing Borrelia burgdorferi OspA prevents murine Lyme borreliosis. Infect. Immun. 63:1611-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusunyan, R. D., J. J. Quinn, Y. Ohno, R. P. MacDermott, and I. R. Sanderson. 1998. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1beta and lipopolysaccharide. Pediatr. Res. 43:84-90. [DOI] [PubMed] [Google Scholar]
- 10.Garhart, C. A., F. P. Heinzel, S. J. Czinn, and J. G. Nedrud. 2003. Vaccine-Induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect. Immun. 71:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grangette, C., H. Müller-Alouf, M. Geoffroy, D. Goudercourt, M. Turneer, and A. Mercenier. 2002. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence. Vaccine 20:3304-3309. [DOI] [PubMed] [Google Scholar]
- 12.Hejnaes, K. R., B. Sehested, H. Worsaae, J. Mølvig, and A. Wollmer. 1990. The effect of N-terminal extension on the structure and function of human interleukin-1 beta. Biol. Chem. Hoppe Seyler 371:23-30. [DOI] [PubMed] [Google Scholar]
- 13.Kajikawa, A., E. Ichikawa, and S. Igimi. 2009. Development of a highly efficient protein-secreting system in recombinant Lactobacillus casei. J. Microbiol. Biotechnol, in press. [PubMed]
- 14.Kajikawa, A., E. Satoh, R. J. Leer, S. Yamamoto, and S. Igimi. 2007. Intragastric immunization with recombinant Lactobacillus casei expressing flagellar antigen confers antibody-independent protective immunity against Salmonella enterica serovar Enteritidis. Vaccine 25:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto, T. 2006. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res. Ther. 8(Suppl. 2):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J. S., H. Poo, D. P. Han, S. P. Hong, K. Kim, M. W. Cho, E. Kim, M. H. Sung, and C. J. Kim. 2006. Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J. Virol. 80:4079-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina, E., and C. A. Guzmán. 2001. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 19:1573-1580. [DOI] [PubMed] [Google Scholar]
- 18.Mohamadzadeh, M., T. Duong, S. J. Sandwick, T. Hoover, and T. R. Klaenhammer. 2009. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl. Acad. Sci. U. S. A. 106:4331-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakae, S., M. Asano, R. Horai, N. Sakaguchi, and Y. Iwakura. 2001. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J. Immunol. 167:90-97. [DOI] [PubMed] [Google Scholar]
- 20.Ohsugi, Y. 2007. Recent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol. Pharm. Bull. 30:2001-2006. [DOI] [PubMed] [Google Scholar]
- 21.Pouwels, P. H., A. Vriesema, B. Martinez, F. J. Tielen, J. F. Seegers, R. J. Leer, J. Jore, and E. Smit. 2001. Lactobacilli as vehicles for targeting antigens to mucosal tissues by surface exposition of foreign antigens. Methods Enzymol. 336:369-389. [DOI] [PubMed] [Google Scholar]
- 22.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 23.Romano-Carratelli, C., C. Bentivoglio, I. Nuzzo, N. Benedetto, E. Buommino, A. Cozzolino, M. Cartenì, F. Morelli, M. R. Costanza, B. Metafora, V. Metafora, and S. Metafora. 2002. Effect of Protein SV-IV on experimental Salmonella enterica serovar Typhimurium infection in mice. Clin. Diagn. Lab. Immunol. 9:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, A., M. Hashiguchi, E. Toda, A. Iwasaki, S. Hachimura, and S. Kaminogawa. 2003. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J. Immunol. 171:3684-3690. [DOI] [PubMed] [Google Scholar]
- 25.Schuerer-Maly, C. C., L. Eckmann, M. F. Kagnoff, M. T. Falco, and F. E. Maly. 1994. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology 81:85-91. [PMC free article] [PubMed] [Google Scholar]
- 26.Shornick, L. P., P. De Togni, S. Mariathasan, J. Goellner, J. Strauss-Schoenberger, R. W. Karr, T. A. Ferguson, and D. D. Chaplin. 1996. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J. Exp. Med. 183:1427-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staats, H. F., and F. A. Ennis, Jr. 1999. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162:6141-6147. [PubMed] [Google Scholar]
- 28.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutterwala, F. S., Y. Ogura, M. Szczepanik, M. Lara-Tejero, G. S. Lichtenberger, E. P. Grant, J. Bertin, A. J. Coyle, J. E. Galán, P. W. Askenase, and R. A. Flavell. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317-327. [DOI] [PubMed] [Google Scholar]
- 30.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 2000. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 68:2034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells, J. M., and A. Mercenier. 2008. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6:349-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin, K. Q., Y. Hoshino, Y. Toda, S. Igimi, Y. Kojima, N. Jounai, K. Ohba, A. Kushiro, M. Kiwaki, K. Hamajima, D. Klinman, and K. Okuda. 2003. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 102:223-228. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto, Y., C. Retzlaff, P. He, T. W. Klein, and H. Friedman. 1995. Quantitative reverse transcription-PCR analysis of Legionella pneumophila-induced cytokine mRNA in different macrophage populations by high-performance liquid chromatography. Clin. Diagn. Lab. Immunol. 2:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]