Abstract
The kinetics of antibody persistence following the administration of a combination meningococcal serogroup C and Haemophilus influenzae type b (Hib) conjugate vaccine (Menitorix) in the second year of life in children primed with two doses of one of three monovalent meningococcal serogroup C (MCC) vaccines was investigated. The study subjects were administered either Menitorix at 12 to 15 months of age, followed by the seven-valent pneumococcal conjugate vaccine (PCV7) and the measles, mumps, and rubella vaccine 4 to 6 weeks later, or all three vaccines concomitantly at 12 to 15 months of age. Blood samples were collected before and 1, 2, 12, and 24 months after the boosting. Sera were analyzed for meningococcal serogroup C serum bactericidal antibody (SBA) and IgG as well as Hib-polyribosylribitol phosphate (PRP)-specific IgG. The antibody persistence data from this study were compared to those of a prior study of Southern et al. (Clin. Vaccine Immunol. 14:1328-1333, 2007) in which children were given three primary doses of a vaccine containing both the MCC and the Hib vaccines but were boosted only with a Hib conjugate vaccine. The magnitude of the meningococcal SBA geometric mean titer was higher for those subjects primed with the MCC vaccine conjugated to tetanus toxoid (NeisVac-C) than for those primed with one of two MCC vaccines conjugated to CRM197 (Menjugate or Meningitec) up to 1 year following boosting. Two years after boosting, the percentages of subjects with putatively protective SBA titers of ≥8 for children primed with NeisVac-C, Menjugate, and Meningitec were 43%, 22%, and 23%, respectively. Additional booster doses of the MCC vaccine may be required in the future to maintain good antibody levels; however, there is no immediate need for a booster during adolescence, as mathematical modeling has shown that persisting herd immunity is likely to control disease for a number of years.
A booster dose of meningococcal serogroup C conjugate (MCC) and Haemophilus influenzae type b (Hib) conjugate vaccine was introduced in September 2006 in England and Wales for children in the second year of life in the form of a combined vaccine, Menitorix (GlaxoSmithKline [GSK]), which has tetanus toxoid (TT) as the carrier protein. In England and Wales, infants receive a combined diphtheria toxoid (D), TT, acellular pertussis (aP5), inactivated poliovirus (IPV), and Hib-TT conjugate vaccine (DTaP5/IPV/Hib-TT; Pediacel; Sanofi Pasteur) at 2, 3, and 4 months of age; MCC vaccination at 3 and either 4 or 5 months of age; and a seven-valent pneumococcal conjugate vaccine (PCV7; Prevenar; Wyeth Vaccines) at 2 and 4 months of age. Three different MCC vaccine manufacturers' vaccines are available: two are conjugated to CRM197, a nontoxigenic natural variant of diphtheria toxin (Meningitec [Wyeth Vaccines] and Menjugate [Novartis Vaccines]), and one is conjugated to TT (NeisVac-C; Baxter Bioscience). Although the data obtained following the administration of the current primary vaccine series in the United Kingdom have been reported (16), antibody persistence following boosting with Menitorix and priming with two doses of each of the licensed MCC vaccines has not been reported. This report details the immunogenicity data for those receiving the MCC and Hib vaccines, by primary MCC vaccine, for before and at 1, 2, 12, and 24 months after a booster dose of Menitorix administered at 12 to 15 months of age. The rates of antibody decline for those receiving the Hib and MCC vaccines are also compared.
MATERIALS AND METHODS
Study population.
Infants eligible for routine vaccination were recruited from general practices (GPs) in Hertfordshire and Gloucestershire, England. The criteria used for study participation included the following: there was no contraindication to vaccination, as specified in the Green Book (3); written informed consent was obtained from the parent or legal guardian; and for the primary vaccination, the infant could be aged no less than 7 weeks exactly and no more than 11 weeks 6 days, as described previously (16).
Treatment and follow-up schedule.
As reported previously (16), the infants were randomized in order of inclusion in the study to one of six groups by treatment schedule by using a computer-generated randomization list with a block size of 16. Groups 1 to 3 received one of the three licensed MCC vaccines (Meningitec, Menjugate, or NeisVac-C) and PCV7 at 2 and 3 months of age. Those in groups 4 to 6 received an MCC vaccine and PCV7 at 2 and 4 months of age. In the booster phase of this study, the subjects were offered Menitorix at 12 to 15 months of age followed by PCV7 and the measles, mumps, and rubella (MMR) vaccine 4 to 6 weeks later, as in the national schedule. In response to the question of whether all three vaccines could be administered at the same visit, the study design was changed so that the subjects were randomized to receive either the vaccines according to the existing national schedule or all three vaccines concomitantly at 12 to 15 months of age. Blood samples were collected before and 1, 12, and 24 months after the booster dose of Menitorix. Blood samples were also collected 2 months following boosting with Menitorix from a subgroup of infants who had received their PCV7 booster and MMR vaccine 1 month after the administration of Menitorix.
Previously unpublished antibody persistence data for children given three primary doses of an MCC vaccine (either NeisVac-C, Meningitec, or Menjugate) at 2, 3, and 4 months of age but who were not given a booster were available from an earlier study (17) and were compared with the MCC persistence data obtained after the Menitorix boosting in the current study. In the earlier study, infants received three doses of a Hib-containing vaccine in infancy, and some of those infants were boosted with a single-antigen Hib-TT vaccine (Hiberix; GSK) in the second year of life. The levels of antibody persistence after the Hib booster obtained in the two studies were compared.
Sera from both studies were analyzed for meningococcal serogroup C antibody by the serum bactericidal antibody (SBA) assay, as described previously (11). The SBA target strain was C11 (C:16:P1.7-1,1), and the complement source was either baby rabbit serum (rSBA; Pel-Freeze Incorporated, Rodgerson, AZ) or, for a subset of 50 subjects, complement-preserved human serum (hSBA; Sera Laboratories International Ltd., West Sussex, United Kingdom) devoid of meningococcal serogroup C bactericidal antibody. The subset of serum samples analyzed with hSBA was selected from the first 50 subjects from whom all blood samples were available for assay. SBA titers were expressed as the reciprocal of the final serum dilution giving ≥50% killing at 60 min. For computational purposes, SBA titers of <4 were assigned a value of 2. The meningococcal serogroup C IgG antibody titer was determined by a standardized enzyme-linked immunosorbent assay (ELISA), as described previously (6). Hib-polyribosylribitol phosphate (PRP)-specific antibodies (IgG) were quantified by a standardized ELISA (12). Sera were titrated against the international anti-Hib quality control serum (Center for Biologics and Evaluation Research [CBER], 1983). The responses to PCV7, assessed at the Immunobiology Unit of the Institute of Child Health, London, United Kingdom, will be reported separately.
Analyses.
In all analyses, the serogroup C rSBA geometric mean titers (GMTs) and the proportions of individuals achieving rSBA titers of ≥8 or ≥128 were calculated. For a subset of serum samples, the proportions achieving serogroup C hSBA titers of ≥4 were calculated. An rSBA titer of ≥8 at 4 weeks after vaccination was shown to predict short-term clinical protection against serogroup C disease in the United Kingdom (1), while an rSBA titer of ≥128 reliably predicted an hSBA titer of ≥4 (2), the accepted hSBA correlate of protection (7, 8). The total serogroup C-specific IgG geometric mean concentrations (GMCs) were also determined. The Hib IgG antibody GMCs and the proportions achieving antibody concentrations of ≥0.15 μg/ml (the putative short-term protective level) or ≥1.00 μg/ml (considered predictive of longer-term protection [9]) were also calculated. Differences in responses according to the MCC vaccine were assessed by the use of logistic regression (for proportions) and linear regression (for logged antibody responses). A significance level of 0.05 was used. In order to compare antibody decay for meningococcal serogroup C rSBA, IgG, and PRP IgG after the primary and the booster vaccinations, data were plotted by using the log of the antibody data against the log of the time, with trend lines being fitted.
Sample size.
To inform vaccination policies, it was decided that the data would be analyzed when blood samples from 100 subjects had been collected 2 years following their Menitorix booster.
Governance.
The study was conducted in accordance with the 1996 International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines, the 2000 Declaration of Helsinki, and the 2004 European Union Clinical Trial Directive. A favorable opinion was given by the United Kingdom Medicines and Healthcare Products Regulatory Authority (MHRA) and the Eastern Multi Centre Research Ethics Committee (MREC). The European Union Drug Regulating Authorities Clinical Trials number was 2004-001049-14, and the study was registered on the public website ClinicalTrials.gov under the identifier NCT00197808. For use of the serum samples from the prior study (17), favorable opinions were gained from the Public Health Laboratory Service ethics committee, the East and North Hertfordshire Local Research Ethics Committee (LREC), and the Gloucestershire LREC.
RESULTS
Study population.
Of the 394 subjects, a total of 280 subjects were included in the analysis because they received the booster dose of Menitorix and at least one blood sample was collected postboosting. The median age at the time of administration of Menitorix was 12.8 months (age range, 12.0 to 14.8 months). A total of 26 children were excluded from the prebooster analysis, as they had received an additional MCC vaccine or Hib conjugate vaccine (1 child had received both) because they had not achieved the putative protective antibody levels 1 month following receipt of their primary vaccination series. A prebooster blood sample was not obtained from an additional 25 subjects because they had received an additional dose of PCV7, as they had been vaccinated under the schedule that used vaccination at 2 and 3 months of age, which was shown to be immunologically inferior to the schedule that used vaccination at 2 and 4 months of age (16). Therefore, only 229 subjects were eligible for analysis preboosting. Of the 280 subjects boosted with Menitorix, blood was collected from 253 subjects at 1 month postboosting, blood was collected from 131 subjects at 2 months postboosting (these subjects received PCV7 1 month following administration of Menitorix), blood was collected from 223 subjects at 1 year postboosting, and blood was collected from 132 subjects at 2 years postboosting.
The total number of subjects for whom meningococcal serogroup C rSBA titers, IgG concentrations, and Hib IgG concentrations were available was less due to insufficient sera for assay. The median intervals from the time of administration of Menitorix for blood samples obtained 1, 2, 12, and 24 month postboosting were 33 days (range, 27 to 56 days), 63 days (range, 56 to 89 days), 377 days (range, 329 to 426 days), and 742 days (range, 705 to 784 days). No significant differences in the responses between males and females were found for any of the serologic measurements.
Responses to MCC vaccine.
Table 1 shows the meningococcal serogroup C rSBA GMTs and the percentage of subjects with rSBA titers of ≥8 and ≥128, by each of the three MCC vaccines used for priming, before and at 1, 2, 12, and 24 months after the administration of Menitorix. The rate of antibody persistence following the two-dose primary series of NeisVac-C but before the Menitorix booster was 48% for subjects with rSBA titers of ≥8, and the rSBA GMT was 8.7 (95% confidence interval [CI], 5.7 to 13.3). Significantly poorer antibody persistence was demonstrated for those primed with two doses of Menjugate or Meningitec than for those primed with NeisVac-C, in which 21% and 17% of subjects receiving Menjugate and Meningitec had rSBA titers of ≥8, respectively; and the rSBA GMTs were 3.7 (95% CI, 2.7 to 5.0) and 3.3 (95% CI, 2.5 to 4.3), respectively.
TABLE 1.
Percentages of subjects achieving meningococcal serogroup C rSBA titers ≥8 or ≥128 and rSBA GMTs before and 1, 2, 12, and 24 months following administration of Menitorix at 12 to 14 months of age, by priming MCC vaccine
| Time from administration of Menitorix (mo) | Parameter | Result obtained with the following priming meningococcal serogroup C conjugate vaccine: |
||
|---|---|---|---|---|
| Meningitec | Menjugate | NeisVac-C | ||
| Preboosting | No. of subjects | 71 | 66 | 65 |
| GMT (95% CI) | 3.3 (2.5-4.3) | 3.7 (2.7-5.0) | 8.7 (5.7-13.3) | |
| % of subjects with a titer ≥8 (95% CI) | 17 (9-28) | 21 (12-33) | 48 (35-60) | |
| % of subjects with a titer ≥128 (95% CI) | 6 (2-14) | 5 (1-13) | 14 (7-25) | |
| 1 | No. of subjects | 80 | 91 | 76 |
| GMT (95% CI) | 368.4 (253.7-534.9) | 467.3 (329.8-662.1) | 2,085.7 (1,475.6-2,948.0) | |
| % of subjects with a titer ≥8 (95% CI) | 95 (88-99) | 96 (89-99) | 100 (95-100) | |
| % of subjects with a titer ≥128 (95 % CI) | 86 (77-93) | 88 (79-94) | 97 (91-100) | |
| 2 | No. of subjects | 36 | 45 | 43 |
| GMT (95% CI) | 128.0 (69.4-235.9) | 343.0 (203.8-577.3) | 1,413.6 (882.7-2,263.8) | |
| % of subjects with a titer ≥8 (95% CI) | 89 (74-97) | 96 (85-99) | 100 (92-100) | |
| % of subjects with a titer ≥128 (95% CI) | 64 (46-79) | 82 (68-92) | 98 (88-100) | |
| 12 | No. of subjects | 63 | 84 | 66 |
| GMT (95% CI) | 6.5 (4.2-10.1) | 7.0 (4.9-10.0) | 82.3 (48.5-139.9) | |
| % ≥8 (95% CI) | 33 (22-46) | 38 (28-49) | 85 (74-92) | |
| % ≥128 (95% CI) | 11 (5-22) | 10 (4-18) | 55 (42-67) | |
| 24 | No. of subjects | 35 | 45 | 42 |
| GMT (95% CI) | 3.6 (2.4-5.6) | 4.3 (2.8-6.7) | 9.0 (5.0-16.0) | |
| % of subjects with a titer ≥8 (95% CI) | 23 (10-40) | 22 (11-37) | 43 (28-59) | |
| % of subjects with a titer ≥128 (95% CI) | 3 (0-15) | 9 (2-21) | 19 (9-34) | |
One month after the administration of Menitorix, 100% of the subjects receiving a primary course of NeisVac-C achieved an rSBA titer of ≥8, and the rSBA GMT was 2,085.7 (95% CI, 1,475.6 to 2,948.0). rSBA GMTs were significantly lower for the subjects primed with Menjugate (rSBA GMT, 467.3; 95% CI, 329.8 to 662.1) or Meningitec (rSBA GMT, 368.4; 95% CI, 253.7 to 534.9). For Menjugate and Meningitec, the proportions of subjects with rSBA titers of ≥8 were 96% and 95%, respectively. The significant difference in rSBA GMTs persisted to 24 months postboosting.
From 1 to 2 months following the administration of Menitorix, 65%, 27%, and 32% decreases in the rSBA GMTs for subjects primed with Meningitec, Menjugate, and NeisVac-C, respectively, were observed. At 2 months following vaccination with Menitorix, the proportions of subjects with rSBA titers of ≥8 for children primed with NeisVac-C, Menjugate, and Meningitec were 100%, 96%, and 89%, respectively. At 12 months following vaccination with Menitorix, the proportions of subjects with rSBA titers of ≥8 for children primed with NeisVac-C, Menjugate, and Meningitec were 85%, 38%, and 33%, respectively. At 24 months following vaccination with Menitorix, the proportions of subjects with rSBA titers of ≥8 for children primed with NeisVac-C, Menjugate, and Meningitec were 43%, 22%, and 23%, respectively.
The hSBA assay was performed with samples from a subset of 50 individuals (for NeisVac-C, n = 16; for Menjugate, n = 15; for Meningitec, n = 19) to compare the percentage of subjects with putative protective titers by the hSBA assay (titer, ≥4) with percentage of subjects with putative protective titers by the rSBA assay (titer, ≥8), regardless of the priming MCC vaccine used. The percentages putatively protected before and at 1, 2, 12, and 24 months after the administration of Menitorix, as determined by the hSBA assay, are given in Table 2 . Significantly fewer subjects were protected as measured by the hSBA assay than as measured by the rSBA assay at 12 months (McNemar's test, P = 0.008), but this difference did not persist to 24 months (P = 0.68).
TABLE 2.
Percentages of subjects with hSBA titers ≥4 and rSBA titers ≥8 before and at 1, 2, 12, and 24 months after administration of Menitorix
| Parameter | Result for the following time (mo) after administration of Menitorix: |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 12 | 24 | |
| % of subjects with hSBA titer ≥4 | 16 (8/49)a | 94 (46/49) | 82 (37/45) | 31 (15/49) | 24 (12/49) |
| % of subjects with rSBA titer ≥8 | 31 (15/49) | 92 (46/50) | 92 (44/48) | 54 (27/50) | 30 (15/50) |
The data in parentheses indicate the number of subjects with the indicated titer/total number of subjects evaluated.
The serogroup C-specific IgG GMCs and 95% CIs are given in Table 3. At both 1 and 2 months following the administration of Menitorix, similar serogroup C-specific IgG GMCs were demonstrated for those primed with either Meningitec or Menjugate, although the GMCs for those primed with NeisVac-C were significantly higher. The serogroup C-specific IgG GMCs declined rapidly from 2 to 12 months after boosting and by 24 months were back to preboosting levels, regardless of the priming MCC vaccine used.
TABLE 3.
Serogroup C-specific IgG GMCs and 95% CIs before and 1, 2, 12, and 24 months following administration of Menitorix at 12 to 14 months of age, by priming MCC vaccine
| Time from administration of Menitorix (mo) | Parameter | Result obtained with the following priming meningococcal serogroup C conjugate vaccine: |
|||
|---|---|---|---|---|---|
| All combined | Meningitec | Menjugate | NeisVac-C | ||
| Preboosting | No. of subjects | 206 | 70 | 66 | 65 |
| GMC (95% CI) | 0.54 (0.48-0.62) | 0.52 (0.42-0.64) | 0.59 (0.47-0.73) | 0.53 (0.42-0.68) | |
| 1 | No. of subjects | 250 | 80 | 91 | 76 |
| GMC (95% CI) | 7.11 (6.35-7.95) | 6.73 (5.47-8.28) | 5.98 (5.04-7.09) | 9.39 (7.63-11.55) | |
| 2 | No. of subjects | 124 | 36 | 44 | 43 |
| GMC (95% CI) | 5.64 (4.78-6.66) | 5.00 (3.67-6.81) | 4.53 (3.44-5.96) | 8.02 (6.09-10.57) | |
| 12 | No. of subjects | 217 | 63 | 83 | 66 |
| GMC (95% CI) | 0.73 (0.63-0.84) | 0.65 (0.53-0.8) | 0.67 (0.52-0.86) | 0.90 (0.69-1.17) | |
| 24 | No. of subjects | 125 | 35 | 45 | 42 |
| GMC (95% CI) | 0.41 (0.34-0.48) | 0.43 (0.32-0.59) | 0.39 (0.30-0.51) | 0.41 (0.30-0.56) | |
Responses to Hib vaccine.
Table 4 shows the PRP IgG GMCs and the percentages of subjects with PRP IgG concentrations of ≥0.15 μg/ml and ≥1.0 μg/ml before and at 1, 2, 12, and 24 months after the administration of Menitorix. As the PRP IgG concentration did not depend on the priming MCC vaccine used, the results were combined for the three priming MCC vaccines. At 1, 2, 12, and 24 months after the administration of Menitorix, regardless of the priming MCC vaccine used, at least 98% of the subjects had the putative short-term correlate of protection, a PRP IgG concentration of ≥0.15 μg/ml. At 24 months postboosting, the PRP IgG concentration remained significantly higher than the levels preboosting.
TABLE 4.
Proportions of subjects achieving Hib-PRP IgG GMCs and percentages of subjects achieving PRP IgG concentration of ≥0.15 μg/ml or ≥1.0 μg/ml 1, 2, 12, and 24 months following administration of Menitorix at 12 to 14 months of age
| Time from administration of Menitorix (mo) | No. of subjects | GMC (95% CI) | % of subjects with PRP IgG concn of (95% CI): |
|
|---|---|---|---|---|
| ≥0.15 μg/ml | ≥1.0 μg/ml | |||
| Preboosting | 210 | 0.58 (0.49-0.69) | 82 (76-87) | 33 (27-40) |
| 1 | 253 | 43.47 (36.56-51.70) | 99 (97-100) | 98 (96-100) |
| 2 | 131 | 23.86 (18.75-30.38) | 98 (95-100) | 98 (93-100) |
| 12 | 221 | 3.34 (2.82-3.96) | 99 (96-100) | 84 (79-89) |
| 24 | 126 | 2.30 (1.87-2.84) | 98 (94-100) | 75 (67-83) |
Antibody kinetics for MCC and Hib conjugate vaccines.
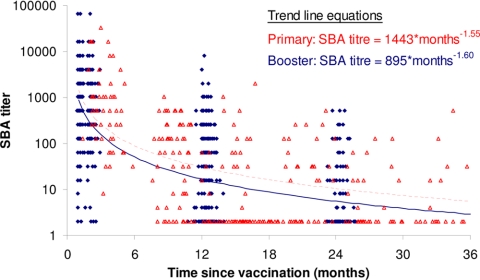
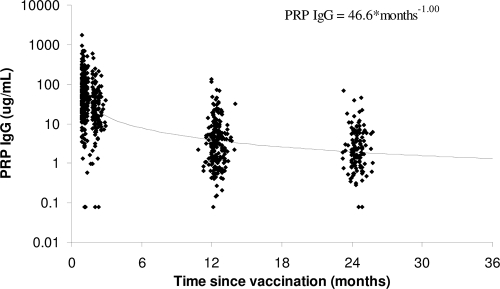
Comparison of the antibody kinetics with boosting (the present study) and without boosting (17) in the second year of life showed similar decay patterns (changes in the log SBA titer per log10 unit of time, −1.60 and −1.55, respectively) (Fig. 1). Thus, for a doubling in time, the rSBA titers decreased by approximately two-thirds, irrespective of whether a booster dose was given in the second year of life. The decay pattern for serogroup C-specific IgG concentrations postboosting was again similar to that after the primary vaccination (changes in the log SBA titer per log10 unit of time, −0.95 and −0.86, respectively) (Fig. 2); with a doubling of time, the IgG concentrations became approximately half. The PRP IgG concentrations after booster decay in the present study (−1.00) and the prior study of Southern et al. (17) (−1.08) were similar to the concentration of serogroup C-specific IgG; with a doubling of time, the IgG concentrations were approximately half (Fig. 3).
FIG. 1.
Meningococcal serogroup C rSBA titers and trend lines by time since primary vaccination (red triangles and dotted trend line) and time since booster vaccination (blue diamonds and solid trend line).
FIG. 2.
Meningococcal serogroup C-specific IgG antibody concentrations and trend lines by time since primary vaccination (red triangles and dotted trend line) and time since booster vaccination (blue diamonds and solid trend line).
FIG. 3.
Hib-PRP IgG antibody concentrations and trend line by time since booster vaccination.
DISCUSSION
This is the first report of antibody persistence up to 24 months following boosting with Menitorix at 12 to 15 months of age in subjects randomized to receive a primary vaccination with two doses of one of the three licensed monovalent MCC vaccines given concomitantly with PCV7 and the DTaP5/IPV/Hib-TT (Pediacel) vaccine. The magnitude of the meningococcal SBA GMT was higher for those subjects primed with NeisVac-C than for those primed with Menjugate or Meningitec up to 1 year following boosting with Menitorix. This may be due to priming and boosting with the same carrier protein, in this case, TT, which is superior to priming and boosting with different carrier proteins. Another explanation is that priming with NeisVac-C is superior to that with Menjugate or Meningitec, regardless of the carrier protein of the conjugate utilized for boosting, reflecting the use of the TT carrier for both the primary and the booster conjugate vaccines (4). A further factor contributing to the better antibody persistence following priming with NeisVac-C than following priming with Menjugate or Meningitec is that NeisVac-C utilizes a de-O-acetylated polysaccharide which has been demonstrated to be a better immunogen than the O-acetylated form (5).
Two years out from vaccination with Menitorix, the proportions of subjects with rSBA titers of ≥8 for children primed with NeisVac-C, Menjugate, and Meningitec were 43%, 22%, and 23%, respectively. This relatively poor antibody persistence was surprising, although to date, the kinetics of antibody persistence of meningococcal antibodies have not been studied. The proportions of subjects maintaining protective levels of antibodies after the administration of other conjugate vaccines such as Hib are more satisfactory following boosting, as demonstrated in this study and elsewhere (17, 18). As the rate of antibody decay is similar for both PRP IgG (−1.00) and meningococcal serogroup C IgG (−0.95) following boosting, the actual magnitude of the booster response appears to determine persistence; the PRP IgG GMCs were substantially higher than the serogroup C IgG GMCs 1 month following boosting. Predictors of long-term humoral immunity following the administration of a primary series of the MCC vaccine have previously been shown to be the absolute level of serogroup C rSBA and IgG and the frequency of memory B cells 1 month after the administration of the primary series (13). The more rapid decay (−1.60) in the rSBA titers than the serogroup C IgG or PRP IgG concentrations following administration of the Menitorix booster (P < 0.001) is of note. It has previously been postulated that rSBA titers disproportionally reflect the titers of IgM antibodies (14), which have a shorter half-life than IgG antibodies. The hSBA assay, in which IgM antibodies are not bactericidal, was performed with a subset of sera to investigate this. However, 1 year following boosting, the proportion of children with putatively protective hSBA titers, 31% (15/49), was lower than the proportion of children with putatively protective rSBA titers, 54% (27/50). Future work of interest would be the performance of the SBA assay for Hib to investigate whether a similar disparity exists for IgG and SBA titers.
A prior study showed that 37% of unprimed children (median age, 2.3 years; age range, 1.4 to 3.2 years) who received a single dose of MCC vaccine (Meningitec) had rSBA titers of ≥8 at a median interval of 1.8 years (range, 1.2 to 2.7 years) after vaccination (15). This is similar to the proportion of 33.9% for children primed with Meningitec 1 year following their booster with Menitorix in this study. However, the age of the unprimed children at the time of vaccination in the previous study was higher than that of the children in the present study, and the age of boosting has previously been shown to influence the magnitude of the response (17).
In England and Wales, there have been no known Menitorix vaccine failures to date among the five meningococcal serogroup C cases in children born on or after 4 September 2005 (Health Protection Agency, unpublished data). This suggests that there is little exposure to serogroup C meningococci, probably due to herd immunity effects (20). Monovalent MCC vaccines also proved effective when they were administered as a single dose in the United Kingdom toddler catch-up and had proven effectiveness for at least 4 years, despite a slight waning of effectiveness after the first year of vaccination (19).
Few data on antibody persistence following two-dose primary courses of MCC and Menitorix boosting have been published. A study in Spain used a two-dose primary course of NeisVac-C at 2 and 4 months of age, with a booster of Menitorix being given at a mean of 13.3 months of age (standard deviation, ±0.5 months) (18). For the Spanish study, the proportions of subjects with rSBA titers of ≥8 before boosting with Menitorix and at 1 month and 18 months after boosting were 87.8%, 99.2%, and 95%, respectively. In the current study, the proportions of subjects with rSBA titers of ≥8 were 48%, 100%, 85%, and 43% before and at 1, 12, and 24 months after boosting with Menitorix, respectively. In the Spanish study, the rSBA GMTs before boosting with Menitorix and at 1 month and 18 months after boosting were 87.8 (95% CI, 80.4 to 93.2), 10,130.0 (95% CI, 7,590.7 to 13,518.8), and 293.8 (95% CI, 212.8 to 405.7), respectively. In the current study, the rSBA GMTs before and at 1, 12, and 24 months after boosting with Menitorix were 8.7 (95% CIs, 5.7 to 13.3), 2,085.7 (95% CIs, 1,475.6 to 2,948.0), 82.3 (95% CIs, 48.5 to 139.9), and 9.0 (95% CIs, 5.0 to 16.0), respectively. The difference in rSBA titers between these studies may be due to differences in the SBA assays performed for the Spanish study in the laboratories of GlaxoSmithKline Biologicals in Rixensart, Belgium, and the current study performed in the Health Protection Agency laboratories in Manchester, United Kingdom. Other confounders include the concomitant use of vaccines; for example, in the Spanish study, PCV7 was not administered and Infanrix hexa (a vaccine for the prevention of diphtheria, tetanus, pertussis [whooping cough], hepatitis B, poliomyelitis, and Hib infection) was given at 2, 4, and 6 months, whereas in the current study, Pediacel was given at 2, 3, and 4 months.
Booster doses of MCC may be required in the future to maintain good antibody levels through adolescence, the age at which meningococci are primarily carried (10). There is, however, no immediate need for a booster vaccination during adolescence, as mathematical modeling has shown that persisting herd immunity will maintain disease control for a number of years (20).
Acknowledgments
This is an independent study funded by the Policy Research Programme in the Department of Health, United Kingdom, grant 039/031.
The views expressed in the publication are those of the authors and not necessarily those of the Department of Health.
We thank the Vaccine Research Nurse teams in Gloucester (Angela Hogan, Ann Maher, Wendy Nedoma, and Diane Webb) and Hertfordshire (Norah Ashwood, Mary Clarke, Diane Hammond, Mary Heath, Lynne Joslin, and Amanda Tew), the laboratory team at the Health Protection Agency/Manchester Medical Microbiology Partnership (Helen Chadha, Daniel Holme, Lesley Mabey, Danielle Thompson, and Kelly Townsend), the immunoassay team (Janet Blake, Bassam Hallis, Mike Hudson, and Carol Powell) at the HPA Centre for Emergency Preparedness and Response, and the administrative team at the HPA Centre for Infections (Teresa Gibbs, Liz Sheasby, and Joan Vurdien) for their assistance with the conduct of this study.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: a re-evaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health Immunisation against Infectious Diseases. 2006. The green book. Department of Health Immunisation against Infectious Diseases, London, United Kingdom. www.dh.gov.uk/greenbook.
- 4.Diez-Domingo, J. 2008. MenC vaccine booster: immunogenicity and factors that affect immune response, p. 9. Abstr. 26th Annu. Meet. Eur. Soc. Paediatr. Infect. Dis.
- 5.Fusco, P. C., E. K. Farley, C. H. Huang, S. Moore, and F. Michon. 2007. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin. Vaccine Immunol. 14:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheesling, L. L., G. M. Carlone, L. B. Pais, P. F. Holder, S. E. Maslanka, B. D. Plikaytis, M. Achtman, P. Densen, C. E. Frasch, H. Käyhty, J. P. Mays, L. Nencioni, C. Peeters, D. C. Phipps, J. T. Poolman, E. Rosenqvist, G. R. Siber, B. Thiesen, J. Tai, C. M. Thompson, P. P. Vella, and J. D. Wenger. 1994. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 32:1475-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldschneider I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayhty, H., H. Peltola, V. Karanko, and P. H. Makela. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147:1100. [DOI] [PubMed] [Google Scholar]
- 10.Maiden, M. C., and J. M. Stuart. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359:1829-1831. [DOI] [PubMed] [Google Scholar]
- 11.Maslanka, S. E., L. L. Gheesling, D. E. LiButti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. N. Devi, C. E. Frasch, J. C. Huang, P. Kris-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. A. M. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipps, D. C., J. West, R. Eby, M. Koster, D. V. Madore, and S. A. Quataert. 1990. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J. Immunol. Methods 135:121-128. [DOI] [PubMed] [Google Scholar]
- 13.Rohner, G. B., M. D. Snape, D. F. Kelly, T. John, A. Morant, L.-M. Yu, A. Borkowski, F. Ceddia, R. Borrow, C.-A. Siegrist, and A. J. Pollard. 2008. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J. Immunol. 180:2165-2173. [DOI] [PubMed] [Google Scholar]
- 14.Santos, G. F., R. R. Deck, J. Donnelly, W. Blackwelder, and D. M. Granoff. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab. Immunol. 8:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snape, M. D., D. F. Kelly, B. Green, E. R. Moxon, R. Borrow, and A. J. Pollard. 2005. Lack of serum bactericidal activity in preschool children 2 years after a single dose of serogroup C meningococcal polysaccharide-protein conjugate vaccine. Pediatr. Infect. Dis. J. 24:128-131. [DOI] [PubMed] [Google Scholar]
- 16.Southern, J., R. Borrow, N. Andrews, R. Morris, P. Waight, M. Hudson, P. Balmer, H. Findlow, J. Findlow, and E. Miller. 2009. Immunogenicity of a reduced schedule of a meningococcal group C conjugate vaccine given concomitantly with the Prevenar and Pediacel vaccines in healthy infants in the United Kingdom. Clin. Vaccine Immunol. 16:194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Southern, J., J. McVernon, D. Gelb, N. Andrews, R. Morris, A. Crowley-Luke, D. Goldblatt, and E. Miller. 2007. Immunogenicity of a fourth dose of Haemophilus influenzae type b (Hib) conjugate vaccine and antibody persistence in young children from the United Kingdom who were primed with acellular or whole-cell pertussis component-containing Hib combinations in infancy. Clin. Vaccine Immunol. 14:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tejedor J. C., M. Moro, J. M. Merino, J. A. Gómez-Campderá, M. García-del-Rio, A. Jurado, F. J. Díez-Delgado, F. Omeñaca, J. García-Sicilia, J. Ruiz-Contreras, A. Martin-Ancel, J. Roca, R. Boceta, P. García-Corbeira, G. Maechler, D. Boutriau, and the Spanish 102547 Study Group. 2008. Immunogenicity and reactogenicity of a booster dose of a novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine given to toddlers of 13-14 months of age with antibody persistence up to 31 months of age. Pediatr. Infect. Dis. J. 27:579-588. [DOI] [PubMed] [Google Scholar]
- 19.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 20.Trotter, C. L., W. J. Edmunds, M. E. Ramsay, and E. Miller. 2006. Modelling future changes to the meningococcal serogroup C conjugate (MCC) vaccine program in England and Wales. Hum. Vaccines 2:68-73. [DOI] [PubMed] [Google Scholar]