Abstract
In France and Finland, farmer's lung disease (FLD), a hypersensitivity pneumonitis common in agricultural areas, is mainly caused by Eurotium species. The presence of antibodies in patients' serum is an important criterion for diagnosis. Our study aimed to improve the serological diagnosis of FLD by using common fungal particles that pollute the farm environment as antigens. Fungal particles of the Eurotium species were observed in handled hay. A strain of Eurotium amstelodami was grown in vitro using selected culture media; and antigen extracts from sexual (ascospores), asexual (conidia), and vegetative (hyphae) forms were made. Antigens were tested by enzyme-linked immunosorbent assay (ELISA), which was used to test for immunoglobulin G antibodies from the sera of 17 FLD patients, 40 healthy exposed farmers, and 20 nonexposed controls. The antigens were compared by receiver operating characteristic analysis, and a threshold was then established. The ascospores contained in asci enclosed within cleistothecia were present in 38% of the hay blades observed; conidial heads of aspergillus were less prevalent. The same protocol was followed to make the three antigen extracts. A comparison of the results for FLD patients and exposed controls showed the area under the curve to be 0.850 for the ascospore antigen, 0.731 for the conidia, and 0.690 for the hyphae. The cutoffs that we determined, with the standard deviation for measures being taken into account, showed 67% for sensitivity and 92% for specificity with the ascospore antigen. In conclusion, the serological diagnosis of FLD by ELISA was improved by the adjunction of ascospore antigen.
Eurotium amstelodami is known to cause hypersensitivity pneumonitis (HP), of which farmer's lung disease (FLD) is a common form (24, 29). Eurotium species, widely isolated from hay harvested in several European countries (22, 30), constitute a major etiological agent of the disease in France and Finland (13, 26). Thus far, the diagnosis of HP has most often relied on an array of unspecific clinical symptoms and signs developed in an appropriate setting and the demonstration of interstitial markings on chest radiographs, serum antibodies against offending antigens, a lymphocytic alveolitis on bronchoalveolar lavage (BAL), and the granulomatous reaction on lung biopsy specimens (17, 21). Enzyme-linked immunosorbent assay (ELISA) is one of the techniques that can be used to look for specific serum antibodies (6). Although ELISA indicates significantly higher immunoglobulin G antibody levels in patients with FLD than in control groups, no clear threshold has been established due to an extreme variability in the results that have been obtained (6, 25). The antigens used in most studies derive from the sonication of crude fungi.
E. amstelodami can be found in any one of three forms (sexual, asexual, or vegetative), and the one that is predominant in the agricultural environment is not known to date. Sexual reproduction is characterized by the production of cleistothecia containing asci with eight ascopores. Asexual reproduction is performed by conidia produced by conidial heads of aspergilli. Vegetative growth is effected by somatic septate hyphae. Ascopores, conidia, and hyphal fragments are all small enough to be inhaled (size, <5 μm) and, hence, to cause FLD, but they do not have the same antigenic capacity (12). The development and dispersal of fungal particles in indoor environments depend on many factors, such as the relative humidity, the turbulence in the air, and the fungal cell wall property (19). The question of what patients actually inhale remains open.
The aim of our study was to improve the serological diagnosis of FLD by using the fungal particles probably inhaled by farmer as antigens. We did this by looking for traces of fungal particles on hay handled by farmers during exposure and testing ascospores, conidia, and hyphae from E. amstelodami as antigens in ELISAs.
MATERIALS AND METHODS
Search for the presence of fungal particles on hay.
Hay sampled from the patients' farms were frozen at −18°C overnight to kill the mites. Ten grams was rinsed with 20 ml of sterile distilled water, shaken vigorously for 1 min, and cultured on petri dishes. The samples were cultured on two culture media, as follows: dichloran-glycerol 18 (Oxoid, Unipath, Basingstoke, United Kingdom) with 0.5% chloramphenicol (Merck, Darmstadt, Germany) at 30°C for mesophilic mold isolation and 3% malt agar (Oxoid) with 10% salt and 0.5% chloramphenicol at 20°C for osmophilic fungal species isolation. The fungi were identified by comparison of their macroscopic and microscopic characteristics to those described in mycology books (5, 15). These characteristics are the color of the colony, growth temperature, potential diffused pigment, characteristics of the mycelium, conidiogenesis mode, and length and aspect of sexual and asexual bodies. The number of CFU per plate was counted after 3 and 7 days of incubation. Among the hay samples, three were selected because of their high concentrations of E. amstelodami (57,600 CFU/g, 118,400 CFU/g, and 860,000 CFU/g per culture, respectively). These hay samples also contained high concentrations of Eurotium herbariorum (synonymous with Eurotium umbrosum) (3,200 CFU/g, 32,000 CFU/g, and 430,000 CFU/g, respectively). Twenty blades of each hay sample were examined microscopically (magnification, ×20) for traces of cleistothecia and conidial heads from the Eurotium genus. Cleistothecia are spherical, bright yellow, and 60 to 160 μm in diameter. Conidial heads are composed of a conidiophore with a swollen apical vesicle which supports flask-shaped phialides openings without collarettes. Phialides are attached directly on the vesicle (uniseriate). The blades of hay were measured to estimate the densities of the cleistothecia and conidial heads (number per cm2 of hay). Moreover, 3 g of the three selected hay samples were rinsed with 20 ml of sterile distilled water, shaken for 2 min, and left to settle for 15 min. Fifty microliters of the surface of the rinsing liquid was taken 10 times and observed microscopically (magnification, ×100) to look for Eurotium sp. conidia (finely roughened, subspherical, 5 to 8 by 3.4 to 5 μm) and ascospores from E. amstelodami (rough walled, with a V-shaped equatorial furrow, lenticular, 4.5 to 7 by 3.5 μm, free or included per height within asci) and E. herbariorum (which have the same characteristics as the ascospores from E. amstelodami but are smooth walled) (5, 15, 27).
Strains and culture conditions.
The strain of Eurotium amstelodami (BBCM/IHEM16286) used in this study was isolated from hay harvested in Franche-Comte, France. It was cultured for 1 week at 30°C on six culture media: brain heart infusion (BHI) liquid (Becton Dickinson), Sabouraud agar (Oxoid), DG18 medium (Oxoid), malt agar (DIFAL, Seysses, France), 3% malt agar (Oxoid, Unipath) with 10% salt (NaCl), and cornmeal agar (Croc Nature, Besancon, France). For the last medium, 42 g of maize was heated in 1 liter of distilled water at 60°C for 1 h and filtered through Whatman paper, and then the volume was restored to 1 liter by adding water. Twelve grams of agar (Pastagar; Bio-Rad, Marnes-la-Coquette, France) was added, and the mixture was sterilized at 121°C for 15 min. All culture media were supplemented with 0.5% chloramphenicol (Merck). Three media were selected to obtain asexual (conidial), sexual (ascospore), and vegetative (hyphal) forms.
Subjects and blood samples.
Three types of subjects were enrolled in the study: (i) 17 patients with FLD, all of whom were dairy farmers who were newly diagnosed with FLD between March 2007 and May 2008 in the university hospital respiratory disease departments in Lausanne (Lausanne, Switzerland) and Besançon (Besançon, France); (ii) 40 exposed controls, which consisted of healthy dairy farmers exposed to fungal allergens; and (iii) 20 nonexposed controls, which consisted of healthy subjects living in rural areas who had never been exposed to an agricultural environment. The FLD patients are described in Table 1. The clinical presentation as acute, subacute, or chronic was given in accordance with the classification of Richerson et al. (28). All subjects with FLD had chronic exposure to moldy material, respiratory symptoms suggestive of the diagnosis, inspiratory crackles, a low CO-diffusing capacity, suggestive high-resolution thoracic computed tomography (CT) scan features, and lymphocytic alveolitis as determined by bronchoalveolar lavage (8, 18). The exposed and nonexposed controls were enrolled during occupational medicine consultations. Spirometry and a standardized medical questionnaire were used to ensure that none of the controls presented any general or respiratory symptoms. The study protocol was approved by the local review boards for research involving human subjects from Besançon and Lausanne. A blood sample was taken from each participating subject, conserved under cold conditions, and centrifuged in less than 4 h following the blood draw. Immunological tests were performed with the sera. The results for precipitins obtained by electrosyneresis of cellulose acetate are presented in Table 2.
TABLE 1.
Description of farmer's lung disease cases
| Case no. | Age (yr) | Smoker | Sexa | Hospital service | Clinical presentationb | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 48 | No | M | Besançon | Acute recurrent | Decrease in exposure, corticosteroids | Recovery |
| 2 | 48 | No | M | Besançon | Subacute | Decrease in exposure | Recovery |
| 3 | 53 | No | M | Besançon | Chronic | Decrease in exposure | Unknown |
| 4 | 51 | No | M | Besançon | Acute recurrent | Decrease in exposure | Residual disease (obstructive respiratory defect) |
| 5 | 47 | No | M | Besançon | Subacute recurrent | Decrease in exposure | Residual disease (restrictive respiratory defect) |
| 6 | 51 | No | F | Besançon | Chronic | Suppression of exposure | Improvement, residual disease (mild restrictive respiratory defect) |
| 7 | 49 | No | F | Besançon | Acute | Suppression of exposure | Improvement, residual disease (mild obstructive respiratory defect) |
| 8 | 47 | No | M | Besançon | Acute | Decrease in exposure, corticosteroids | Recovery |
| 9 | 59 | No | M | Besançon | Subacute | Decrease in exposure, short course (5 days) of corticosteroids | Recovery |
| 10 | 36 | No | M | Besançon | Subacute | Decrease in exposure, corticosteroids | Recovery |
| 11 | 51 | No | M | Besançon | Subacute | Decrease in exposure | Recovery |
| 12 | 51 | No | F | Besançon | Subacute severe | Decrease in exposure, corticosteroids | Improvement but persistent active disease, moderate restrictive respiratory defect |
| 13 | 43 | No | M | Besançon | Chronic | Decrease in exposure | Recovery |
| 14 | 53 | Yes | M | Besançon | Subacute | Decrease in exposure, corticosteroids | Improvement but persistent active disease, moderate restrictive respiratory defect |
| 15 | 56 | No | F | Besançon | Subacute | Suppression of exposure | Improvement, residual disease (mild obstructive respiratory defect) |
| 16 | 63 | No | M | Neuchatel, Switzerland | Chronic | Decrease in exposure, corticosteroids | Improvement, residual disease |
| 17 | 65 | No | M | Neuchatel, Switzerland | Subacute | Suppression of exposure | Recovery |
M, male; F, female.
According to the classification of Richerson et al. (28).
TABLE 2.
Precipitin results for farmer's lung cases by electrosyneresis on cellulose acetate method
| Case no. | No. of precipitin arcs for: |
|||||
|---|---|---|---|---|---|---|
| Absidiacorymbifera | Wallemiasebi | Eurotiumamstelodami | Saccharopolysporarectivirgula | MesophilicStreptomyces | Hay extract | |
| 1 | 2 | 4 | 2 | 0 | 0 | 2 |
| 2 | 1 | 2 | 3 | 1 | 2 | 2 |
| 3 | 1 | 4 | 1 | 3 | 0 | 2 |
| 4 | 1 | 4 | 4 | 1 | 2 | 2 |
| 5 | 0 | 5 | 1 | 0 | 1 | 1 |
| 6 | 2 | 1 | 4 | 15 | 4 | 6 |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 |
| 8 | 2 | 0 | 0 | 4 | 1 | 2 |
| 9 | 1 | 3 | 0 | 0 | 2 | 2 |
| 10 | 1 | 0 | 0 | 0 | 2 | 2 |
| 11 | 1 | 3 | 2 | 1 | 0 | 4 |
| 12 | 1 | 2 | 1 | 7 | 2 | 6 |
| 13 | 2 | 6 | 1 | 0 | 1 | 4 |
| 14 | 2 | 0 | 2 | 0 | 1 | 7 |
| 15 | 0 | 0 | 0 | 0 | 1 | 2 |
| 16 | 0 | 4 | 1 | 3 | 1 | 2 |
| 17 | 0 | 2 | 0 | 1 | 1 | 5 |
Antigen extracts for ascospores, conidia, and hyphae.
Ascospores, conidia, and hyphae were first suspended in a 10 mM sodium phosphate-0.9% NaCl buffer, pH 6 (0.09 mM sodium phosphate monobase [Sigma-Aldrich, St. Louis, MO], 0.01 mM sodium hydrogen phosphate dehydrate [Merck], 0.9% NaCl [Sigma-Aldrich], pH 6.0). For the ascospores and conidia, 3 ml of 10 mM sodium phosphate-0.9% NaCl buffer was added to the culture, spread gently with a glass rake, and aspirated. For each extract, five culture media were rinsed by this method with 5 to 15 ml of rinsing liquid. The hyphae, which grew on a liquid medium, were recovered by filtration and added at 15 ml to 10 mM sodium phosphate-0.9% NaCl buffer. Each suspension was examined by microscopy to verify that it contained only the specific form of the fungus. After 5 min of centrifugation (4°C, 6,000 × g; JA 20.1 rotor; Beckman Coulter) to pellet the ascospores, conidia, and hyphae, the cells were suspended in 5 ml of 0.1 M Tris-HCl buffer at pH 8.5 supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM o-phenanthroline, 1 mM pepstatin [Sigma-Aldrich]). The mixtures were incubated for 1 h at room temperature with a recombinant β-1,3-glucanase (20 U/μl; lyticase; MP Biomedicals, Irvine, CA). During the incubation, they underwent continuous light movement, which allowed the proteins on the surface to be released into the buffer. Soluble proteins were separated by two successive centrifugations (4°C, 3 min, 13,000 × g; JA 20.1 rotor; Beckman Coulter). The supernatant was retained, and then 100 μl of 0.1% deoxycholic acid solution (Sigma-Aldrich) was added per milliliter and the preparation was incubated for 5 min at room temperature. The proteins were pelleted with 70 μl of 0.6 M trichloroacetic acid (Sigma-Aldrich) per milliliter of supernatant on ice for 15 min. The precipitated proteins were subsequently pelleted by centrifugation (4°C, 15 min, 30,000 × g; JA 20.1 rotor; Beckman Coulter). Protein extracts were purified with a sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) clean-up kit (Roche Diagnostics, Basel, Switzerland), as recommended by the manufacturer, and suspended in an ELISA coating buffer (see below). The protein concentration of each extract was determined by a protein dosage program (280 nm) with a spectrophotometer (ND-1000 Nanodrop; Thermo Fisher Scientific, Waltham, MA).
2D gel electrophoresis and staining procedure.
Protein fractions, generated as described above, containing 54 μg of protein were resuspended in a two-dimensional (2D) PAGE lysis buffer (5 mM magnesium acetate, 7 M urea, 2 M thiourea, 4% [wt/vol] 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 30 mM Tris, pH 8). After sonication and centrifugation, the supernatants were diluted in an equal volume of the same buffer supplemented with 2 mg/ml dithiothreitol (DTT) and 2% (vol/vol) Pharmalyte 3 to 10 (GE Healthcare, Freiburg, Germany). Immobiline DryStrip gels (pH 3 to 10, 11 cm; GE Healthcare) were rehydrated according to the manufacturer's instructions prior to first-dimension separation with cup-loading sample application. Isoelectric focusing was carried out on an Ettan IPGphor electrophoresis system (or on an IPGphor isoelectric focusing unit [GE Healthcare]) with a total focusing of 30,000 V-h, according to the manual and the instructions of the manufacturer. Prior to the running of the second dimension, strips were equilibrated for 10 min in a reducing buffer containing 6 M urea, 2% (wt/vol) SDS, 30% (vol/vol) glycerol, 32 mM DTT, and 100 mM Tris, pH 8. This was followed by a 10-min alkylation in a buffer containing 6 M urea, 2% (wt/vol) SDS, 30% (vol/vol) glycerol, 240 mM iodoacetamide, and 100 mM Tris, pH 8. Second-dimension separation was carried out on a precast 8 to 16% polyacrylamide gel (13.3 by 8.7 cm; Criterion; Bio-Rad). The IPG strip from the first dimension was loaded onto the slab gels together with a molecular mass size marker and was run at 80 V across the stacking gel for 30 min and at 120 V for 90 min. Following electrophoresis, the proteins were stained with colloidal Coomassie blue and image acquisition was performed with a visible light densitometer. Spot detection and alignment with the Western blot image were performed by using the Melanie (version 7.0) program (Genebio, Geneva, Switzerland).
ELISA.
Ninety-six-well plates (PolySorp Immunomodule; Nalge Nunc, Rochester, NY) were coated with 200 μl of a 1-μg/ml antigen solution in 50 mmol/liter K2HPO4 buffer (Sigma-Aldrich), pH 8.5, at 4°C for 48 h. Excess binding sites were blocked at 37°C for 1 h with 250 μl of 50 mmol/liter NaH2PO4 (Sigma-Aldrich) containing 0.5% bovine serum albumin (Sigma-Aldrich) and 60 g/liter sorbitol (Sigma-Aldrich). One hundred-microliter serum samples diluted 1:200 were added in triplicate to all wells. The plates were incubated at 37°C for 1 h under constant agitation. The plates were washed four times with washing buffer (100 mmol/liter Tris-HCl, pH 7.5, 0.25% Tween). Next, 100 μl of peroxidase-conjugated goat anti-human IgG (Sigma-Aldrich) diluted 1:4,000 was added to all wells, and the plates were incubated at 37°C for 1 h. The washing procedure was repeated once, and 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) solution (TMB One-Step Substrate system; RD Biotech, Besançon, France) at room temperature was added to the wells for 10 min. To stop the color reaction, 100 μl of an acid solution (0.5 N H2SO4) was added. The wells were read spectrophotometrically at 450 nm (Multiskan; Titertek, Huntsville, AL), and the results were expressed in optical density (OD) units.
Statistics and validation.
The concentrations of serum and antigens used were preliminarily determined by dilution assays. Sera were deposited on plates in triplicate; and the coefficients of variation, corresponding to the standard deviation divided by the mean for the triplicate samples, were calculated. If a coefficient of variation was above 15%, the serum sample was tested again. This was done only for samples with ODs above 0.050.
The mean for the triplicate samples was used for analysis. Due to the variability in the optical densities observed between plates, a reference pool of sera was used and the results were expressed as the difference between the OD for the reference serum sample and the OD for the serum sample tested (ΔOD). The reference pool of sera was constituted by mixing the sera from three exposed controls, selected to yield an OD near 0.400 for Eurotium antigens.
Receiver operating characteristic (ROC) curve analysis was chosen to compare the diagnostic performance characteristics of the immunological tests (32). The optimum cutoff level was determined with sensitivity and specificity tables provided by STATA software (release 9; StataCorp LP, College Station, TX). The value selected corresponded to the maximum sensitivity and specificity. The area under the curve indicated the discriminating capacity of the antigens. A high value for the area under the ROC curve indicated good sensitivity and specificity. Values under 0.5 indicated that the test could not discriminate patients from controls.
RESULTS
Search for the presence of fungal organs in hay samples.
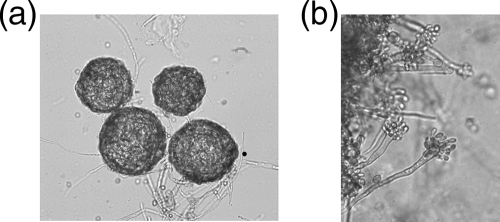
Microscopic examination was used to look for fungal particles in three samples of hay from Franche-Comte hay handled by farmers with HP and shown to contain a large amount of E. amstelodami. Analysis of 20 blades per sample revealed numerous Eurotium yellow globosus cleistothecia with diameters of 90 to 150 μm (Fig. 1). Among the 60 blades observed, 23 (38%) presented cleistothecia with a density of 54 per square centimeter of hay blade. Conidial heads were observed on only two blades (one head per blade), but the species of Aspergillus could not be determined. Ascospores characterized by a lenticular form and distinct equatorial ridges were observed after the hay samples were rinsed with water. Two types of ascospores (lengths, 4.5 to 5 μm) were distinguishable: smooth-walled ones and rough-walled ones. These two types may belong to the Eurotium herbariorum (smooth) and Eurotium amstelodami (rough) species, which were isolated in cultures. Other species able to produce cleistothecia, such as Neosartorya spp., Emericella spp., and Fennellia spp. (teleomorphs of Aspergillus) and Eupenicillium spp. and Talaromyces spp. (teleomorphs of Penicillium), were not found by culture in the hay samples selected.
FIG. 1.
Direct observation of cleistothecia on hay by microscope examination. Magnification, ×20.
Production of E. amstelodami antigens from ascospores, conidia, and hyphae.
To improve the serological diagnosis of FLD, we separately produced antigens from the ascospores, conidia, and hyphae. Six culture media were tested for the selective production of E. amstelodami forms. A dense production of conidia was obtained with malt agar culture medium (Fig. 2b). Numerous cleistothecia containing ascospores were obtained with DG18 medium (Fig. 2a). Both cleistothecia and conidia were observed on Sabouraud agar and salted malt agar. Only hyphae were obtained on BHI medium, and only a weak growth of the fungus was observed on cornmeal agar. Consequently, antigens from ascospores, conidia, and hyphae were produced as described in Materials and Methods with E. amstelodami grown on the DG18, malt agar, and BHI media, respectively.
FIG. 2.
Cleistothecia containing ascospores (a) and aspergillus heads and conidia (b) obtained on DG18 and malt agar media, respectively.
2D gel electrophoresis.
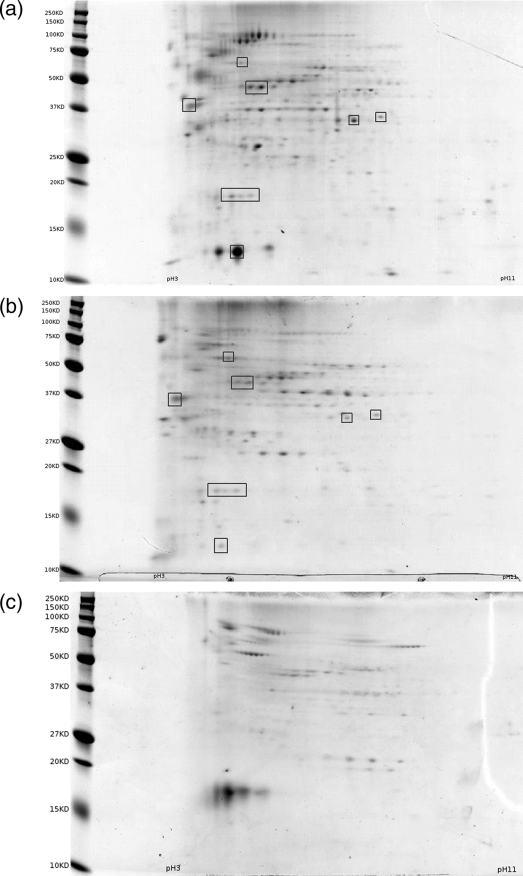
Three 2D gel electrophoreses were performed with ascospore, conidia, and hyphae antigens (Fig. 3). The total number of spots was higher for the ascospore antigen (n = 261) than for the conidial antigen (n = 143) and the hyphal antigen (n = 81). For the three antigen extracts, most of the proteins were located between 25 kDa and 100 kDa. The number of spots within the four classes of molecular weights (≤25, 25 to 37, 37 to 100, >100) were as follows: 55, 60, 125, and 21 spots, respectively, for the ascospore antigen; 31, 38, 68, and 6 spots, respectively, for the conidial antigen; and 17, 17, 44, and 3 spots, respectively, for the hyphal antigen. The 2D gel electrophoresis patterns for the ascospore and conidial antigens showed similarities and were matched with 2D image analysis software after a few landmark spots that seemed to be the same on both gels were chosen (Fig. 3). Thirty-three spots were matched between ascospores and conidial antigens and were distributed over the range of molecular weights. Without identification of the proteins by mass spectrometry and despite spot migration on the three gels at the same time, we cannot say if these spots correspond to identical proteins in both antigens.
FIG. 3.
2D electrophoresis gel patterns of Eurotium amstelodami antigens stained with colloidal Coomassie blue. (a) Ascospore antigen; (b) conidial antigen; (c) hyphal antigen. Boxes surround the spots used to match the gels in panels a and b.
The gel pattern for hyphae was markedly different and could not be matched to the patterns for the ascospores and conidia.
Serological diagnosis of FLD with E. amstelodami antigens from ascospores, conidia, and hyphae.
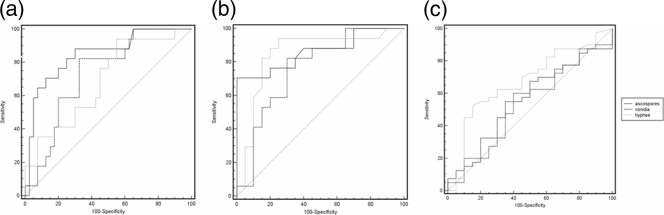
The results of the ELISA with the three antigens were evaluated by ROC curve analysis. The area under the curve and the sensitivity and specificity values for the threshold chosen are indicated in Table 3. With the largest area under the curve, the ascospore antigen was the best able to discriminate FLD patients from both exposed and nonexposed controls (Fig. 4). The same threshold values (≥0.109 for the ascospore antigen and ≥0.013 for the conidial antigen) were established by ROC curve analysis for the discrimination of FLD patients from exposed and nonexposed controls. The hyphal antigen was more efficient than the ascospore or conidial antigen in discriminating exposed from nonexposed controls.
TABLE 3.
ROC curve analyses comparing the capacity to discriminate FLD patients from exposed and nonexposed controls for the three types of E. amstelodami antigens
| Study groupa and antigen type | Threshold (ΔOD)b | Sensitivity (%) | Specificity (%) | Area under the curve (95% CI) |
|---|---|---|---|---|
| FLD vs exposed controls | ||||
| Ascospores | ≥0.113 | 71 | 87 | 0.850 (0.735-0.965) |
| Conidia | ≥0.013 | 82 | 67 | 0.731 (0.597-0.865) |
| Hyphae | ≥0.022 | 53 | 70 | 0.690 (0.540-0.839) |
| FLD vs nonexposed controls | ||||
| Ascospores | ≥0.113 | 71 | 100 | 0.863 (0.736-0.991) |
| Conidia | ≥0.013 | 82 | 70 | 0.762 (0.602-0.921) |
| Hyphae | ≥0.002 | 76 | 85 | 0.847 (0.706-0.988) |
| Exposed controls vs nonexposed controls | ||||
| Ascospores | ≥0.015 | 30 | 65 | 0.442 (0.288-0.597) |
| Conidia | ≥−0.026 | 60 | 60 | 0.534 (0.376-0.692) |
| Hyphae | ≥−0.051 | 82 | 40 | 0.673 (0.524-0.822) |
For patients with FLD, n = 18; for exposed healthy control individuals, n = 40; for nonexposed control individuals, n = 20.
Refers to a pool of sera.
FIG. 4.
ROC curve indicating the capacity to discriminate FLD patients from exposed controls (a), FLD patients from nonexposed controls (b), and exposed from nonexposed controls (c) for the three types of E. amstelodami antigens.
Threshold determination.
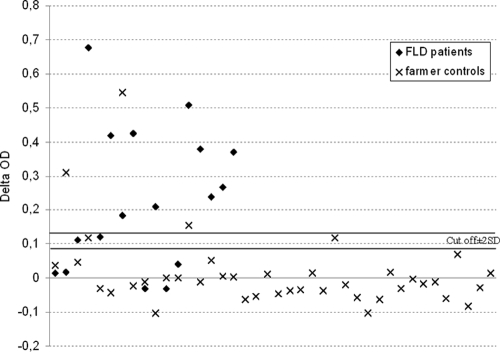
For the ascospore antigen, we established a cutoff of +0.113. In regard to the variability of the triplicate values (mean standard deviation [SD] = 0.013 for the ascospore antigen), an imprecise zone of ±2 SDs can be applied. Two thresholds of +0.087 (+0.113 to 2 SDs) and +0.139 (+0.113 + 2 SDs) were thus established. The test results for the ODs were interpreted as follows: OD values below +0.087 were considered a negative result (no immunological argument for FLD), OD values between +0.087 and +0.139 were classified as an unspecified result, and ODs up to +0.139 were considered a positive result (an immunological argument in favor of FLD). The results based on these thresholds are presented in Fig. 5. The sensitivity of the test was 67%, and the specificity was 92%. Test results could not be specified for three patients. The five patients with values below the lower cutoff had one arc or no arcs in electrosyneresis on cellulose acetate with E. amstelodami antigen, indicating that they had less precipitin antibodies than the threshold against this fungus required (three arcs) (24, 25).
FIG. 5.
Results (in ΔOD) of the ELISA with the ascospore antigen for FLD patient and farmer control sera.
Reproducibility of results.
Similar results were obtained in a second assay with new batches of antigens. In a comparison of FLD patients and exposed controls, the ascospore antigen (ROC curve areas = 0.834 and 0.850, respectively) was more efficient than either the conidial antigen (ROC curve areas = 0.728 versus 0.731, respectively) or the hyphal antigen (ROC curve areas = 0.543 and 0.690, respectively).
The Pearson correlation between the two assays was high (rho = 0.90) for the ascospore antigen, intermediate for the hyphal antigen (rho = 0.36), and low (rho = 0.15) for the conidial antigen.
DISCUSSION
Microscopic examination of hay showed that the sexual forms of E. amstelodami, ascospores contained in asci enclosed within cleistothecia, were highly prevalent on hay. A strain of E. amstelodami was grown in vitro in selected culture media. Numerous ascospores contained in asci within cleistothecia were obtained on DG18 medium. Conidia densely produced by conidial heads were obtained with malt agar culture medium. Only hyphae were obtained on BHI liquid medium. Antigen extracts from sexual (ascospore), asexual (conidial), and vegetative (hyphal) forms were made. Two-dimensional electrophoresis gels showed a higher number of proteins in the ascospore antigen than in the conidial or the hyphal antigen. The ascospore antigen allowed the better discrimination of FLD patients from exposed and nonexposed controls than the other two antigens, and the results were reproducible for the ascospore antigen. In view of these results, the ascospore antigen was the best extract for use with the ELISA method.
With no standardized, validated diagnostic criteria, the diagnosis of HP is difficult (9). Among the well-documented proposals for diagnostic criteria, serological tests are not always given the same weight (11, 18). Indeed, the presence of precipitins in a healthy farmer does not predict an increased risk of FLD (4, 10). The large variations in sensitivity and specificity observed in different studies have led some authors to consider precipitins a marker for exposure and not for the presence of the disease. These discrepancies are due to the diversity of immunological methods and antigens used and to the lack of knowledge about the precise nature of the antibodies detected. Several points require mention. First, the etiological agents of HP are varied, and the use of antigens from microorganisms not actually present in the patient's environment can result in a false-negative immunological test result (14, 31). Second, many different serological methods—ELISA, Western blotting, and the Ouchterlony test, to name a few—have been used to detect specific antibodies. The fact that so many different methods are used makes it difficult to draw comparisons between studies. Third, processes for making antigens are not standardized, so different kinds of antigens (metabolic, somatic, total antigens, or purified fractions) are used to diagnose HP (20, 23). Finally, tobacco consumption decreases the level of precipitin production (3), and the duration of exposure influences the immunoglobulin titer (16). Despite these difficulties, however, identification of the antibodies in sera helps with the diagnosis of HP by indicating exposure to the antigens involved in the disease. A multicenter study by Lacasse et al. identified serum precipitins as a significant predictor of HP, regardless of exposure (odds ratio = 5.3; 95% confidence interval [CI] = 2.7 to 10.4) (18).
In previous studies, the use of metabolic antigens made with entire crushed fungi failed to yield a reliable serological test by the ELISA method (24, 25). In the present study, antigen extracts were made by enzymatic digestion of the cell wall and precipitation of the proteins. The quality and the purity of the antigens were sufficient to make 2D electrophoresis gels. The ascospore antigen seemed to have value for the serodiagnosis of FLD. Because several fungi and actinomycetes can be involved in FLD, the sensitivity may be higher if a panel of relevant antigens instead of one antigen is used. One of our previous studies showed that the serological diagnosis of FLD can be made by using electrosyneresis on cellulose acetate with a panel of four antigens found on the fungi and actinomycetes present in hay (Saccharopolyspora rectivirgula, Absidia corymbifera, E. amstelodami, and Wallemia sebi). Although testing with each antigen individually is relatively inefficient, combining the results obtained with these four antigens into a score gives a specificity value of 90% and negative predictive values ranging from 81% to 88% (8). The sensitivity was improved and the specificity did not change when serological scores were used. In that study, the low sensitivity of the serological score (76%) can be explained by the high percentage of cases of HP most likely caused by antigens not included in the panel. In the present study, the serological diagnosis of the disease was improved by using a specific fungal particle present in hay as the antigen in an ELISA. The cutoff determined, including the standard deviation for measures, showed 67% for sensitivity and 92% for specificity. The specificity was high but the sensitivity was low with respect to what we expected for a serological diagnosis test; this was due to the use of antigens from E. amstelodami, a microorganism that was probably not responsible for all cases of FLD. Indeed, six patients had no precipitins by electrosyneresis on cellulose acetate with E. amstelodami antigen. Improving the antigen issued from each of the four microorganisms used in the panel and constituting a score on the basis of the results obtained with the most efficient antigens are two ways to ensure a more reliable serological diagnosis of HP.
In a Finnish study of Aspergillus umbrosus, Kaukonen et al. showed that immunolabeling and the use of gold-conjugated antibodies from patients with FLD resulted in reactions with conidia and hyphae but not with cleistothecia (12). Although A. umbrosus is close to E. amstelodami, the results of that study would appear to contradict the results of our study. However, that study was performed with sera from three patients, and only the outer layer of the cell wall was available for immunodetection. Other authors have shown that it is easier to obtain substances that induce alveolitis in animal models from intracellular membranes and the cytoplasm than from the cell wall (1). In our study, antigens from ascospores, conidia, and hyphae were produced under similar conditions; and better ELISA results were obtained with the ascospore antigen. These results would seem to indicate that ascospores are more antigenic than either conidia or hyphae. The use of the ascospore antigen and ELISA methods to detect specific immunoglobulin G allowed the good discrimination of FLD patients from both exposed and nonexposed controls, but it did not discriminate exposed from nonexposed controls. Thus, the presence of IgG against ascospore antigen would appear to be a marker of disease and not a marker of exposure.
Many factors contribute to the variability of fungal extracts. Among these are the strains, the culture conditions, and the extraction process used (2, 7). To improve the serological diagnosis of FLD and other types of HP, standardized fungal antigens must be used. Hence, we have undertaken another study to determine the proteins of ascospores involved in antibody detection and to produce recombinant antigens useful for the serological diagnosis of FLD.
Acknowledgments
This work was supported by the French National Agency for Research (ANR no. 05977), INTERREG IIIA (the ODIPHAAR project), and the PAPPA group (Réseau des Pathologies Pulmonaires Agricoles).
We are grateful to E. Lamy for help during occupational medicine consultations and Nancy Richardson-Peuteuil for editorial assistance.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Blyth, W. 1978. The occurrence and nature of alveolitis-inducing substances in Aspergillus clavatus. Clin. Exp. Immunol. 32:272-282. [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, R. K. 1993. Fungal extracts in clinical practice. Allergy Proc. 14:385-390. [DOI] [PubMed] [Google Scholar]
- 3.Cormier, Y., J. Belanger, and P. Durand. 1985. Factors influencing the development of serum precipitins to farmer's lung antigen in Quebec dairy farmers. Thorax 40:138-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormier, Y., L. Letourneau, and G. Racine. 2004. Significance of precipitins and asymptomatic lymphocytic alveolitis: a 20-yr follow-up. Eur. Respir. J. 23:523-525. [DOI] [PubMed] [Google Scholar]
- 5.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2001. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, and Universitat Rovira i Virgili, Pays-Bas-Reus, Spain.
- 6.Erkinjuntti-Pekkanen, R., M. Reiman, J. I. Kokkarinen, H. O. Tukiainen, and E. O. Terho. 1999. IgG antibodies, chronic bronchitis, and pulmonary function values in farmer's lung patients and matched controls. Allergy 54:1181-1187. [DOI] [PubMed] [Google Scholar]
- 7.Esch, R. E. 2004. Manufacturing and standardizing fungal allergen products. J. Allergy Clin. Immunol. 113:210-215. [DOI] [PubMed] [Google Scholar]
- 8.Fenoglio, C. M., G. Reboux, B. Sudre, M. Mercier, S. Roussel, J. F. Cordier, R. Piarroux, and J. C. Dalphin. 2007. Diagnostic value of serum precipitins to mould antigens in active hypersensitivity pneumonitis. Eur. Respir. J. 29:706-712. [DOI] [PubMed] [Google Scholar]
- 9.Fink, J. N., H. G. Ortega, H. Y. Reynolds, Y. Cormier, L. L. Fan, T. J. Franks, K. Kreiss, S. Kunkel, D. Lynch, S. Quirce, C. Rose, R. P. Schleimer, M. R. Schuyler, M. Selman, D. Trout, and Y. Yoshizawa. 2005. Needs and opportunities for research in hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 171:792-798. [DOI] [PubMed] [Google Scholar]
- 10.Gariepy, L., Y. Cormier, M. Laviolette, and A. Tardif. 1989. Predictive value of bronchoalveolar lavage cells and serum precipitins in asymptomatic dairy farmers. Am. Rev. Respir. Dis. 140:1386-1389. [DOI] [PubMed] [Google Scholar]
- 11.Girard, M., Y. Lacasse, and Y. Cormier. 2009. Hypersensitivity pneumonitis. Allergy 64:322-334. [DOI] [PubMed] [Google Scholar]
- 12.Kaukonen, K., L. J. Pelliniemi, J. Savolainen, and E. O. Terho. 1996. Identification of the reactive subunits of Aspergillus umbrosus involved in the antigenic response in farmer's lung. Clin. Exp. Allergy 26:689-696. [PubMed] [Google Scholar]
- 13.Kaukonen, K., J. Savolainen, M. Viander, M. Kotimaa, and E. O. Terho. 1993. IgG and IgA subclass antibodies against Aspergillus umbrosus in farmer's lung disease. Clin. Exp. Allergy 23:851-856. [DOI] [PubMed] [Google Scholar]
- 14.Kaukonen, K., J. Savolainen, M. Viander, and E. O. Terho. 1994. Avidity of Aspergillus umbrosus IgG antibodies in farmer's lung disease. Clin. Exp. Immunol. 95:162-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klich, M. A. 2002. Identification of common Aspergillus species. Centraalbureau Voor Schimmelcultures, Utrecht, The Netherlands.
- 16.Kusaka, H., Y. Homma, H. Ogasawara, M. Munakata, K. Tanimura, H. Ukita, N. Denzumi, and Y. Kawakami. 1989. Five-year follow-up of Micropolyspora faeni antibody in smoking and nonsmoking farmers. Am. Rev. Respir. Dis. 140:695-699. [DOI] [PubMed] [Google Scholar]
- 17.Lacasse, Y., E. Israel Assayag, M. Laviolette, and Y. Cormier. 2004. Clinical and immunopathological aspects of hypersensitivity pneumonitis. Rev. Mal. Respir. 21:769-781. [DOI] [PubMed] [Google Scholar]
- 18.Lacasse, Y., M. Selman, U. Costabel, J. C. Dalphin, M. Ando, F. Morell, R. Erkinjuntti-Pekkanen, N. Muller, T. V. Colby, M. Schuyler, and Y. Cormier. 2003. Clinical diagnosis of hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 168:952-958. [DOI] [PubMed] [Google Scholar]
- 19.McGinnis, M. R. 2007. Indoor mould development and dispersal. Med. Mycol. 45:1-9. [DOI] [PubMed] [Google Scholar]
- 20.Mundt, C., W. M. Becker, and M. Schlaak. 1996. Farmer's lung: patients' IgG2 antibodies specifically recognize Saccharopolyspora rectivirgula proteins and carbohydrate structures. J. Allergy Clin. Immunol. 98:441-450. [DOI] [PubMed] [Google Scholar]
- 21.Patel, A. M., J. H. Ryu, and C. E. Reed. 2001. Hypersensitivity pneumonitis: current concepts and future questions. J. Allergy Clin. Immunol. 108:661-670. [DOI] [PubMed] [Google Scholar]
- 22.Radon, K., B. Danuser, M. Iversen, E. Monso, C. Weber, J. Hartung, K. Donham, U. Palmgren, and D. Nowak. 2002. Air contaminants in different European farming environments. Ann. Agric. Environ. Med. 9:41-48. [PubMed] [Google Scholar]
- 23.Ramasamy, M., Z. U. Khan, and V. P. Kurup. 1987. A partially purified antigen from Faenia rectivirgula in the diagnosis of farmer's lung disease. Microbios 49:171-182. [PubMed] [Google Scholar]
- 24.Reboux, G., R. Piarroux, F. Mauny, A. Madroszyk, L. Millon, K. Bardonnet, and J. C. Dalphin. 2001. Role of molds in farmer's lung disease in eastern France. Am. J. Respir. Crit. Care Med. 163:1534-1539. [DOI] [PubMed] [Google Scholar]
- 25.Reboux, G., R. Piarroux, S. Roussel, L. Millon, K. Bardonnet, and J. C. Dalphin. 2007. Assessment of four serological techniques in the immunological diagnosis of farmers' lung disease. J. Med. Microbiol. 56:1317-1321. [DOI] [PubMed] [Google Scholar]
- 26.Reboux, G., M. Reiman, S. Roussel, K. Taattola, L. Millon, J. C. Dalphin, and R. Piarroux. 2006. Impact of agricultural practices on microbiology of hay, silage and flour on Finnish and French farms. Ann. Agric. Environ. Med. 13:267-273. [PubMed] [Google Scholar]
- 27.Reboux, G., S. Roussel, and F. Grenouillet. 2006. Moisissures de l'environnement agricole. J. Mycol. Med. 16:246-262. [Google Scholar]
- 28.Richerson, H. B., I. L. Bernstein, J. N. Fink, G. W. Hunninghake, H. S. Novey, C. E. Reed, J. E. Salvaggio, M. R. Schuyler, H. J. Schwartz, and D. J. Stechschulte. 1989. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J. Allergy Clin. Immunol. 84:839-844. [DOI] [PubMed] [Google Scholar]
- 29.Roussel, S., G. Reboux, J. C. Dalphin, K. Bardonnet, L. Millon, and R. Piarroux. 2004. Microbiological evolution of hay and relapse in patients with farmer's lung. Occup. Environ. Med. 61:e3. [PMC free article] [PubMed] [Google Scholar]
- 30.Roussel, S., G. Reboux, J. C. Dalphin, D. Pernet, J. J. Laplante, L. Millon, and R. Piarroux. 2005. Farmer's lung disease and microbiological composition of hay: a case-control study. Mycopathologia 160:273-279. [DOI] [PubMed] [Google Scholar]
- 31.Salvaggio, J. 1972. Diagnostic significance of serum precipitins in hypersensitivity pneumonitis. Chest 62:242. [DOI] [PubMed] [Google Scholar]
- 32.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]