Abstract
The history of the pneumococcal polysaccharide enzyme-linked immunosorbent assay (ELISA) is characterized by a continuous search for increased specificity. A third-generation ELISA that uses 22F polysaccharide inhibition has increased the specificity of the assay, particularly at low antibody concentrations. The present work compared various 22F ELISAs and non-22F ELISAs. The comparisons involved three different laboratories, including a WHO reference laboratory, and included sera from subjects from different geographic areas immunized with different pneumococcal conjugate vaccines, including the licensed 7-valent Prevenar vaccine and the 10-valent Synflorix vaccine. All comparisons led to the same conclusion that the threshold defined as 0.35 μg/ml for the WHO non-22F ELISA is lower when any 22F ELISA is used. The use of highly purified polysaccharides for coating further improved the specificity of the assay. In conclusion, we confirm that the 22F ELISA can be recommended as a reference method for the determination of antibodies against pneumococcal polysaccharides.
It has been amply demonstrated that protection against the various manifestations of pneumococcal disease is mediated by antibodies induced to the type-specific pneumococcal polysaccharides (PSs) (10, 19). The biological function of these antibodies is to bind onto the surface of the pneumococcal cell and in so doing induce complement activation, leading to the uptake and killing of the pneumococcal cells by human phagocytic cells, particularly polymorphonuclear leukocytes (26). Such antibodies are referred to as being opsonic, and the whole process of uptake and killing is referred to as opsonophagocytosis. With the development of new pneumococcal conjugate vaccines (PCVs), it is critical that highly specific immunoassays be used to measure the vaccine-induced antibodies that are associated with protective immunity. This implies that the widely used anti-PS enzyme-linked immunosorbent assay (ELISA) should selectively measure those antibodies with opsonic activity. For that purpose, several improvements have been made to the initial ELISA, principally through the recognition of the role played by the antibodies directed against pneumococcal determinants other than the PSs.
One of these determinants is the common cell wall polysaccharide (CWPS). CWPS is covalently linked to the serotype-specific capsular PS through as yet to be determined linkages (4, 9). Thus, when the pneumococcal serotype PS is purified, the CWPS is copurified. The PSs distributed by the American Type Culture Collection (ATCC) to laboratories that perform ELISA for the quantitation of antipneumococcal antibodies are the 23 PSs included in the 23-valent PS vaccine manufactured by Merck (Whitehouse Station, NJ) and contain CWPS as a contaminant.
The preadsorption of postimmunization sera from adults and children with CWPS alone may not be sufficient for the measurement of antibody concentrations that are predictive of vaccine efficacy (24, 31), the reason being that there is a poor correlation between the antibody concentration and opsonic activity (coefficient of correlation [r] = about 0.5 rather than 0.8 or higher). Yu et al. reported in 1999 (31) that many heterologous pneumococcal PSs reduced the level of antibody binding of sera from PS vaccine-immunized adults to the serotype 6B PS over that removed by CWPS alone. They concluded that the antibodies removed by the heterologous pneumococcal PSs were against a novel epitope not found on CWPS. Importantly, they found that these antibodies against novel epitopes were not opsonic. They went on to suggest that the specificity of the pneumococcal PS ELISA could be improved by preadsorption with an unrelated pneumococcal PS, in addition to the CWPS. Soininen at al. (24) examined PSs obtained from the ATCC and from three manufacturers. They observed that the amount of cross-reactive antibody removed by preadsorption with heterologous pneumococcal PS in sera from both adults and infants differed depending upon the manufacturer of the purified pneumococcal PS used in the ELISA. This is in line with the observation that ATCC PS batches have varing degrees of contaminants (30). Xu et al. reported that CWPS is present in two forms: one form is bound and the other form is unbound to the capsular PS (29). Thus, it would be expected that those methods of PS purification that disassociate unbound CWPS would yield purer capsular PS.
From the observations made above, Concepcion and Frasch recommended that each serum be doubly adsorbed: with CWPS, as previously recommended for the second-generation pneumococcal PS ELISA, and with a heterologous pneumococcal serotype PS (2). They recommended that the 22F PS be used for the second adsorption, because the 22F PS was not being considered for use in any of the pneumococcal conjugate vaccines and it was available from the ATCC. The use of 22F as the second adsorbent has now been evaluated in a number of laboratories (5, 7, 21, 23) and is included in the WHO-recommended third-generation pneumococcal ELISA (25).
Some countries that have introduced the pneumococcal conjugate vaccine into their routine immunization schedules had instituted a reduced, accelerated schedule. For example, the United Kingdom has instituted two primary doses at 2 and 4 months of age and a booster at about 12 months of age. As noted above, adults have rather high levels of IgG to CWPS, and maternal antibodies will be more evident in the infant when a compressed or shorter immunization schedule is used. This means that the added 22F adsorption will be required for the measurement of serotype-specific antibodies after primary immunization when such schedules are used.
Recommendations for the serological criteria to be considered for the licensure of new pneumococcal conjugate vaccines for use in infants were given as an appendix in volume 927 of the WHO Technical Report Series, published in 2005 (28). The serological criteria included the demonstration of noninferiority against a registered vaccine, with the primary end point being the IgG antibody concentration, as measured by ELISA, in sera from infants collected 4 weeks after a three-dose primary series, with a single threshold of IgG antibody concentration of 0.35 μg/ml for all serotypes. Of note, the threshold value was defined by using pooled immunogenicity and efficacy data from three different efficacy trials with invasive disease end points. The reference value was first proposed to be 0.20 μg/ml, on the basis of a vaccine efficacy trial conducted in Northern California (1, 8). When pooled data from three different efficacy trials with invasive disease end points performed in Northern California, in South Africa, and among Navajo Indians were taken into account (1, 8, 11, 14), an antibody concentration of 0.35 μg/ml, aggregated across the seven serotypes, was determined. However, the WHO agreed that alternative methods may be used for the validation of new conjugate vaccine formulations, and this may eventually lead to the establishment of an alternative threshold (28), but no guidance for bridging was provided. It was further agreed that other clinical end points (e.g., pneumonia or otitis media) would not be taken into account. Nevertheless, this reference threshold value, determined by using data from an ELISA without 22F adsorption, does not necessarily predict protection in an individual subject. A possible correlation between antibody concentrations in the range of 0.20 to 0.35, as measured by ELISA, and a titer of 1:8, as measured by an opsonophagocytosis assay (OPA), was mentioned. At last, the demonstration of the functional capacity of the antibody through determination of opsonophagocytic activity, as measured by OPA after a three-dose priming series, was defined as an important secondary end point. These recommendations published in 2005 have recently been confirmed in a 2009 WHO publication, based upon a consensus meeting held in July 2008 in Ottawa, Ontario, Canada (3).
There is an important inconsistency in the 2003 WHO recommendations for serological criteria. The WHO-recommended third-generation ELISA, approved by WHO in 2000 and set up in two WHO reference laboratories, includes the routine use of the 22F adsorbent, as described by Wernette et al. (25). However, as noted above, the recommended threshold concentration was derived without the use of the 22F adsorbent.
While the 22F adsorbent did not affect the antibody concentrations in sera from infants in the Northern California efficacy trial (20), 22F adsorption reduced the antibody concentrations over 30%, on average, in vaccinated children in the South African trial (13). In the case in which a reduced primary immunization schedule is used, it will be important to remove maternal unspecific nonopsonizing antibody. Henckaerts et al. (5) made observations comparable to those derived from the South African study.
In order to further investigate the impact of 22F adsorption and improved ELISA coating conditions, a series of comparison ELISA measurements was carried out. These involved three different laboratories and used serum samples from pediatric subjects after immunization with different PCVs, including the Prevenar and Synflorix vaccines.
MATERIALS AND METHODS
Studies, serum samples, and laboratories (see also Table 1). (i) Study A. (a) Serum samples.
TABLE 1.
Details of the studies
| Study | No. of serum samples | Vaccine | Study parameters |
Assay details |
Location of results | |||
|---|---|---|---|---|---|---|---|---|
| Country | Immunization schedulea | Age at sampling | ELISA | Laboratory | ||||
| A | 70 | 11-valent candidate PCV (GSK) | Germany | 3, 4, 5 mo | 6 mo | WHO non-22F | ICH | Fig. 1 |
| Lithuania | 3, 4.5, 6 mo | 7 mo | GSK 22F | GSK | ||||
| Argentina | 2, 4, 6 mo | 16-19 mo | ||||||
| Boost at 15-18 mo | ||||||||
| Costa Rica | 2, 4, 6 mo | 13-16 mo | ||||||
| Boost at 12-15 mo | ||||||||
| B | 140 | Prevenar | Germany | 2, 3, 4 mo | 5 mo | GSK 22F | GSK | Fig. 2 |
| 140 | Meningitec | Germany | 2, 3, 4 mo | 5 mo | ||||
| C | 118 | Prevenar | Germany | 2, 3, 4 mo | 5 mo | WHO non-22F | ICH | Fig. 3 |
| GSK 22F | GSK | |||||||
| WHO 22F | ICH | |||||||
| D | 78 | Investigational nine-valent | South Africa | 6, 10, 14 wk | 17-20 wk | THL non-22F | THL | Fig. 4 |
| PCV (Wyeth) | THL 22F | THL | ||||||
| E | 50 | Prevenar | Poland | 2, 4, 6 mo | 5 mo | WHO non-22F | GSK | Fig. 5 |
| GSK 22F | GSK | |||||||
| 50 | Synflorix | Poland | 2, 4, 6 mo | 5 mo | ||||
| F | 32 | Nonvaccinated | Philippines | 6 wk | WHO non-22Fb | GSK | Table 4 | |
| Israel | 2 mo | GSK 22Fb | GSK | |||||
Age of immunization.
ATCC PS and GSK PS were compared in both assays.
The study published by Henckaerts et al., which used serum samples from German and Lithuanian infants (5), was extended by using samples from Latin American infants vaccinated with the same 11-valent candidate pneumococcal vaccine (GlaxoSmithKline Biologicals [GSK], Rixensart, Belgium). In addition to the original 30 serum samples, we included 18 prebooster and 22 postbooster serum samples from infants in Argentina and Costa Rica taken 1 month after administration of the fourth vaccine dose. Sera were selected to represent the full range of antibody concentrations with sufficient values around the threshold concentration. The 11-valent candidate pneumococcal vaccine was coadministered with the diphtheria-tetanus-acellular pertussis (DTPa)-hepatitis B virus (HBV)-inactivated poliovirus (IPV)-Haemophilus influenzae type b (Hib) vaccine (GSK) or the diphtheria-tetanus-whole-cell pertussis (DTPw)-HBV-Hib-oral poliovirus (OPV) vaccine (GSK) in Argentina or with DTPw-HBV-OPV (GSK) in Costa Rica.
(b) Laboratories and assays.
The testing laboratories were the WHO reference laboratory at the Institute of Child Health (ICH), London, United Kingdom (headed by David Goldblatt), which used the WHO non-22F ELISA, and the GSK Biologicals laboratory, which used the GSK 22F-ELISA.
(ii) Study B. (a) Serum samples.
In study B, we used serum samples from 140 German infants vaccinated with Prevnar (Wyeth Lederle Vaccines, Pearl River, NY), together with 140 control serum samples from infants vaccinated with Meningitec (Wyeth). Both of those vaccines were coadministered with Infanrix Hexa (GSK).
(b) Laboratories and assays.
In study B, the testing laboratory was GSK, which used the GSK 22F ELISA.
(iii) Study C. (a) Serum samples.
For study C, 118 serum samples from German infants, obtained after vaccination with Prevenar, were used. The vaccine was coadministered with the Infanrix Hexa vaccine (GSK). Sera were selected to represent the full range of antibody concentrations with sufficient values around the threshold concentration.
(b) Laboratories and assays.
The testing laboratories in study C were the laboratory at ICH, which used the WHO non-22F ELISA and the WHO 22F ELISA, and the GSK laboratory, which used the GSK 22F ELISA.
(iv) Study D. (a) Serum samples.
Study D involved 78 serum samples from infants from South Africa obtained after vaccination with an investigational CRM197-conjugated nine-valent PCV from Wyeth. The pneumococcal vaccine contained 2 μg of each of the polysaccharides of serotypes 1, 4, 5, 9V, 14, 18C, 19F, and 23F and 4 μg of the polysaccharide of serotype 6B, all of which were independently linked to a total of 20 μg of the nontoxic mutant diphtheria toxin protein (CRM197). Other vaccines were coadministered, i.e., the trivalent oral polio vaccine (Polioral; Biovac), diphtheria-tetanus toxoid and whole-cell pertussis vaccine (DTwP; Sanofi-Pasteur), hepatitis B vaccine (Hepaccine-B; Cheil Foods and Chemicals), and the Haemophilus influenzae type b conjugate vaccine (HibTITER, Wyeth; Vaxem-Hib, Chiron).
(b) Laboratories and assays.
The testing laboratory in study D was the Finnish National Institute for Health and Welfare (THL), which used the THL non-22F ELISA and the 22F ELISA. The serotypes included were 1, 6B, 14, 19F, and 23F (13).
(v) Study E. (a) Serum samples.
Samples that were from a Polish study involving 50 infants vaccinated with Prevenar and 50 infants vaccinated with Synflorix and that covered the full range of antibody levels with sufficient values around the threshold were used. Both vaccines were coadministered with Infanrix Penta and a Hib-meningococcal serogroup C (Hib-MenC) vaccine (Menitorix; GSK).
(b) Laboratories and assays.
The testing laboratory in study E was GSK, which used the WHO non-22F ELISA and the GSK 22F ELISA.
(vi) Study F. (a) Serum samples.
Study F involved samples from 6-week-old (Philippines) and 2-month-old (Israel) unvaccinated infants.
(b) Laboratories and assays.
The testing laboratory in study F was GSK, which used the WHO non-22F ELISA and the GSK 22F ELISA.
Non-22F ELISAs.
The reference WHO non-22F pneumococcal ELISA applied by the ICH and GSK laboratories in this study was performed as described previously (5). The protocol used for the assay was similar to the standard protocol used by Wyeth during the three efficacy trials that established the aggregate threshold of 0.35 μg/ml (17). As specified in the WHO reference protocol (27), microtiter plates were coated with serotype-specific pneumococcal PS (ATCC). Serum samples and standard serum sample 89-SF (U.S. Food and Drug Administration [FDA]) were preadsorbed with 10 μg/ml of inhibiting CWPS (Statens Serum Institut [SSI], Copenhagen, Denmark). Bound antibodies were detected by an alkaline phosphatase-labeled anti-human IgG. The serotype-specific IgG concentrations of the samples were expressed in micrograms per milliliter, on the basis of the standard serum sample 89-SF.
The THL non-22F ELISA was performed as described previously (16).
22F ELISAs.
Several slightly different protocols for the 22F ELISA were used in this study.
(i) GSK 22F ELISA.
The 22F ELISA used by the GSK laboratory was the same as that described previously (5) and is essentially based on the procedure published by Concepcion and Frasch (2), but with some modifications to increase the specificity (5). Briefly, microtiter plates were coated with purified serotype-specific pneumococcal PS sourced from GSK and mixed with methylated human serum albumin (0.5 to 7.5 μg/ml, depending on the PS). Serum samples were diluted in adsorption buffer (phosphate-buffered saline [PBS], 10% fetal calf serum [FCS], 0.1% polyvinyl alcohol) containing 10 μg/ml of CWPS (SSI) and 2 μg/ml of PS of serotype 22F (ATCC) and were incubated overnight at 4°C. The 89-SF reference serum sample (FDA) was diluted in the same adsorption buffer but with 10 μg/ml of CWPS only, and the mixture was incubated overnight at 4°C. An internal reference serum sample, calibrated against standard serum sample 89-SF, was included in every plate. Unlike the working standard serum sample, in line with the WHO recommendations (27), the 89-SF reference serum sample was preadsorbed only with CWPS and not with 22F because the values for the anti-PS concentrations of the 89-SF reference serum sample were assigned without the use of 22F PS. Bound antibodies were detected by using alkaline phosphatase-conjugated anti-human IgG.
In study F, the same protocol described above was applied, except that ATCC PSs and purified GSK PS were compared.
(ii) WHO 22F ELISA.
The 22F ELISA method applied at the ICH laboratory (27) was developed on the basis of the WHO non-22F ELISA described above and used the serotype-specific pneumococcal PS from ATCC to coat the microtiter plates (<10 μg/ml, depending on the PS). Serum samples were diluted in adsorption buffer (PBS, 0.02% NaN3, 0.05% Tween 20) containing 5 μg/ml of CWPS (SSI) and 5 μg/ml of the PS of serotype 22F (ATCC) and were incubated for 30 min at 4°C. The 89-SF reference serum sample (FDA) was diluted in the same adsorption buffer but with 5 μg/ml of CWPS only, and the mixture was incubated for 30 min at 4°C. Bound antibodies were detected by using alkaline phosphatase-conjugated anti-human IgG.
(iii) THL 22F ELISA.
The 22F ELISA applied by THL in Finland was performed as described by Simell et al. (21) and used serotype-specific pneumococcal PS from ATCC to coat the microtiter plates (<10 μg/ml, depending on the PS). Serum samples were diluted 1:100 in PBS-10% FCS (PBS-F) containing 10 μg/ml of CWPS (SSI) and 30 μg/ml of PS of serotype 22F (ATCC) and were incubated overnight at 4°C. The 89-SF reference serum sample (FDA) was diluted in PBS-F containing 10 μg/ml of CWPS only, and the mixture was incubated overnight at 4°C. The bound antibodies were detected by using alkaline phosphatase-conjugated anti-human IgG.
Assay cutoff and LOQs of the ELISAs.
To increase the confidence in the positive/negative status of a serum sample, an assay cutoff value greater than the limit of quantification (LOQ) was used for all ELISAs performed at GSK and was set equal to 0.05 μg/ml. Antibody concentrations below this cutoff were attributed half the cutoff value, i.e., 0.025 μg/ml. For the ELISAs carried out by the THL and ICH laboratories, an LOQ value specific for each PS was used. Antibody concentrations below the LOQs were attributed half the LOQ values.
Statistical analyses.
Sera were selected to have sufficient data close to the threshold values to gain precision on the derived threshold value. However, the full range of antibody titers was represented in the serum sample sets. From a statistical viewpoint, the ideal power was obtained for our comparative studies since the following criteria were met: the value of the threshold of the new assays was derived from the analysis of aggregate reverse cumulative distribution curves (RCDCs) (18), obtained by combining the concentrations of antibodies against multiple serotypes. It is recommended that aggregate RCDCs be drawn by using pediatric sera obtained after vaccination and tested both by the WHO non-22F ELISA and by the new assay. In the context of assay comparison, serum samples were selected in order to guarantee the coverage of a broad range of antibody concentrations (0.05 μg/ml to 15 μg/ml) and to allow comparison of the assays for the most clinically relevant antibody concentrations, especially those around the threshold values. The proportion of serum samples with low antibody concentrations was increased on purpose to allow an appropriate measurement of the rate of agreement between the two laboratories at the low antibody concentrations. It is recommended that a significant number of values be in the range of 0.05 to 0.5 μg/ml. Hence, the antibody concentration in the serum samples selected should not necessarily be representative of the immune response in the vaccinated population.
The new threshold was calculated by comparing the percentage of serum samples with an antibody concentration above the 0.35-μg/ml threshold, as measured by the WHO non-22F ELISA, to the RCDC obtained with the new assay.
When the results obtained by two different laboratories were compared, the statistical analyses included the concordance and the correlation. Study of the concordant pairs of antibody concentrations below or above the respective thresholds determined the percent agreement. In addition, the coefficient of correlation and the accuracy were used to measure the correlation between the two laboratories. The concordance correlation coefficient (CCC) is the product of the correlation and the accuracy (12).
RESULTS
Comparison of GSK 22F ELISA and WHO non-22F ELISA performed at two different laboratories with serum samples from infants immunized with 11-valent PCV (study A).
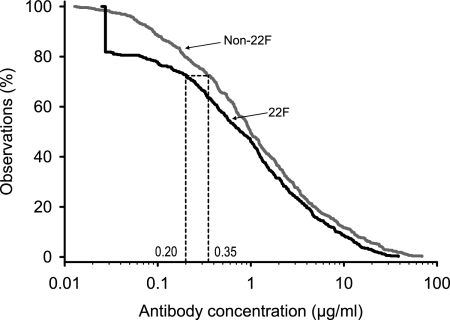
In order to confirm the observations made by using a set of 30 serum samples from Europe (5), an additional set of 40 serum samples from Latin America was evaluated, and the results for those 40 samples were analyzed together with those for the 30 samples from the original study. The WHO non-22F ELISA was carried out at ICH and the GSK 22F ELISA was carried out at the GSK laboratory with all 70 serum samples, as described previously (5). The range of antibody concentrations for the serum samples was from <0.05 to 39 μg/ml. The aggregate RCDCs of the antibody responses across serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F for both ELISAs are presented in Fig. 1. At antibody concentrations of >1 μg/ml, the curves were almost parallel, with the GSK 22F ELISA curve running underneath the WHO non-22F ELISA curve. At antibody concentrations of <1 μg/ml, a greater divergence occurred: the curve for the GSK 22F ELISA shifted farther toward lower concentrations. Antibody concentrations of ≥0.35 μg/ml were seen in 72% of the cases by the non-22F assay, while the corresponding concentration in the GSK 22F ELISA was 0.20 μg/ml.
FIG. 1.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in the sera of infants from Europe and Latin America after immunization with a pneumococcal conjugate vaccine for comparison of the GSK 22F ELISA and the WHO non-22F ELISA (study A). Aggregate reverse cumulative distribution curves were plotted by using the concentrations of antibodies against pneumococcal serotype 4, 6B, 9V, 14, 18C, 19F, and 23F polysaccharides.
Evaluation of the GSK 22F ELISA with samples from infants immunized with Prevenar or a control vaccine (study B).
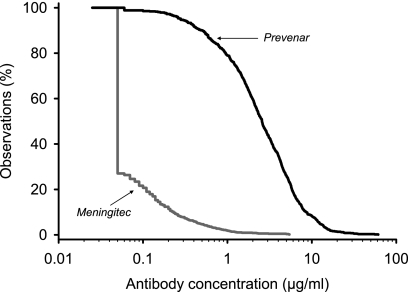
The GSK 22F ELISA was evaluated with pediatric sera obtained after the primary immunization with Prevenar or the control vaccine, Meningitec, to compare the vaccine response with the remaining natural antibody background in nonimmunized infants. The distribution of antibody concentrations after vaccination was analyzed by the use of RCDCs. The aggregate RCDCs across the seven anti-PSs for the GSK 22F ELISA showed that approximately 10% of the control infants vaccinated with Meningitec had a concentration of antibody to pneumococcal PS of >0.20 μg/ml (Fig. 2).
FIG. 2.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in pediatric sera after immunization with the Prevenar or the Meningitec vaccine by using the GSK 22F ELISA (study B).
Comparison of GSK 22F ELISA and WHO non-22F ELISA with samples from infants immunized with Prevenar (study C).
The bridging of the GSK 22F ELISA with the WHO non-22F ELISA was further extended by the analysis of serum samples from Prevenar-vaccinated infants. For that purpose, 118 serum samples obtained from infants after the primary vaccination were tested for the seven serotypes in the Prevenar vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, 23F) in a blinded manner at ICH by the WHO non-22F ELISA and at the GSK laboratory by the GSK 22F ELISA. As before, samples were selected in order to cover a broad range of antibody concentrations and to include a high number of samples with concentrations around the thresholds.
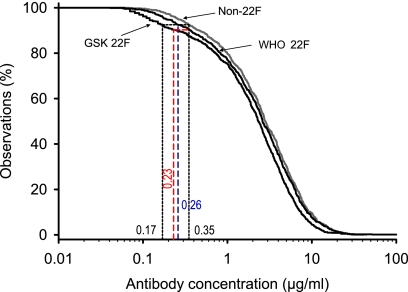
The distribution of antibody concentrations after Prevenar vaccination was analyzed by the use of RCDCs (Fig. 3). The aggregate RCDC for the GSK 22F ELISA ran below the curve calculated for the WHO non-22F assay. The two curves were almost parallel down to a concentration of 1 μg/ml. The difference became more pronounced at antibody concentrations of <1 μg/ml. The comparison of the area of low antibody concentrations confirmed that the proposed threshold concentration of 0.35 μg/ml (93% of the samples had concentrations above this threshold concentration) determined by the WHO non-22F ELISA corresponds to the concentration of 0.17 μg/ml obtained by the GSK 22F ELISA.
FIG. 3.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in pediatric sera after immunization with the Prevenar vaccine for comparison of the WHO non-22F ELISA and the WHO 22F ELISA, both of which were performed at ICH, with the GSK 22F ELISA, which was performed at GSK (study C). In addition to our standard comparison of the WHO non-22F ELISA (threshold, 0.35 μg/ml) with the two 22F ELISAs (GSK 22 [dotted lines] and WHO 22F [blue dashed line]), a comparison of the WHO 22F ELISA (by use of a 0.35-μg/ml threshold) and the GSK 22F ELISA was also performed (red dashed line).
Statistical analyses were performed to compare the results obtained by the two laboratories. As was observed in the previous comparisons, a very good correlation and a high degree of concordance between the two assays were observed for all serotypes when the respective thresholds were used (Table 2 and Table 3). The level of agreement reached 100% for some serotypes, and the correlation between the two methodologies in the two different laboratories was high, ranging from 0.85 for serotype 6B to 0.96 for serotypes 4 and 19F. The accuracy, which evaluates how far the regression line is from the perfect concordance line, was between 0.87 and 1.00; and the concordance correlation coefficient, the product of concordance and accuracy, was largely greater than 0.8 (except that it was 0.74 for serotype 6B), which indicates the validity of the comparison of the two tests.
TABLE 2.
Analysis of concordance of GSK 22F ELISA and WHO non-22F ELISA performed in two different laboratories (study C)
| Serotype | Concordancea | % Agreement (95% CI)b |
|---|---|---|
| 14 | 118/118 | 100 (96.92-100) |
| 18C | 114/118 | 96.61 (91.55-99.07) |
| 19F | 118/118 | 100 (96.92-100) |
| 23F | 110/118 | 93.22 (87.08-97.03) |
| 4 | 117/118 | 99.15 (95.37-99.98) |
| 6B | 103/118 | 87.29 (79.9-92.71) |
| 9V | 115/118 | 97.46 (92.75-99.47) |
Concordance is the number of samples positive at both laboratories plus the number of samples negative at both laboratories divided by the total number of samples (positivity thresholds, 0.35 μg/ml for WHO non-22F ELISA and 0.20 μg/ml for GSK 22F ELISA).
% Agreement, concordance expressed in percent; CI, confidence interval.
TABLE 3.
Analysis of correlation of GSK 22F ELISA and WHO non-22F ELISA (study C)
| Serotype | ra | 95% CIb |
Accuracy | Concordance correlation coefficient | |
|---|---|---|---|---|---|
| LL | UL | ||||
| 4 | 0.96 | 0.93 | 0.97 | 0.98 | 0.94 |
| 6B | 0.85 | 0.76 | 0.91 | 0.87 | 0.74 |
| 9V | 0.93 | 0.90 | 0.95 | 1.00 | 0.93 |
| 14 | 0.94 | 0.91 | 0.96 | 0.87 | 0.82 |
| 18C | 0.92 | 0.89 | 0.95 | 1.00 | 0.92 |
| 19F | 0.96 | 0.94 | 0.97 | 0.94 | 0.89 |
| 23F | 0.90 | 0.86 | 0.93 | 0.93 | 0.84 |
r, coefficient of correlation (precision), calculated on the basis of the numbers of positive results by both assays (positivity thresholds, 0.35 μg/ml for WHO non-22F ELISA and 0.20 μg/ml for GSK 22F ELISA). Lin's CCC is the product of the correlation (r) and the accuracy (12).
CI, confidence interval; LL, lower limit; UL, upper limit.
Comparison of WHO 22F ELISA and WHO non-22F ELISA with samples from infants immunized with Prevenar (study C).
To exclude any interlaboratory effect, a comparison of the 22F and non-22F assays was run by the same laboratory. This comparison was conducted by the WHO laboratory, which used the 118 samples from the German Prevenar vaccinees described earlier. The sera were tested for the seven serotypes in the Prevenar vaccine by the WHO non-22F ELISA and the WHO 22F ELISA in a blinded manner. This additional comparison confirmed the results presented above, showing that the aggregate RCDC for the WHO 22F ELISA ran below the curve for the WHO non-22F assay (Fig. 3). The two curves were mostly parallel, but a slight divergence occurred around the threshold levels, with the consequence being that the threshold of 0.26 μg/ml achieved when the WHO 22F ELISA was used was shown to be equivalent to the threshold of 0.35 μg/ml achieved when the WHO non-22F ELISA was used.
Comparison of WHO 22F ELISA and GSK 22F ELISA with samples from infants immunized with Prevenar (study C).
A similar exercise was performed to compare the 0.35-μg/ml threshold for the WHO 22F ELISA (although the original threshold of 0.35 μg/ml for the WHO assay was derived by the use of a non-22F ELISA) with an equivalent threshold for the GSK 22F ELISA. When the 0.35-μg/ml threshold from the RCDC established with the WHO 22F ELISA was compared to the RCDC established with the GSK 22F ELISA, the related threshold was 0.23 μg/ml (dashed red line in Fig. 3).
Comparison of THL 22F and non-22F ELISAs with infant samples after vaccination with an investigational nine-valent PCV (study D).
To define whether the 22F inhibition effect could be generalized, samples from an African population that included samples from HIV-positive and HIV-negative children were chosen. A recent publication (13) described a nested study in the phase III trial that evaluated the efficacy of an experimental nine-valent pneumococcal CRM197 conjugate vaccine (Wyeth), which was conducted in South Africa. Samples from that study were analyzed by both the 22F ELISA and the non-22F ELISA at THL (13).
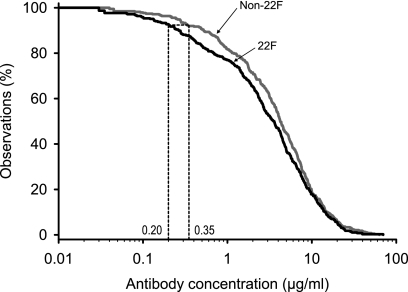
A total of 78 samples were tested for five serotypes (serotypes 1, 6B, 14, 19F, and 23F). For the five anti-PSs, the aggregate RCDC calculated for the THL 22F ELISA was lower than the distribution curve calculated for the non-22F ELISA (Fig. 4). Since the study was conducted with samples from HIV-positive and -negative children, two threshold values, one prepared with and one prepared without the data for the HIV-positive children, were calculated. Both values were <0.35 μg/ml (0.21 and 0.19 μg/ml, respectively) for the 22F ELISA. The RCDC was compiled by combining all data, and a threshold value of 0.20 μg/ml was observed.
FIG. 4.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in pediatric sera after immunization with a nine-valent pneumococcal conjugate vaccine for comparison of the THL non-22F ELISA and 22F ELISA (study D) by using the concentration of antibodies against pneumococcal serotypes 1, 6B, 14, 19F, and 23F. Data for two groups of vaccinated children, HIV-infected children (n = 20) and non-HIV-infected children (n = 58), were pooled. The data from both groups combined were used to prepare the RCDCs.
Comparison of GSK 22F ELISA and WHO non-22F ELISA performed at the GSK laboratory with samples from infants immunized with Prevenar or Synflorix (study E).
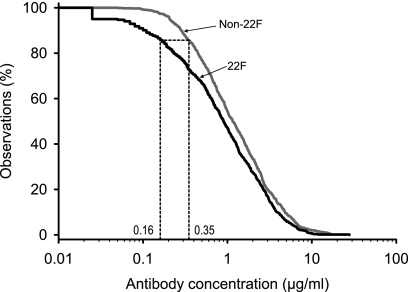
Another comparison of the 22F and non-22F ELISAs with the goal of excluding any interlaboratory effect was performed by the GSK laboratory with samples from both Prevenar- and Synflorix-vaccinated infants. In this study, 100 serum samples from Polish infants (50 vaccinated with Prevenar and 50 vaccinated with Synflorix) were evaluated at the GSK laboratory by use of the WHO non-22F ELISA (for which the ATCC PSs were used) and the GSK 22F ELISA. The aggregate RCDC was lower for the GSK 22F ELISA than for the WHO non-22F assay (Fig. 5). A threshold of 0.16 μg/ml for the GSK 22F ELISA was shown to be equivalent to the threshold of 0.35 μg/ml obtained with the WHO non-22F ELISA.
FIG. 5.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations in pediatric sera measured after immunization with the Synflorix or the Prevenar vaccine for comparison of the WHO non-22F ELISA and the GSK 22F ELISA, both of which were performed at the GSK laboratory (study E).
Coating of ELISA plates with purified PS increases assay specificity (study F).
Frequent explanations for the lack of assay specificity are the varying quality and the inconsistency of the coating antigens. In an attempt to further improve the specificity of the 22F ELISA, the effect of the purified PS used to coat the ELISA plates was investigated with sera from unvaccinated children. The non-22F and 22F ELISAs were carried out after coating of the plates with PSs from ATCC or with PSs purified at the GSK laboratory. Geometric mean ratios were calculated for the IgG antibody concentrations measured by each assay (Table 4). A ratio of 1 would suggest no difference between the two measurements. The ratio for the 22F ELISA antibody/non-22F ELISA antibody was generally below 1, independent of the source of PS used for the coating. With both the non-22F and the 22F ELISAs, coating with purified PS yielded lower ELISA antibody values (ratio, <1) for all PSs but one (the serotype 14 PS). Since unspecific contamination of PSs may vary among different ATCC vaccine lots (30), the specificities of both the non-22F and the 22F ELISAs and the effectiveness of the preadsorbance with PS can be improved by using purified PS for plate coating.
TABLE 4.
Antipneumococcal antibody concentrations measured by non-22F and 22F ELISAs with ATCC PSs or GSK purified PSs (study F)a
| Serotype | No. of serum samples | ATCC PS |
GSK PS |
GSK PS/ATCC PS |
|||||
|---|---|---|---|---|---|---|---|---|---|
| GMC (μg/ml) |
GMR (1) | GMC (μg/ml) |
GMR (2) | GMR (3) | GMR (4) | ||||
| Non-22F | 22F | Non-22F | 22F | ||||||
| 4 | 21 | 0.37 | 0.26 | 0.70 | 0.13 | 0.08 | 0.62 | 0.35 | 0.31 |
| 6B | 23 | 0.48 | 0.3 | 0.63 | 0.31 | 0.26 | 0.84 | 0.65 | 0.87 |
| 9V | 26 | 0.31 | 0.28 | 0.90 | 0.19 | 0.22 | 1.16 | 0.61 | 0.79 |
| 14b | 26 | 1.22 | 1.15 | 0.94 | 1.97 | 1.44 | 0.73 | 1.61 | 1.25 |
| 18C | 32 | 0.58 | 0.42 | 0.72 | 0.48 | 0.37 | 0.77 | 0.83 | 0.88 |
| 19F | 20 | 1.66 | 1.13 | 0.68 | 1.23 | 1.03 | 0.84 | 0.74 | 0.91 |
| 23F | 23 | 0.71 | 0.43 | 0.61 | 0.63 | 0.39 | 0.62 | 0.89 | 0.91 |
GMC, geometric mean concentration; GMR, geometric mean of the individual ratios; (1), 22F ATTC PS/non-22F ATTC PS; (2), 22F GSK PS/non-22F GSK PS; (3), non-22F GSK PS/non-22F ATTC PS; (4), 22F GSK PS/22F ATTC PS.
Further experiments showed that the addition of polyvinyl alcohol to the dilution buffer had a clear impact on the specificity of the ELISA for serotype 14 (geometric mean of the individual ratios, 0.64) without affecting the assay for the other serotypes (geometric mean of the individual ratios range, 0.79 to 1.01), which prompted the use of polyvinyl alcohol in the protocol for the 22F ELISA.
DISCUSSION
To show that a new pneumococcal conjugate vaccine is equivalent to a vaccine with demonstrated efficacy, the primary purpose, from a regulatory perspective, should be to use the comparative serological responses to a threshold antibody concentration for those serotypes in common. The chosen threshold cannot be considered a universal protective antibody cutoff for several reasons, the first one being that the use of a single antibody threshold is based upon a series of simplifying assumptions (8, 20). In addition, the threshold value would be valid only for invasive pneumococcal disease, and it is known that different serotypes require antibody at concentrations more or less than the threshold to protect individuals against invasive disease. Finally, the universal protective threshold value of 0.35 μg/ml proposed by the WHO was based upon a meta-analysis of efficacy data from three efficacy trials in which the estimates of the antibody concentrations for the percent efficacy found for each specific trial were widely divergent (20). Concerning this point, the rationale for pooling the data was that the 95% confidence intervals overlapped, but each confidence interval was very broad, essentially being from nearly 0 to 100%. The original 0.20-μg/ml threshold proposed by Jódar et al. (8) was based on the Kaiser Permanente PCV7 efficacy trial (1), which aligned with the observed efficacy and in which the control group showed a low rate of seropositivity.
In the present work, the 0.20-μg/ml threshold for the 22F ELISA, first described elsewhere (5) as being equivalent to the WHO non-22F ELISA 0.35-μg/ml threshold, was confirmed in an extended study with samples from German and Latin American infants vaccinated with an experimental 11-valent candidate pneumococcal vaccine. Another bridging of the GSK 22F ELISA with the WHO non-22F ELISA was performed with pediatric serum samples after vaccination with the licensed heptavalent vaccine to make a link with vaccine efficacy. The same samples were tested by the GSK 22F ELISA (performed at the GSK laboratory) and the WHO non-22F ELISA (performed at ICH). At low antibody concentrations, the analysis showed that the proposed threshold of 0.35 μg/ml, as determined with the WHO non-22F ELISA, corresponded to 0.17 μg/ml when the GSK 22F ELISA was used. These results are in line with those from previous comparisons performed with sera from infants immunized with an experimental 11-valent formulation. The fact that the present study, which used sera from Prevenar vaccinees, led to the same conclusion rules out any bias in favor of one or the other vaccine. In addition, it confirmed the validity of the 0.20-μg/ml threshold when the GSK 22F ELISA was used for both a new candidate vaccine and the Prevenar vaccine. These comparisons were performed with well-validated assays which allowed the conclusion that the observed differences are not due to interlaboratory variability but, rather, are the result of the improved ELISA specificity due to the preadsorption of sera with 22F. Indeed, the generation of lower threshold values after addition of 22F is known to reflect increased specificity, as determined in previous studies (2, 5).
To further illustrate the vaccine- and laboratory-independent nature of 22F inhibition, both the 22F and the non-22F ELISAs were carried out in a third independent laboratory (THL) with samples from South African infants vaccinated with an investigational nine-valent PCV. Again, an equivalent threshold of about 0.20 μg/ml was found when the measurements were made with the 22F ELISA and compared with those obtained by the non-22F ELISA, which had a threshold of 0.35 μg/ml. Surprisingly, Siber et al. (20) did not find a similar effect of 22F with sera from South Africa originating from the same study, but only a very few serum samples from South Africa were tested. Technical differences between the WHO laboratory and the THL laboratory may account for this discrepancy. At THL, where ATCC polysaccharides are used, a more vigorous adsorption step (30 μg/ml 22F with overnight incubation) was found to be necessary to increase specificity. The WHO 22F ELISA uses 5 μg/ml 22F and 30 min of incubation.
Although statistical analyses excluded any interlaboratory effect when the WHO non-22F assay was compared with the GSK 22F assay, it was nevertheless decided that the same 118 serum samples from Prevenar vaccinees would be used to evaluate a 22F ELISA performed at the WHO reference laboratory. This allowed a more direct comparison of the two assays, as they were performed in the same laboratory. The WHO 22F ELISA protocol differs from the GSK 22F ELISA protocol mainly in that it uses ATCC PSs and methylated human serum albumin is not implemented during the coating procedure. The results obtained with the WHO 22F ELISA defined an aggregate threshold at 0.26 μg/ml to be equivalent to the 0.35-μg/ml value obtained by the WHO non-22F ELISA, again highlighting the effect of 22F. With the same serum samples, the GSK 22F ELISA led to an equivalent threshold at 0.17 μg/ml, similar to previous findings (5). This shows that, in addition to 22F, the specificity of the assay is increased by using an improved coating procedure that makes use of purified PS. This aspect was also evidenced by direct comparison of the two 22F ELISAs: although the WHO noninferiority threshold of 0.35 μg/ml was derived with the Wyeth non-22F ELISA, for completion of the analysis, we also compared the WHO 22F ELISA with a threshold at 0.35 μg/ml for equivalence to the GSK 22F ELISA, which resulted in equivalence at 0.23 μg/ml.
The claim of higher specificity primarily refers to experiments comparing the susceptibilities of 22F and non-22F assays to homologous or heterologous PS inhibition, as described by Concepcion and Frasch (2) and Henckaerts et al. (5). In the latter paper, serum samples from different sources were analyzed pairwise for anti-serotype 4 PS antibodies as an example after preincubation without or with homologous serotype 4 PS and different heterologous PSs (serotypes 6B, 9V, 14, 18C, 19F, and 23F). The 22F PS improved the specificity of the ELISA, as demonstrated by a reduction in the nonspecific inhibition by heterologous PSs (5). As said before, the purity of the PSs used for coating is another factor that affects the specificity of an assay. Although a direct comparison of the purities of the GSK PSs and the ATCC PSs has not been performed, indirect evidence of the higher purity of GSK PS has been obtained by demonstrating that cross-reactive antibodies from nonimmunized infants showed almost no binding to the serotype 6B PS from GSK but showed high-level binding to the PS from ATCC (24). A different approach to the testing of assay specificity depending on the source of PSs is illustrated in the present study by comparison of the antipneumococcal antibody concentrations obtained after coating with ATCC PS and those obtained after coating with GSK PSs. With the latter PSs, the antibody concentrations were consistently lower, indicating that the GSK PSs had better assay specificity than the ATCC PSs, while assay performance, as calculated from the correlation, accuracy, and CCC, were satisfactory (Table 3), excluding a loss of sensitivity.
When a decreased threshold for a 22F ELISA is established, it is important to ensure that this does not artificially increase the number of vaccine responders. To this end, nonvaccinated children were examined at the age when vaccinated children were evaluated for their antibody responses after they received a conjugate vaccine. Low proportions of sera with antibody concentrations of >0.20 μg/ml were demonstrated when they were measured by the GSK 22F ELISA. The low level of seropositivity for the controls was comparable to the percentage of antibody concentrations of >0.35 μg/ml shown by the WHO non-22F ELISA (20).
As stated previously, the purpose of the addition of 22F PS preadsorption was to more selectively measure antibodies with serotype specificity and in so doing improve the correlation between the antibody concentration and opsonic activity (2). The mechanism by which the 22F PS is able to increase the serotype specificity was recently examined by Skovsted et al. (22). They identified the active component in the 22F PS preparation as cell wall polysaccharides containing two phosphocholines per pentasaccharide repeat unit. This type of cell wall polysaccharide is referred to as CWPS2, whereas CWPS defines the prominent type with one phosphocholine per pentasaccharide repeat. CWPS2 was purified from a pneumococcal strain that expressed this form of cell wall polysaccharide. Both the CWPS and the CWPS2 moieties contained unique epitopes, and adsorption with both was needed to achieve the serotype specificity obtained by adsorption with CWPS plus the 22F PS. Interestingly, they found that adsorption by CWPS plus CWPS2 could replace the need for 22F adsorption, but this observation needs to be replicated in other laboratories.
There has been some confusion as to the need for 22F adsorption for the analysis of pediatric serum samples because the added common epitope contained in the 22F PS is a carbohydrate epitope. The ability of an individual to respond to carbohydrate epitopes matures with age, and infants do not normally make antibodies to PS epitopes in their native state, but they do so if the PS is conjugated to a protein carrier molecule, such as that in a conjugate vaccine. Thus, infants make little or no natural antibody to the common epitope contained in the 22F PS from ATCC, while somewhat older children and adults make high levels of antibody to this epitope. In an extended immunization schedule (at 2, 4, and 6 months of age), sera obtained from infants from the Northern California Kaiser trials after immunization with a conjugate vaccine were not affected by the added 22F adsorption step (1, 20). It now appears that the rate of development of antibodies to the common epitope found in the 22F PS is related to the level of exposure to the pneumococcus, as well as to remaining maternal antibodies. In many developing countries, pneumococcal carriage rates are very high (6). In such cases, there is a demonstrable effect of the added 22F adsorption step. This was found by Madhi et al. (13). They found that, on average, use of the added 22F adsorption step reduced the anti-PS antibody concentrations in sera from South African infants after immunization with a pneumococcal conjugate vaccine by 30% over that achieved with CWPS alone.
In conclusion, several comparisons involving the WHO reference laboratory at ICH, the THL laboratory, and the GSK laboratory were performed with sera from subjects from Latin America, South Africa, and Europe who had been immunized with one of four different vaccines: the 7-valent Prevenar vaccine (Wyeth), a 9-valent conjugate vaccine (Wyeth), the 10-valent Synflorix vaccine (GSK), or an 11-valent PCV (GSK). Different assays in different laboratories may lead to different thresholds, but all comparisons led to the same conclusion that the threshold, which is 0.35 μg/ml for the WHO non-22F assay, is lower when any 22F ELISA is used. The results of multiplex assays, such as the Luminex immunoassay (15), appear to confirm the possibility that the specificity of an antibody assay may be increased over that of the current WHO reference assay.
The present study confirms previous observations (5). The comparisons were performed by using well-validated assays and included all the appropriate controls, which allowed us to conclude that the differences observed were not due to either interlaboratory variability or the vaccine or the population used but were the result of the improved specificity of the ELISA due to 22F adsorption and the use of highly purified polysaccharides.
Acknowledgments
We thank Martine Van Campenhout for her excellent technical support. We are grateful to Ulrike Krause and Pascal Cadot for writing and editorial assistance. We thank Cédric Bievelet and Angélique Baclin for the statistical analyses. We are also grateful to Lindsay Ashton and David Goldblatt from the WHO Reference Laboratory for performance, under contract, of the various blinded ELISAs that formed an essential part of our studies.
S.A.M. received research support from Wyeth and has served on the speakers bureau for Wyeth and GSK. H.K. has provided consultancies on advisory boards for GlaxoSmithKline; has had travel paid by GlaxoSmithKline, Wyeth Vaccines, and Novartis as an invited speaker or expert at symposia; and has received honoraria from GlaxoSmithKline. C.E.F. has participated on advisory boards for GlaxoSmithKline and Novartis and has received honoraria from GlaxoSmithKline and Novartis. J.T.P., P.L., and I.H. are employees of GlaxoSmithKline.
Footnotes
Published ahead of print on 4 November 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, and Northern California Kaiser Permanente Vaccine Study Center Group. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feavers, I., I. Knezevic, M. Powell, and E. Griffiths. 2009. Challenges in the evaluation and licensing of new pneumococcal vaccines, 7-8 July 2008, Ottawa, Canada. Vaccine 27:3681-3688. [DOI] [PubMed] [Google Scholar]
- 4.Fischer, W., T. Behr, R. Hartmann, J. Peter-Katalinic, and H. Egge. 1993. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur. J. Biochem. 215:851-857. [DOI] [PubMed] [Google Scholar]
- 5.Henckaerts, I., D. Goldblatt, L. Ashton, and J. Poolman. 2006. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin. Vaccine Immunol. 13:356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill, P. C., A. Akisanya, K. Sankareh, Y. B. Cheung, M. Saaka, G. Lahai, B. M. Greenwood, and R. A. Adegbola. 2006. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin. Infect. Dis. 43:673-679. [DOI] [PubMed] [Google Scholar]
- 7.Inostroza, J., S. Villanueva, K. Mason, L. E. Leiva, and R. U. Sorensen. 2005. Effects of absorption with pneumococcal type 22F polysaccharide on maternal, cord blood, and infant immunoglobulin G antipneumococcal polysaccharide antibodies. Clin. Diagn. Lab. Immunol. 12:722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jódar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson, C., P.-E. Jansson, and U. B. Skov Sørensen. 1999. The pneumococcal common antigen C-polysaccharide occurs in different forms. Mono-substituted or di-substituted with phosphocholine. Eur. J. Biochem. 265:1091-1097. [DOI] [PubMed] [Google Scholar]
- 10.Klugman, K. P., F. Cutts, R. A. Adegbola, S. Black, S. A. Madhi, K. L. O'Brien, M. Santosham, H. Shinefield, and J. A. C. Sterne. 2008. Meta-analysis of the efficacy of conjugate vaccines against invasive pneumococcal disease, p. 317-326. In G. R. Siber, K. P. Klugman, and P. H. Makela (ed.), Pneumococcal vaccines: the impact of conjugate vaccine. ASM Press, Washington, DC.
- 11.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, N. Pierce, and Vaccine Trialists Group. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 12.Lin, L. I. 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255-268. [PubMed] [Google Scholar]
- 13.Madhi, S. A., L. Kuwanda, C. Cutland, A. Holm, H. Käyhty, and K. P. Klugman. 2005. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr. Infect. Dis. J. 24:410-416. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355-361. [DOI] [PubMed] [Google Scholar]
- 15.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 16.Puumalainen, T., M. R. Zeta-Capeding, H. Käyhty, M. G. Lucero, K. Auranen, O. Leroy, and H. Nohynek. 2002. Antibody response to an eleven valent diphtheria- and tetanus-conjugated pneumococcal conjugate vaccine in Filipino infants. Pediatr. Infect. Dis. J. 21:309-314. [DOI] [PubMed] [Google Scholar]
- 17.Quataert, S. A., C. S. Kirch, L. J. Quackenbush Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed, G. F., B. D. Meade, and M. C. Steinhoff. 1995. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96:600-603. [PubMed] [Google Scholar]
- 19.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 20.Siber, G. R., I. Chang, S. Baker, P. Fernsten, K. L. O'Brien, M. Santosham, K. P. Klugman, S. A. Madhi, P. Paradiso, and R. Kohberger. 2007. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 25:3816-3826. [DOI] [PubMed] [Google Scholar]
- 21.Simell, B., M. Lahdenkari, A. Reunanen, H. Käyhty, and M. Väkeväinen. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skovsted, I. C., M. B. Kerrn, J. Sonne-Hansen, L. E. Sauer, A. K. Nielsen, H. B. Konradsen, B. O. Petersen, N. T. Nyberg, and J. Ø. Duus. 2007. Purification and structure characterization of the active component in the pneumococcal 22F polysaccharide capsule used for adsorption in pneumococcal enzyme-linked immunosorbent assays. Vaccine 25:6490-6500. [DOI] [PubMed] [Google Scholar]
- 23.Soininen, A., M. Karpala, S.-L. Wahlman, H. Lehtonen, and H. Käyhty. 2002. Specificities and opsonophagocytic activities of antibodies to pneumococcal capsular polysaccharides in sera of unimmunized young children. Clin. Diagn. Lab. Immunol. 9:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soininen, A., G. van den Dobbelsteen, L. Oomen, and H. Käyhty. 2000. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin. Diagn. Lab. Immunol. 7:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Käyhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkelstein, J. A. 1981. The role of complement in the host's defense against Streptococcus pneumoniae. Rev. Infect. Dis. 3:289-298. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2004. Training manual for enzyme-linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA) (WHO13). World Health Organization, Geneva, Switzerland. http://www.vaccine.uab.edu/. Accessed 4 December 2009.
- 28.World Health Organization. 2005. Recommendations for the production and control of pneumococcal conjugate vaccines. WHO Tech. Rep. Ser. 927(Annex 2):64-98. [Google Scholar]
- 29.Xu, Q., C. Abeygunawardana, A. S. Ng, A. W. Sturgess, B. J. Harmon, and J. P. Hennessey, Jr. 2005. Characterization and quantification of C-polysaccharide in Streptococcus pneumoniae capsular polysaccharide preparations. Anal. Biochem. 336:262-272. [DOI] [PubMed] [Google Scholar]
- 30.Yu, J., D. E. Briles, J. A. Englund, S. K. Hollingshead, W. P. Glezen, and M. H. Nahm. 2003. Immunogenic protein contaminants in pneumococcal vaccines. J. Infect. Dis. 187:1019-1023. [DOI] [PubMed] [Google Scholar]
- 31.Yu, X., Y. Sun, C. Frasch, N. Concepcion, and M. H. Nahm. 1999. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]