Abstract
Johne's disease, a chronic enteritis of ruminants, is caused by infection with Mycobacterium avium subsp. paratuberculosis. Three distinct forms have been observed in sheep: paucibacillary disease (PB), multibacillary disease (MB), and asymptomatic infection (AS). In this study, immune parameters for animals naturally infected with M. avium subsp. paratuberculosis and identified postmortem as having PB, MB, or AS were compared to provide a further understanding of the immunological reactivity contributing to or resulting from these different disease states in sheep. PB was associated with strong ex vivo M. avium subsp. paratuberculosis antigen-stimulated gamma interferon responses, pronounced increases in CD25+ T-cell frequencies in circulation, antibody production, and a B-cell population that expanded significantly upon ex vivo antigenic stimulation. The MB group featured the highest antibody levels and a lack of cellular immune responsiveness to the M. avium subsp. paratuberculosis antigen. The AS group expressed an immunological phenotype intermediate between that for noninfected control animals and that for the PB group. The relationship between immune responses and disease severity within the PB group was investigated more closely; significant positive correlations were observed between disease severity and both the CD8+ population in the circulating blood and the expression of interleukin-4 mRNA in antigen-stimulated blood samples ex vivo. Together, these data point toward distinct immune profiles in sheep that correspond to different Johne's disease states, which can be determined from circulating blood and/or from localized intestinal tract tissue samples.
Paratuberculosis, or Johne's disease (JD), has emerged over the last 2 decades as an economically important disease in farmed ruminants, including cattle (16), sheep (5), goats (8), and deer (19). The infectious agent is Mycobacterium avium subsp. paratuberculosis. Clinical disease in ruminants features chronic weight loss, typically associated with diarrhea, which is due to the inflammation of M. avium subsp. paratuberculosis-infected tissues of the intestinal tract epithelium and submucosa (3), which leads to the loss of tissue integrity and protein malabsorption. The typical pathology observed at necropsy includes thickening and corrugation of the intestinal epithelia and enlargement of draining lymphatic ducts and the associated lymph nodes (7).
The incidence of ovine paratuberculosis has been estimated to be 0.9 to 1.3% per annum among farmed sheep in New Zealand (20) and 5 to 15% per annum in Australia (24). It is difficult to accurately determine the true prevalence of Johne's disease due to the subclinical disease state (which can last for several years) and limitations in the available diagnostic techniques. No definitive symptoms or immune markers are found in all clinically and subclinically affected animals. Immunodiagnosis is further complicated by the fact that paratuberculosis has two immunologically distinct forms: multibacillary (MB) and paucibacillary (PB).
In ruminants, the cell-mediated immune (CMI) responses that are predominant in animals with paucibacillary disease are thought to be directed primarily by type 1 CD4+ T cells (6, 7). These are associated with the increased production of Th1 cytokines, such as gamma interferon (IFN-γ), by peripheral blood mononuclear cells and lymph node and intestinal lymphocytes, but they also comprise circulating antibodies directed against M. avium subsp. paratuberculosis antigens (2, 3, 18, 21, 25, 26). In contrast, the CMI responses that are predominant in ruminants with multibacillary disease are thought to be directed primarily by type 2 CD4+ T cells (6, 7). Multibacillary disease typically features high circulating antibody titers, higher levels of Th2 cytokine expression in ileal lesions and peripheral blood mononuclear cells (10, 26, 28), and a downregulated type 1 CMI response in the ileum and the mesenteric lymph nodes (2, 17, 26, 28). However, these data are predominantly derived from studies of bovine paratuberculosis, in which the disease typically progresses from a subclinical phase through a paucibacillary phase during early disease to multibacillary paratuberculosis at the severe end point of disease. By comparison, ovine paucibacillary and multibacillary diseases are generally thought to be distinct, separate forms of the end point of disease (26).
The subclinical or asymptomatic presentation of early-stage M. avium subsp. paratuberculosis infection also differs between ruminant species. In many cattle with subclinical M. avium subsp. paratuberculosis infection, there are obvious histopathological changes to the intestinal tract tissues which indicate disease (28), whereas in sheep, those animals diagnosed with subclinical/asymptomatic JD have a normal intestinal tract histology and no lesions. Subclinically infected cattle feature an inherent proinflammatory gene expression profile in peripheral blood mononuclear cells, although this is downregulated in blood cells that are stimulated ex vivo with M. avium subsp. paratuberculosis recall antigens, concurrent with an increased level of expression of the anti-inflammatory cytokine interleukin-10 (IL-10) (9, 11). In cattle, IL-10-secreting regulatory T cells have been hypothesized to be stimulated by M. avium subsp. paratuberculosis to limit proinflammatory type 1 protective responses (12); however, in sheep there is little evidence for the modified expression of IL-10 during asymptomatic disease (26).
Since the CMI responses presenting during different forms of ovine paratuberculosis remain to be fully described, the aim of the study described here was to further characterize the immune parameters associated with the asymptomatic, paucibacillary, or multibacillary disease forms occurring in naturally infected sheep. The immune markers present in the blood and draining lymph nodes were monitored at the time of sampling; additionally, the immune responses in mononuclear cells ex vivo upon stimulation with M. avium subsp. paratuberculosis antigen were assessed. The objective was to achieve a better understanding of the CMI responses across the disease spectrum in sheep.
MATERIALS AND METHODS
Experimental animals.
Sheep suspected of having JD were selected from two farms in the Southland/Otago region of southern New Zealand that have an ongoing history of chronic JD. Sheep from a third farm with suspected JD were tested for blood responses to Johnin purified protein derivative (PPDj) by the use of current ovine immunodiagnostic tests (IFN-γ enzyme-linked immunosorbent assay [ELISA] and antibody ELISA), and those animals with high PPDj-specific responses were selected as being potentially infected. Control animals were selected from the AgResearch facility, Invermay, New Zealand, which are free from M. avium subsp. paratuberculosis infection. In order to examine tissues, animals were euthanized humanely by the intravenous administration of sodium pentobarbitone or stunning with a captive bolt gun followed by exsanguination. All procedures were conducted according to the Invermay AgResearch Animal Ethics Committee (approval number 10500 and Animal Ethics Tissue Collection number 17).
Histology.
At necropsy, 1- to 2-cm tissue samples were excised from the posterior jejunal lymph nodes (PJLNs), the mid- and anterior jejunal lymph nodes, the ileocecal lymph node (ICLN), and the associated intestinal epithelia and submucosa. Tissue samples were fixed in 10% buffered formalin, processed on an automatic Shandon Citadel 1000 tissue processor, and set in Paraplast Plus tissue-embedding medium. Sections were cut at 5 μm with a Leica RM2135 microtome and stained with hematoxylin-eosin or Ziehl-Neelsen stain. The slides were examined and scored by using a grading system outlined for use for describing the pathophysiological aspects of JD (15). That system utilizes a ranked scoring system from 0 to 13 (with 0 representing no lesions or overt pathology and 13 representing extensive granulomatous lesions in intestinal mucosa, submucosa and serosa, mesenteric granulomata, pericapsular lymph node granulomata, and sheet-like granulomata in lymph nodes [15, 22]). The pauci- or multibacillary classification was based subjectively on the density of acid-fast organisms observable in the stained tissue sections, as described in detail elsewhere (7).
Bacteriological culture.
Nonfixed tissue samples were frozen at −20°C until they were required. Culture was carried out at AgResearch, Wallaceville, New Zealand, by using the automated Bactec system for M. avium subsp. paratuberculosis organisms (Becton Dickinson, North Ryde, NSW, Australia). The samples were incubated for a maximum of 60 days to allow the differentiation of positive versus negative samples.
Isolation of leukocytes from blood.
Blood was obtained by jugular venipuncture and placed into 10-ml heparinized Vacutainer tubes, which were then centrifuged at 1,500 × g for 15 min. The buffy coats were removed and the erythrocytes were depleted through hypotonic lysis. Residual cell suspensions were washed by centrifugation twice in phosphate-buffered saline (PBS), and then the pellets were resuspended in 8 ml RPMI 1640 base medium (Gibco, Invitrogen, Auckland, New Zealand) supplemented with 10% fetal calf serum (FCS; ICP Biologicals, New Zealand) plus 2 mM l-glutamine and 5 μg/ml gentamicin sulfate.
Isolation of mononuclear leukocytes from lymph node tissues.
The lymph nodes were excised from the animals immediately after slaughter and were placed into petri dishes with 25 ml of RPMI 1640 medium-2% FCS. The samples were manually macerated with a scalpel and forceps, and the cell suspensions were filtered through 70-μm-pore-size cell strainers (Becton Dickinson) and layered over 7.5 ml Histopaque (density, 1.077 g/ml at 25°C; Sigma-Aldrich). The mononuclear cells were removed from the interface layer after centrifugation, washed in PBS, and resuspended in 8 ml RPMI 1640 medium-10% FCS with l-glutamine and gentamicin.
Antibody ELISA for plasma samples.
An ELISA for the detection of M. avium subsp. paratuberculosis-specific ovine IgG antibodies in plasma was adapted from the assays described by Begg et al. (1) and Griffin et al. (13, 14); the responses in the sera from animals with M. avium subsp. paratuberculosis exposure were compared with those observed by using pooled sera from the control (noninfected) group of animals which had bred and maintained on a restricted-entry farm with no history of JD or prior mycobacterial infection. Flat-bottomed microtiter plates (Maxisorp Immuno plates; Nunc) were coated with 50 μl of antigen, either PPDj (CIDC Lelystad, The Netherlands) at 12.5 μg/ml or paratuberculosis protoplasmic antigen (PPAg; Allied Monitor Inc.) at 2 μg/ml. The plates were washed six times, and 50-μl plasma samples, diluted 1/100 with wash buffer, were added to both PPDj- and PPAg-coated wells. The plates were incubated at 37°C for 1 h and washed six times, and 50 μl of rabbit antisheep-horseradish peroxidase (HRP) conjugate antibody (Dakocytomation, Glostrup, Denmark) diluted 1/2,000 was added to each well. The plates were incubated at 37°C for 30 min and washed six times, and antibody binding was visualized with an o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich) substrate system. The color reactions were recorded at 490 nm, and the data were expressed as ELISA units (EUs), where 1 EU equals (sample OD − negative control OD) × 100 (14).
IFN-γ ELISA for antigen-stimulated blood cells.
Whole heparinized sheep blood (1.5 ml) was aliquoted in duplicate and placed into the wells of a 24-well tissue culture plate (Becton Dickinson). PPDj was added to one aliquot to a final concentration of 20 μg/ml, and an equal volume of PBS was added to the nonstimulated control aliquot. The plates were incubated at 37°C with 5% CO2 for 24 h, before the removal and −20°C storage of 1 ml of cell-free supernatant from each aliquot. Blood from all groups of M. avium subsp. paratuberculosis-exposed animals but not from the control group (in which no cytokine reactivity would be expected) was tested.
A capture ELISA was carried out to detect ovine IFN-γ by using mouse anti-bovine IFN-γ antibody (MCA1964; Serotec) at 1.67 μg/ml for capture and biotinylated mouse anti-bovine IFN-γ antibody (MCA1783B; Serotec) at 0.125 μg/ml for detection. Interferon was detected with a streptavidin-peroxidase conjugate (Roche) and a tetramethylbenzidine (TMB; Invitrogen) chromogen solution (Invitrogen); the plates were read at 450 nm, and the IFN-γ levels were expressed as EUs, in which the sample comprised cells cultured with antigen and the negative control comprised cells cultured without antigen.
Stimulation of blood or lymph node leukocytes for cytokine expression or phenotype analysis.
Leukocytes were adjusted to 2.5 × 106 cells/ml in RPMI 1640 medium-10% FCS, aliquoted in 1-ml volumes, and placed in 24-well tissue culture plates. The samples were stimulated with 5 μg/ml PPDj at 37°C with 5% CO2 for 24 h prior to quantitative PCR (qPCR) analysis or 72 h prior to flow cytometric analysis.
Flow cytometric analysis.
The leukocytes were aliquoted into 96-well round-bottom plates (Nunc) at 1 × 106 cells per well and pelleted at 394 × g. Murine antibodies targeting the following ovine cell surface markers were diluted as required in PBS-1% FCS and then added: anti-CD4 (44.97 and 44.38 in a 1:1 ratio) and anti-γδ T-cell receptor (TCR; 86D) (supplied by the Centre for Animal Biotechnology, University of Melbourne), anti-CD8 (MCA2216; Serotec), anti-CD25 (MCA2218; Serotec), and a B-cell marker (BAQ155A; supplied by Veterinary Medical R&D, Pullman, WA). Surface labeling was visualized by using fluorescein isothiocyanate-conjugated donkey anti-mouse IgG (Jackson Immunochemicals), prior to fixation of the cells in 1% paraformaldehyde for analysis in a FACScan flow cytometer (Becton Dickinson). Upon acquisition for fluorescence-activated cell sorter (FACS) analysis, the cells were first gated on the basis of their physical characteristics (forward scatter versus side scatter) to select just mononuclear leukocytes for phenotypic analysis. The phenotypic data were expressed as the frequency of positive labeling for each phenotype among the mononuclear cell population, based on 10,000 recorded cell events.
RNA recovery and treatment.
RNA was extracted from circulating peripheral blood leukocytes. Mononuclear leukocytes were separated from 1 ml of whole heparinized blood over Histopaque 1077 and washed in PBS; the cell pellet was lysed in 1 ml Trizol reagent (Invitrogen), and the lysate was stored frozen until analysis. RNA was also recovered from thin lymph node dissections snap-frozen in Trizol reagent.
In addition, RNA was recovered from blood or lymph node leukocytes following ex vivo antigen stimulation of the mononuclear cells. The samples were stimulated with PPDj, as described above, and then centrifuged, and the cell pellets were lysed in Trizol reagent.
After Trizol lysis and recovery, the purified RNA was treated with DNase by using the New England Biolabs DNase 1 products and protocols. cDNA was prepared from approximately 1,500 ng of DNase-treated RNA by using a Superscript first-strand synthesis system by oligo(dT) priming, according to the manufacturer's instructions, and was used for reverse transcription-PCR (Invitrogen).
Quantitative PCR.
TaqMan probes and primers were designed by the Assays-by-Design Service (Applied Biosystems, New Zealand). TaqMan assays were designed to span intron sequences (Table 1), as predicted by alignment of the ovine sequences against the complete bovine genome database. qPCRs were carried out in MicroAmp Fast optical 96-well reaction plates (Applied Biosystems) sealed with ABsolute qPCR seals (ABgene) by combining the TaqMan universal PCR master mix (Applied Biosystems) with the primer at 900 nM and the probe at 100 nM. All samples were assayed in duplicate by using a 7500 Fast Real-Time PCR system thermocycler (Applied Biosystems).
TABLE 1.
Quantitative PCR targets, GenBank accession numbers, primer sequences, probe sequences, amplicon sizes, and size of the predicted intron spanned by the assay
| Gene target (GenBank accession no.) | Primer and probe sequences (5′-3′)a | Amplicon size (bp) | Intron size (bp)b |
|---|---|---|---|
| β2-Microglobulin (NM 001009284) | Fwd: GGCTGCTGTCGCTGTCT | 72 | 9,555 |
| Rev: GCGGGTGTCTTGAGTATACCT | |||
| Probe: FAM-CCATCCAGCGTATTCC-MGBNFQ | |||
| IFN-γ (NM 001009803) | Fwd: CTGGAGGACTTCAAAAGGCTGATT | 63 | 2,393 |
| Rev: GGCTTTGCGCTGGATCTG | |||
| Probe: FAM-ATCCACCGGAATTTG-MGBNFQc | |||
| IL-4 (NM 001009313) | Fwd: TGCCTGTAGCAGACGTCTTTG | 64 | 4,735 |
| Rev: GCCCTGCAGAAGGTTTCCT | |||
| Probe: FAM-CTCAGTTGCGTTCTTTG-MGBNFQ | |||
| IL-10 (NM 001009327) | Fwd: TGGAGCAGGTGAAGAGAGTCT | 68 | 1,125 |
| Rev: AAACTCACTCATGGCTTTGTAGACA | |||
| Probe: FAM-CCCTCTCTTGGAGCATAT-MGBNFQ |
Fwd, forward primer; Rev, reverse primer; FAM, 6-carboxyfluorescein.
Intron sizes were inferred from the bovine whole-genome sequence database.
The probe binds to the complementary strand.
Data were analyzed by relative quantification. A modified threshold cycle (ΔΔCT) formula was used (4) to take into account variations in the efficiencies of the reactions. The equation compared the results for the samples to those for a reference gene to account for variations in the amount of cDNA present in each reaction mixture and then compared the results for the normalized samples to those for the appropriate control. β2-Microglobulin was used as the reference gene. The mean efficiency of the target genes was calculated with LinRegPCR (version 7.5) software (23); the results for the PPDj-stimulated samples were compared to the result for the paired nonstimulated sample so that each sample had an individual control. The levels of mRNA in samples from circulating blood or lymph node samples were compared to those in the one animal with the lowest level of mRNA detected, which was chosen as the control for all samples.
Statistical analysis.
Statistical analyses were carried out by using the GraphPad InStat program (version 3.0b). One-way analysis of variance (ANOVA) was used to compare several groups, followed by a Bonferroni multiple-comparisons test to compare paired sets of data when the data sets were normally distributed. Alternatively, a Kruskal-Wallis nonparametric test was used, followed by a Dunn's multiple-comparisons test, for nonnormalized data sets. Data from repeat samples were compared by repeated-measures ANOVA, followed by a Bonferroni multiple-comparisons test (if the data were normally distributed) or by a Friedman repeated measures test followed by Dunn's multiple-comparisons tests (if the data were nonnormally distributed). Correlations within a data set were tested by linear correlation (normally distributed data).
RESULTS
Necropsy.
Sheep slaughtered from farms with confirmed or suspected cases of JD were assessed for clinical disease at necropsy. The animals were subsequently assigned to groups retrospectively on the basis of the culture and the histopathology findings (Table 2) and comprised six animals with PB and seven animals with MB. Asymptomatic animals (n = 4) were classified as culture positive and histopathology negative. The group of control animals (n = 4), derived from an agricultural facility free from M. avium subsp. paratuberculosis infection, was confirmed to be culture and histopathology negative.
TABLE 2.
Classification of animals studied
| Groupa | Animal tag no. | Histology finding | Culture result | Flock JD status |
|---|---|---|---|---|
| Noninfected controls | 5060 | 0 | − | − |
| 5061 | 0 | − | − | |
| 5066 | 0 | − | − | |
| 5067 | 0 | − | − | |
| AS | 7048 | 0 | + | + |
| 7032 | 0 | + | + | |
| 7068 | 0 | + | + | |
| 7089 | 0 | + | + | |
| PB | 0520 | 6PB | + | + |
| 5 | 9PB | + | + | |
| 0527 | 11PB | + | + | |
| 7067 | 12PB | + | + | |
| 0783 | 12PB | + | + | |
| 0987 | 13PB | + | + | |
| MB | 7091 | 5MB | + | + |
| 7 | 10MB | + | + | |
| 6 | 13MB | + | + | |
| 0902 | 13MB | + | + | |
| 0831 | 13MB | + | + | |
| 0622 | 13MB | + | + | |
| 0985 | 13MB | + | + |
Sheep from farms with ongoing ovine JD were sorted according to histology and culture results into groups representing a noninfected control group, an infected nondiseased asymptomatic group (AS), a PB group, and an MB group.
Antibody ELISA.
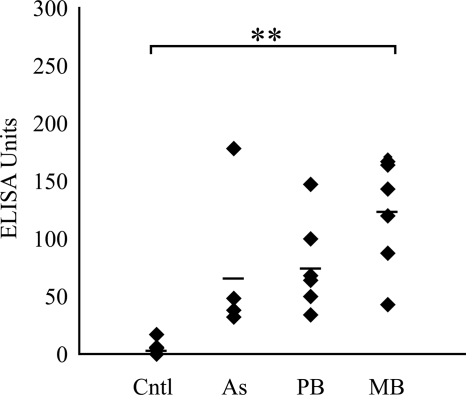
All infected animals had detectable levels of PPDj-specific IgG antibody in their plasma, whereas the control group had low levels (mean, 7 EUs). The group with MB disease had the highest mean antibody level detected (128 EUs) and was the only group for which the results were statistically significantly greater than those for the control group (P < 0.01) (Fig. 1).
FIG. 1.
PPDj-specific IgG antibody in plasma samples, as detected by ELISA. The mean is indicated by a black bar. Cntl, control group; As, asymptomatic group; **, P < 0.01.
IFN-γ ELISA.
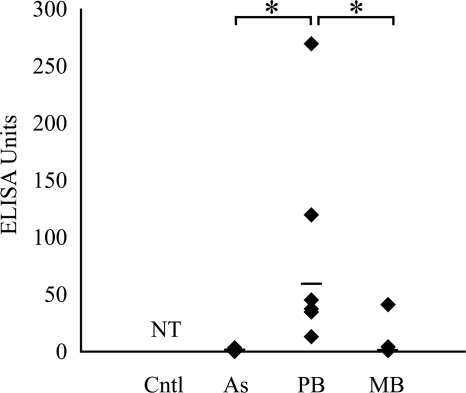
Low levels of IFN-γ were detected in cultures of PPDj-stimulated blood from the asymptomatic group (mean, 1 EU) and the group with MB disease (mean, 7 EUs) (Fig. 2). Within these groups, the amount of detectable IFN-γ did not rise above the background level of the assay in several animals (asymptomatic group, two of four animals; group with MB disease, four of seven animals). High levels of IFN-γ were detected in the group with PB disease (mean, 50 EUs), with the levels being statistically significantly higher than those for both the asymptomatic and the MB disease groups (P < 0.05) (Fig. 2).
FIG. 2.
IFN-γ production by whole-blood samples following PPDj stimulation, as detected by ELISA. The mean is indicated by a black bar. NT, not tested; *, P < 0.05.
Flow cytometric analysis of antigen-stimulated blood and PJLN samples.
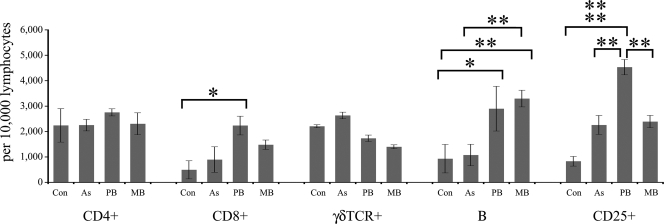
The mononuclear cell populations (cell debris and granulocytes were excluded by FACS gating) in PPDj-stimulated and nonstimulated blood samples were monitored. The change in the frequencies of cell phenotypes after stimulation was calculated by subtracting the cell count after 72 h of culture in the absence of antigen from the cell count after 72 h of culture with antigen (i.e., the PPDj-stimulated phenotype frequency minus the frequency of nonstimulated cells for each animal). The absolute frequencies of each cell population after 72 h of stimulation with PPDj are presented in Fig. 3.
FIG. 3.
Absolute numbers of lymphocytes in blood samples that stained for CD4+, CD8+, γδTCR+, B cells, or CD25+ after 72 h of in vitro PPDj stimulation. Con, control. Significant between-group differences are indicated: *, P < 0.05; **, P < 0.01;  , P < 0.001. The results are presented as the means ± standard errors of the means.
, P < 0.001. The results are presented as the means ± standard errors of the means.
For CD4+ cells from blood, there were no statistically significant changes in the cell frequency in response to 72 h stimulation with PPDj; however, a noticeable decrease in the proportion of CD4+ cells was observed in the PB group upon antigenic stimulation, and the magnitude of this decrease was statistically significant compared to the change in magnitude of CD4+ cells (PPDj stimulated versus nonstimulated) from the MB group (P < 0.01; data not shown). For CD8+ cells, there were no statistically significant changes in cell frequency in response to 72 h stimulation with PPDj and no significant between-group differences in cell frequency for nonstimulated blood samples. However, the absolute frequency of CD8+ cells in PPDj-stimulated blood samples was the highest in samples from the PB group, which reached statistical significance compared with the absolute frequency of CD8+ cells in the PPDj-stimulated blood samples from the control group (P < 0.05). There were no significant between-group differences in the frequencies of γδTCR-positive (γδTCR+) cells among PPDj-stimulated and nonstimulated blood samples. For B cells, nonstimulated blood samples from the group with MB disease had a high B-cell frequency (MB group > control, asymptomatic, and PB groups [P < 0.05]), but there was no significant change in the magnitude of the B-cell frequency within this group's samples upon exposure to antigen. In contrast, nonstimulated cell samples from the group with PB disease had a low frequency of B cells, similar to the frequencies for the control and the asymptomatic groups, but this did increase significantly more than the frequencies for all other groups following PPDj stimulation (change following PPDj stimulation, PB group > control group [P < 0.01] and the asymptomatic and MB groups [P < 0.05]; data not shown). Among the blood samples stimulated with PPDj, the absolute B-cell frequencies were the highest among samples from animals with PB and MB disease (MB group > control and asymptomatic groups [P < 0.01] and PB group > control group [P < 0.05]) (Fig. 3). For CD25+ cells, there were no significant between-group differences in cell frequency in nonstimulated blood samples, but upon PPDj stimulation, the CD25+ cell frequencies in blood samples from all infected animals increased noticeably. Among the blood samples stimulated with PPDj, the absolute CD25+ cell frequencies were significantly higher in blood samples from the group with PB disease than in blood samples from the other groups (P < 0.001 compared with the results for the control group; P < 0.01 compared with the results for the asymptomatic and the MB disease groups).
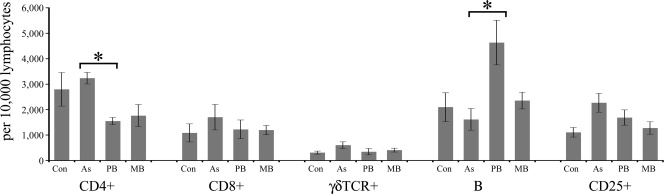
The cell populations in PPDj-stimulated and nonstimulated PJLN samples were monitored as described above for blood cells. The change in cell phenotype frequency after stimulation (i.e., the cell phenotype frequency after PPDj stimulation minus the cell phenotype frequency with no stimulation) was also calculated. The absolute frequencies of each cell population after 72 h stimulation with PPDj are presented in Fig. 4.
FIG. 4.
Absolute numbers of lymphocytes from PJLN samples that stained for CD4+, CD8+, γδTCR+, B cells, or CD25+ after 72 h of in vitro PPDj stimulation. Significant between-group differences are indicated: *, P < 0.05. The results are presented as the means ± standard errors of the means.
For CD4+ cells from PJLNs, there were no statistically significant between-group differences in cell frequency for nonstimulated samples; however, in response to 72 h of stimulation with PPDj, a noticeable decrease in the proportion of CD4+ cells was observed in the PB group, and both the magnitude of this decrease and the absolute CD4+ cell frequency in the PPDj-stimulated samples were statistically significantly different from those for the asymptomatic group (P < 0.05; Fig. 4). There was a high frequency of B cells among samples from the PB disease group for both PPDj-stimulated PJLN samples (PB group > asymptomatic group [P < 0.05]; Fig. 4) and for nonstimulated cell samples (PB group > control, asymptomatic, and MB groups [P < 0.01]; data not shown). The frequencies of CD8+, CD25+, and γδTCR+ cells in PJLN samples were similar between groups and did not change significantly in response to PPDj stimulation.
To further analyze the shifts in major cell populations on the basis of the disease state, the ratio of CD4+ T cells to B cells was compared between groups. In PPDj-stimulated blood and PJLN samples, the CD4+ cell/B-cell ratio in the PB and MB disease groups tended to be a higher than that in the control and asymptomatic groups (Table 3). This trend reached significance in PJLN samples, in which the PB disease group had a significantly higher CD4+ cell/B-cell ratio than the control and asymptomatic groups (P < 0.05).
TABLE 3.
Mean ratio of major cell subpopulations (T and B lymphocytes) in blood and PJLN samples after PPDj stimulation
| Specimen type | CD4+ cell/B-cell ratioa |
|||
|---|---|---|---|---|
| Control | AS | PB | MB | |
| Blood | 1:0.49 ± 0.11 | 1:0.50 ± 0.14 | 1:1.76 ± 0.79 | 1:1.79 ± 0.45 |
| PJLN | 1:0.90* ± 0.27 | 1:0.49** ± 0.12 | 1:3.06*,** ± 0.55 | 1:1.72 ± 0.35 |
The results are means ± standard errors of the means. * and **, significant differences between pairs of groups (paired groups sharing similar symbols denote the between-group significance; P < 0.05 in all cases). AS, asymptomatic group.
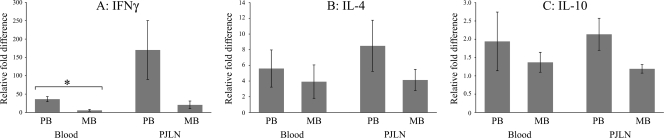
qPCR analysis of stimulated blood and PJLN samples.
The PB disease group had higher levels of IFN-γ mRNA expression than the MB disease group in both blood and PJLN samples after stimulation with PPDj; this reached statistical significance for the blood samples (P < 0.05) (Fig. 5A). There were no significant differences between the groups in the amount of IL-4 or IL-10 mRNA expressed after PPDj stimulation of blood or PJLN samples (Fig. 5B and C).
FIG. 5.
Relative fold differences in levels of cytokine mRNA expression following PPDj stimulation for IFN-γ (A), IL-4 (B), and IL-10 (C). All mRNA values are normalized to the value for β2-microglobulin mRNA and are compared to the reference value. The mean PCR amplification efficiencies were 2.01, 2.00, 1.99, and 2.00 for β2-microglobulin, IFN-γ, IL-4, and IL-10, respectively. *, P < 0.05 for significant changes after antigen stimulation. The results are presented as the means ± standard errors of the means.
Flow cytometric analysis of circulating blood and freshly isolated PJLN samples.
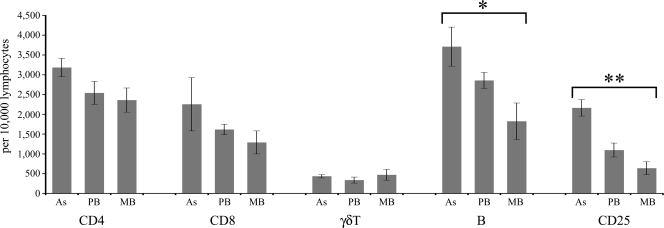
Blood and PJLN samples were analyzed as soon as possible after sampling to allow analysis of the circulating (blood) and resident (PJLN) cell populations inherently present in infected animals (Fig. 6). Data for the control group were not available.
FIG. 6.
Numbers of lymphocytes labeled positively for CD4+, CD8+, γδTCR+, B cells, or CD25+ present in PJLN samples freshly excised from animals. *, P < 0.05 for values significantly different between asymptomatic and MB groups; **, P < 0.01 for values significantly different between asymptomatic and MB groups. The results are presented as the means ± standard errors of the means.
For circulating blood samples, there were no between-group differences in the frequency of any cell population (data not shown). For PJLN samples, there were no between-group differences in the frequency of CD4+, CD8+, or γδTCR+ cells (Fig. 6); however, the frequency of CD25+ cells was higher in the asymptomatic group than in the PB or MB disease group, although this was statistically significant only for the comparison of the results with those for the MB disease group (P > 0.01). The asymptomatic group also had the highest proportion of B cells, again reaching significance only in comparison to the results for the MB disease group (P < 0.05).
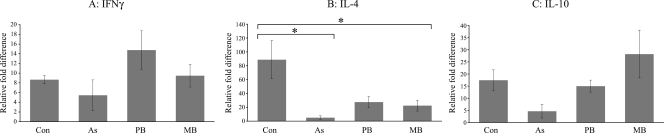
qPCR analysis of cytokine expression in circulating blood and PJLN samples.
There were no significant between-group differences in the levels of expression of IFN-γ or IL-10 mRNA in blood or PJLN samples, although there was a trend for higher levels of IFN-γ in PJLN samples from the PB disease group. There was a trend for higher levels of IL-4 mRNA in blood and PJLN samples from the control group, although this reached significance only for blood samples but not PJLN samples in comparison to the asymptomatic and MB disease groups (Fig. 7).
FIG. 7.
Mean levels of cytokine mRNA for IFN-γ (A), IL-4 (B), and IL-10 (C) detected in circulating blood samples normalized to the value for β2-microglobulin and compared to the reference values. The mean PCR amplification efficiencies were 1.99, 2.00, 1.96, and 1.98 for β2-microglobulin, IFN-γ, IL-4, and IL-10, respectively. *, P < 0.05 for IL-4 mRNA values in the asymptomatic and MB groups that were significantly different from those for the controls. The results are presented as the means ± standard deviations.
To further analyze the shifts in the levels of the major cytokines related to the disease state, the ratio of the expression of a major Th1 cytokine (IFN-γ) to that of a major Th2 cytokine (IL-4) was assessed between groups for both blood and PJLN cell samples. All M. avium subsp. paratuberculosis-infected groups had lower IFN-γ mRNA/IL-4 mRNA ratios than the control group (Table 4). However, this reached significance only for PJLN samples, in which the PB and MB disease groups had ratios significantly lower than the ratio for the control group (P < 0.05).
TABLE 4.
Mean ratio of Th1 cytokine mRNA/Th2 cytokine mRNA levels in circulating blood samples and PJLN samples
| Specimen type | IFN-γ mRNA/IL-4 mRNA ratioa |
|||
|---|---|---|---|---|
| Control | AS | PB | MB | |
| Blood | 1:11.60 ± 4.23 | 1:2.21 ± 1.54 | 1:1.99 ± 1.02 | 1:2.24 ± 0.64 |
| PJLN | 1:50.16*,** ± 10.18 | 1:31.08 ± 12.93 | 1:9.91** ± 3.18 | 1:8.42* ± 4.16 |
The results are means ± standard errors of the means. * and **, significant difference between pairs of groups (paired groups sharing similar symbols denote the between-group significance; P < 0.05 in all cases). AS, asymptomatic group.
PB disease severity compared to immune readouts.
The random sampling of animals resulted in a PB group containing six animals with different severities of disease: 6, 9, 11, 12 (n = 2), and 13 on the scale of the histopathology score (15). This caused higher variability within the PB disease group but also allowed further analysis of immune parameters within the PB disease group which was not possible within the MB disease group. It was not possible to undertake a correlative analysis of the severity of multibacillary disease according to the histopathology score and immune parameters, as most animals had the same severe grade of disease.
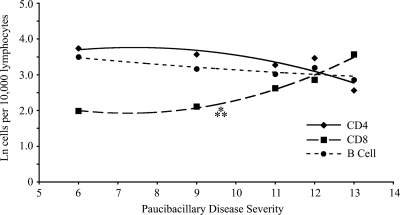
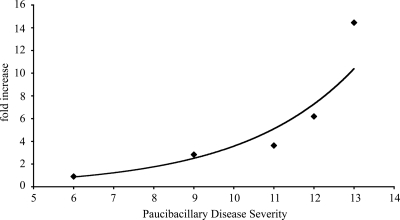
Analysis of the cell subpopulations within circulating blood samples from animals classified as having PB disease indicated a nonvariable relationship between CD4+ T-cell and B-cell frequencies and an increasing histopathology score (Fig. 8). In contrast, there was a significant positive correlation between the CD8+ T-cell frequency and disease severity (P < 0.005). There was also a significant positive correlation between IL-4 mRNA expression in PPDj-stimulated blood samples and the severity of the histopathology (P < 0.05) (Fig. 9).
FIG. 8.
Relationship between major blood lymphocyte numbers and disease severity. The natural logarithms of the proportion of lymphocytes staining positively for CD4+, CD8+, or B cells in circulating blood samples of animals with paucibacillary disease were correlated with disease severity in this group (n = 5). Each point represents an individual animal except at disease severity rank 12, where n is equal to 2. ⁂, significant correlation between the natural log of the CD8 T-cell count and histopathology (P < 0.005). Data were fitted with polynomial regression curves: for CD4+, r2 = 0.76; for CD8+, r2 = 0.98; for B cells, r2 = 0.78.
FIG. 9.
Relationship between Th2 cytokine expression and disease severity. The fold change in the level of IL-4 mRNA expression in the blood of paucibacillary diseased animals after PPDj stimulation was compared to disease severity (n = 5). The mean PCR amplification efficiencies were 2.01 and 1.99 for β2-microglobulin and IL-4, respectively. Each point represents an individual animal. There was a significant correlation between cytokine expression and histopathology (P < 0.05). The data were fitted with an exponential trend line (r2 = 0.94).
DISCUSSION
Ovine paratuberculosis can be classified into three forms of disease (26). The results of the present study reinforce that notion with immunological data, in that paucibacillary disease features T-cell activity comprising a mixed Th1-Th2 cytokine profile and is concurrent with low bacterial numbers; multibacillary disease features a response with only a Th2-type cytokine profile and antibody-based reactogenicity, characterized by high bacterial numbers; and the asymptomatic form features infection with no pathology and an indeterminate immune response. This immunological/microbiological/pathological spectrum differs from that observed in bovine paratuberculosis, in which the paucibacillary state represents an early form of disease which progresses to the more severe multibacillary disease; in the ovine system, both paucibacillary disease and multibacillary disease are the end points of disease. Asymptomatic infection in sheep also differs from the equivalent in cattle, which features both infection and subclinical pathologies (26, 28). The aim of this study was to investigate the underlying patterns of immune reactivity in sheep that either lead to or reflect these different manifestations of disease.
In this study, sheep with paucibacillary disease demonstrated immune reactivity comprising both type 1 and type 2 T-cell responses. Animals with paucibacillary disease had elevated levels of antibody and large populations of B cells in both blood and lymph node samples after PPDj stimulation. Animals with paucibacillary disease were the only ones to have significant levels of IFN-γ, as detected by ELISA, and they also exhibited larger increases in the levels of IFN-γ mRNA expression after PPDj stimulation of blood samples than the multibacillary disease group and the highest levels of endogenous IFN-γ mRNA in PJLN samples overall. The production of IFN-γ is typically associated with this form of disease in ruminants (2, 3). Immune responses during paucibacillary disease are understood to be mediated by CD4+ T cells in ruminants (6, 7, 10). Ovine immunohistochemistry data have shown increased numbers of CD4+ and γδTCR+ cells and decreased numbers of CD8+ cells in the intestinal mucosa, ileum, and jejunal Peyer's patches of animals with paucibacillary disease (18, 25). A study of M. avium subsp. paratuberculosis-infected goats reported decreased numbers of CD4+ T cells and increased numbers of CD8+ T cells in mesenteric lymph nodes compared to the numbers in noninfected control animals (21). The data from the current study have also revealed a low frequency of CD4+ T cells in the PJLNs of animals with paucibacillary disease, although the numbers of CD8+ and γδTCR+ cells were equivalent between sheep with PB disease and noninfected control animals. Furthermore, PPDj stimulation of blood or PJLN samples caused the proportion of CD4+ T cells to decrease in the paucibacillary disease group (to a statistically significant degree in comparison to the proportion in the MB or the asymptomatic group); a similar decrease in blood-borne CD4+ T cells after PPDj stimulation has also been observed in this laboratory in sheep vaccinated against M. avium subsp. paratuberculosis (S. Gillan and J. F. T. Griffin, 2007, unpublished data). This decrease may be due to terminal differentiation of the active CD4+ T cells in the samples after in vitro antigen stimulation for 3 days. Interestingly, the data presented here indicate that the largest CD8+ T-cell population in the blood after PPDj stimulation was in the paucibacillary disease group, possibly indicating an active role for these cells in the PB disease state. High levels of CD8+ T cells have previously been associated with a protective immune response against M. avium subsp. paratuberculosis in goats and cattle (8, 9, 28); it has been hypothesized that CD8+ T cells can limit the suppressive activity of immunoregulatory γδTCR+ T cells on M. avium subsp. paratuberculosis-reactive CD4+ T cells, which are themselves necessary to clear infection (8, 9, 26, 27). However, the data generated in this study did not entirely fit this hypothesis, as the animals with the highest CD8+ T-cell frequency also had the most severe paucibacillary disease. Furthermore, the animals in the paucibacillary disease group were found to have a spectrum of disease severity, on the basis of histopathological data, that ranged from 6 to 13 on the scale described previously (15). By correlating disease severity with immune parameters, it was noted that increasing paucibacillary disease severity correlated positively with the frequency of blood-borne CD8+ T cells and with the amount of IL-4 mRNA produced after PPDj stimulation of blood samples; together, these data support the theory of the involvement of CD8+ T cells, possibly with a Th2 profile, in the paucibacillary disease state (which is nonprotective but in which disease severity and bacterial replication are limited).
The immune profile of the multibacillary disease group featured type 2 immune reactivity, little type 1 reactivity, and signs of a suppressed immune response, as has been reported in other studies (2, 7, 10). That group had the highest levels of plasma antibody (although they were statistically nonsignificantly different from the levels for the PB group), almost no production of inducible IFN-γ by antigen stimulation of blood leukocytes, and very little induction of IFN-γ mRNA expression after stimulation with PPDj. Low levels of IFN-γ mRNA have previously been observed in ovine multibacillary disease (3), suggesting that a Th1 profile is either overridden or absent in this disease state in sheep. After stimulation with PPDj, there were no obvious changes in cell phenotype frequencies in either blood or PJLN samples in the present study, which is in contrast to the observations for cattle, in which multibacillary disease is associated with decreased CD4+ T-cell populations and increased γδTCR T-cell populations in the ileum, as detected by immunohistochemistry (17). In goats, multibacillary disease is associated with a decreased CD4+ T-cell frequency and an increased CD8+ cell frequency in the intestinal lamina propria and mesenteric lymph nodes (21). In the present study, a high frequency of blood-borne B cells was observed concurrently with high levels of circulating antibody in the MB group; however, in contrast, sheep with MB disease had a high ratio of CD4+ T cells/B cells in the PJLN tissues, concurrent with a low frequency of B cells overall, possibly indicating B-cell activation and trafficking from the intestinal tissues back into the circulation.
In studies of cattle with JD, IL-4 and IL-10 mRNA expression in the ileum (28) and IL-4 mRNA expression in peripheral blood mononuclear cells (10) have been associated with multibacillary disease. The cytokine data for PJLN samples presented here demonstrated a trend toward higher levels of IL-10 in the PJLNs of the sheep with multibacillary disease but similar levels of IFN-γ mRNA and low levels of IL-4 compared to those in the control group. Unexpectedly, the sheep with paucibacillary disease tended to produce more IL-4 mRNA in response to PPDj stimulation. This could reflect the presence of a strengthening mixed immune response in the paucibacillary disease group, whereas the IL-4 mRNA levels in the multibacillary disease group are sufficient to stimulate a type 2 response without the confounding effect of a type 1 response. These data support the hypothesis that multibacillary disease develops due to a lack of balance from cross-regulatory Th1 components, which fails to limit infection and which, in turn, leads to the presence of extensive and severe lesions and little immunological control of bacterial replication.
As outlined previously, the subclinical or asymptomatic phase of M. avium subsp. paratuberculosis infection differs between species. In cattle, subclinically infected animals exhibit histopathological changes indicating disease (28), whereas sheep diagnosed with subclinical/asymptomatic JD have a normal histology and no lesions. In cattle, analysis of the inherent cytokine mRNA profile revealed that peripheral blood mononuclear cells from subclinically infected animals express a proinflammatory gene profile, but following stimulation with M. avium subsp. paratuberculosis antigen in vitro, only the immunoregulatory cytokine IL-10 is consistently upregulated (11). In sheep, tumor necrosis factor alpha mRNA expression is significantly upregulated and IL-18 expression is downregulated in ileal samples compared to their levels of expression in control uninfected ileal samples, although the expression of IL-10 was not found to differ between the two groups (26). Nevertheless, it has been hypothesized that in ruminants M. avium subsp. paratuberculosis infection stimulates the production of IL-10-secreting regulatory T cells (Treg), which, as a consequence, limits proinflammatory type 1 protective responses (17); however, this does not seem to be the case in ovine paratuberculosis, as identified in the present study. An increase in the level of IL-10 mRNA expression, which is typically observed in blood samples taken from subclinically infected cattle (12), was not observed in subclinically infected sheep in the present study.
Asymptomatic sheep have been described as being immunologically similar to uninfected animals, even though they are capable of responding to M. avium subsp. paratuberculosis antigens, and as being distinguishable from uninfected animals by their cytokine profile (26). This description matches the composite immunological observations made in the present study, in that while the asymptomatic group had a majority of immune readouts in common with those for the control (noninfected) group, they differentially exhibited detectable levels of M. avium subsp. paratuberculosis-specific antibody and a high CD25+ cell frequency following PPDj stimulation. The latter responses were similar to those of the paucibacillary disease group; however, the asymptomatic group lacked the large CD8+ T-cell population present in the blood of animals with PB disease. The asymptomatic group tended to have the highest CD4+ and CD25+ T-cell frequencies in the PJLNs, perhaps indicating that cells expressing these phenotypes (which are typically regulatory by nature) are required to mediate a balanced immune response and prevent disease. By comparison, blood samples from the PB disease group showed a decrease in CD4+ T-cell frequency (in comparison to the frequency for the MB disease group) following PPDj stimulation, while PJLN cells from animals in the MB disease group had a consistently lower CD4+ T-cell frequency than the asymptomatic group. These observations together support the notion of an active role for CD4+ T cells in the mediation of disease progression. As the asymptomatic animals in this study were defined as such by combined bacteriological culture and tissue histopathology findings postmortem, it cannot be determined if they would have gone on to develop clinical disease later or if the asymptomatic state described represented a permanent state of permissive infection.
In summary, the aim of the experiments described here was to define the immune parameters associated with the different forms of paratuberculosis observed in sheep. It was found that animals with multibacillary disease had an immunological response profile comprising type 2 components and that animals with paucibacillary disease had responses comprising mixed type 1 and type 2 components; the profile for the asymptomatic group was somewhat similar to that for the control group, but with a few components being more representative of the responses observed in sheep with paucibacillary disease. Overall, these data underscore the differences between ovine and bovine paratuberculosis, indicating that the ovine model requires further investigation, especially to determine if the asymptomatic form is a phenotype representative of immune protection. If so, then animals typed as such could be important in defining the protective immune responses to M. avium subsp. paratuberculosis.
Acknowledgments
We thank the following individuals for their contributions to this work: Phil Farquhar (AgResearch, Invermay, New Zeland) for animal husbandry, Colin Mackintosh (AgResearch) for veterinary assistance and animal handling, and Gary Clark (AgResearch) for undertaking the histology. We also thank Frank Cross (University of Otago) for his assistance with the preparation of the manuscript.
This research was supported, in part, by grant funding awarded to J.F.T.G. from the New Zealand Foundation of Research, Science & Technology.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Begg, D. J., R. O'Brien, C. G. Mackintosh, and J. F. Griffin. 2005. Experimental infection model for Johne's disease in sheep. Infect. Immun. 73:5603-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne's disease). Vet. Immunol. Immunopathol. 68:139-148. [DOI] [PubMed] [Google Scholar]
- 3.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1998. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne's disease). The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet. Immunol. Immunopathol. 66:343-358. [DOI] [PubMed] [Google Scholar]
- 4.Bustin, S. A. 2004. A-Z of quantitative PCR, vol. 5. International University Line Press, La Jolla, CA.
- 5.Carrigan, M. J., and J. T. Seaman. 1990. The pathology of Johne's disease in sheep. Aust. Vet. J. 67:47-50. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini, R. J. 1996. Immunology: resistance to paratuberculosis. Vet. Clin. North Am. Food Anim. Pract. 12:313-343. [DOI] [PubMed] [Google Scholar]
- 7.Clarke, C. J., and D. Little. 1996. The pathology of ovine paratuberculosis: gross and histological changes in the intestine and other tissues. J. Comp. Pathol. 114:419-437. [DOI] [PubMed] [Google Scholar]
- 8.Corpa, J. M., J. Garrido, J. F. Garcia Marin, and V. Perez. 2000. Classification of lesions observed in natural cases of paratuberculosis in goats. J. Comp. Pathol. 122:255-265. [DOI] [PubMed] [Google Scholar]
- 9.Coussens, P. M., A. Jeffers, and C. Colvin. 2004. Rapid and transient activation of gene expression in peripheral blood mononuclear cells from Johne's disease positive cows exposed to Mycobacterium paratuberculosis in vitro. Microb. Pathog. 36:93-108. [DOI] [PubMed] [Google Scholar]
- 10.Coussens, P. M., C. B. Pudrith, K. Skovgaard, X. Ren, S. P. Suchyta, J. R. Stabel, and P. M. Heegaard. 2005. Johne's disease in cattle is associated with enhanced expression of genes encoding IL-5, GATA-3, tissue inhibitors of matrix metalloproteinases 1 and 2, and factors promoting apoptosis in peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 105:221-234. [DOI] [PubMed] [Google Scholar]
- 11.Coussens, P. M., N. Verman, M. A. Coussens, M. D. Elftman, and A. M. McNulty. 2004. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 72:1409-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Almeida, D. E., C. J. Colvin, and P. M. Coussens. 2008. Antigen-specific regulatory T cells in bovine paratuberculosis. Vet. Immunol. Immunopathol. 125:234-245. [DOI] [PubMed] [Google Scholar]
- 13.Griffin, J. F., J. P. Cross, D. N. Chinn, C. R. Rodgers, and G. S. Buchan. 1994. Diagnosis of tuberculosis due to Mycobacterium bovis in New Zealand red deer (Cervus elaphus) using a composite blood test and antibody assays. N. Z. Vet. J. 42:173-179. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, J. F., E. Spittle, C. R. Rodgers, S. Liggett, M. Cooper, D. Bakker, and J. P. Bannantine. 2005. Immunoglobulin G1 enzyme-linked immunosorbent assay for diagnosis of Johne's disease in red deer (Cervus elaphus). Clin. Diagn. Lab. Immunol. 12:1401-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin, J. F., A. D. Hughes, S. Liggett, P. A. Farquhar, C. G. Mackintosh, and D. Bakker. 2009. Efficacy of novel lipid-formulated whole bacterial cell vaccines against Mycobacterium avium subsp. paratuberculosis in sheep. Vaccine 27:911-918. [DOI] [PubMed] [Google Scholar]
- 16.Hines, S. A., C. D. Buergelt, J. H. Wilson, and E. L. Bliss. 1987. Disseminated Mycobacterium paratuberculosis infection in a cow. J. Am. Vet. Med. Assoc. 190:681-683. [PubMed] [Google Scholar]
- 17.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Muller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little, D., H. M. Alzuherri, and C. J. Clarke. 1996. Phenotypic characterisation of intestinal lymphocytes in ovine paratuberculosis by immunohistochemistry. Vet. Immunol. Immunopathol. 55:175-187. [DOI] [PubMed] [Google Scholar]
- 19.Mackintosh, C. G., R. E. Labes, R. G. Clark, G. W. de Lisle, and J. F. Griffin. 2007. Experimental infections in young red deer (Cervus elaphus) with a bovine and an ovine strain of Mycobacterium avium subsp. paratuberculosis. N. Z. Vet. J. 55:23-29. [DOI] [PubMed] [Google Scholar]
- 20.Morris, C. A., S. M. Hickey, and H. A. Henderson. 2006. The effect of Johne's disease on production traits in Romney, Merino and Merino × Romney-cross ewes. N. Z. Vet. J. 54:204-209. [DOI] [PubMed] [Google Scholar]
- 21.Navarro, J. A., G. Ramis, J. Seva, F. J. Pallares, and J. Sanchez. 1998. Changes in lymphocyte subsets in the intestine and mesenteric lymph nodes in caprine paratuberculosis. J. Comp. Pathol. 118:109-121. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien, R., C. G. Mackintosh, D. Bakker, M. Kopecna, I. Pavlik, and J. F. Griffin. 2006. Immunological and molecular characterization of susceptibility in relationship to bacterial strain differences in Mycobacterium avium subsp. paratuberculosis infection in the red deer (Cervus elaphus). Infect. Immun. 74:3530-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakers, C., J. M. Ruijter, R. H. L. Deprez, and A. F. M. Moorman. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339:62-66. [DOI] [PubMed] [Google Scholar]
- 24.Reddacliff, L., J. Eppleston, P. Windsor, R. Whittington, and S. Jones. 2006. Efficacy of a killed vaccine for the control of paratuberculosis in Australian sheep flocks. Vet. Microbiol. 115:77-90. [DOI] [PubMed] [Google Scholar]
- 25.Reddacliff, L. A., S. J. McClure, and R. J. Whittington. 2004. Immunoperoxidase studies of cell mediated immune effector cell populations in early Mycobacterium avium subsp. paratuberculosis infection in sheep. Vet. Immunol. Immunopathol. 97:149-162. [DOI] [PubMed] [Google Scholar]
- 26.Smeed, J. A., C. A. Watkins, S. M. Rhind, and J. Hopkins. 2007. Differential cytokine gene expression profiles in the three pathological forms of sheep paratuberculosis. BMC Vet. Res. 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storset, A. K., H. J. Hasvold, M. Valheim, H. Brun-Hansen, G. Berntsen, S. K. Whist, B. Djonne, C. M. Press, G. Holstad, and H. J. Larsen. 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 80:271-287. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka, S., M. Sato, T. Onitsuka, H. Kamata, and Y. Yokomizo. 2005. Inflammatory cytokine gene expression in different types of granulomatous lesions during asymptomatic stages of bovine paratuberculosis. Vet. Pathol. 42:579-588. [DOI] [PubMed] [Google Scholar]