Abstract
σ-Receptor (σR) antagonists have been reported to block certain effects of psychostimulant drugs. The present study examined the effects of σR ligands in rats trained to self-administer cocaine (0.032–1.0 mg/kg/inj i.v.) under fixed-ratio 5-response schedules of reinforcement. Maximal rates of responding were maintained by 0.32 mg/kg/inj cocaine, or by the σR agonists, 1,3-di-(2-tolyl)guanidine (DTG; 1.0 mg/kg/inj) or 2-(4-morpholinethyl) 1-phenylcyclohexane-1-carboxylate hydrochloride (PRE-084; 0.32 mg/kg/inj), when substituted for cocaine. Lower response rates were maintained at higher and lower doses of the compounds. No dose of the σR antagonists [N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine (BD 1008), N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine (BD 1047), N-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine (BD 1063)] maintained responding appreciably above levels obtained when responding had no consequences. Presession treatment with σR agonists dose-dependently shifted the cocaine self-administration dose-effect curve leftward. The dopamine-uptake inhibitor, (−)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane (WIN 35,428), dose-dependently shifted the DTG and PRE-084 self-administration dose-effect curves leftward. Treatment with the σR antagonists dose-dependently decreased response rates maintained by DTG or PRE-084, but did not affect cocaine self-administration. Response rates maintained by maximally effective DTG or PRE-084 doses were decreased by σR antagonists at lower doses than those that decreased response rates maintained by food reinforcement. Although σR antagonists block some cocaine-induced effects, the lack of effect on cocaine self-administration suggests that the primary reinforcing effects of cocaine do not involve direct effects at σRs. However, the self-administration of σR agonists in cocaine-trained subjects, facilitation of cocaine self-administration by σR-agonist pretreatment, and the facilitation of σR-agonist self-administration by WIN 35,428, together suggest enhanced abuse-related effects resulting from concomitant dopaminergically mediated actions and σR-mediated actions of the drugs.
Interactions between cocaine and σ-receptor (σR) ligands have been well documented. For example, the σR antagonists, BMY 14802 and rimcazole, antagonized the locomotor-stimulant effects of cocaine at doses that were inactive when given alone, suggesting a specific antagonist effect (Menkel et al., 1991). Several other σR antagonists have been reported to block cocaine's locomotor-stimulant effects (Witkin et al., 1993; McCracken et al., 1999; Matsumoto et al., 2001), as well as its acute toxic effects (Matsumoto et al., 2003). Furthermore, an antisense oligodeoxynucleotide directed against σRs attenuated the locomotor and toxic effects of cocaine (Matsumoto et al., 2003), and σR agonists potentiated the acute toxicity of cocaine (Matsumoto et al., 2001).
Interactions of σR ligands with effects of cocaine related to reinforcement have been more complex. Several studies reported dose-dependent antagonism of cocaine-induced place conditioning in mice by the σR antagonists, BD 1047 and NE-100 (Romieu et al., 2000, 2002). However, BD 1047 failed to substantially affect rates of cocaine (0.25 mg i.v.) self-administration in rats (Martin-Fardon et al., 2007). In the same study, responding was established with food or cocaine reinforcement under one set of stimulus conditions, and extinguished under another. Subsequent restoration of the original stimulus conditions under extinction increased rates of responding (reinstatement). Doses of 20 or 30 mg/kg BD 1047 attenuated the reinstatement of responding previously maintained by cocaine or food, respectively (Martin-Fardon et al., 2007). The modest preferential effect of BD 1047 on cocaine reinstatement might suggest a role for σRs in the abuse-related effects of cocaine, but the direct effects of BD 1047 on cocaine self-administration do not.
Other studies suggest a role of σRs in adaptations to repeated cocaine or methamphetamine. Such treatment produced a sensitization to locomotor-stimulant effects that was blocked by coadministration of σR antagonists (Ujike et al., 1992a; Witkin et al., 1993). Moreover, response-contingent (self-administration) methamphetamine produced an up-regulation of σR protein in the midbrain and altered levels of σR mRNA in the frontal cortex and hippocampus of rats, effects not obtained in rats that received the drug noncontingently (yoked controls), or those that received saline (Stefanski et al., 2004). However, in another study, noncontingent cocaine up-regulated σR expression, and the σR antagonist, BD 1063, blocked that up-regulation (Liu and Matsumoto, 2008).
Consistent with the modulation of the behavioral effects of cocaine by drugs acting at σRs are several reports of a regulation of dopaminergic actions by σRs. For example, the dopaminergic agonist, apomorphine, suppressed the firing of both A9 and A10 dopamine neurons, an effect reversed by the σR antagonist, BMY 14802 (Wachtel and White, 1988). Furthermore, σR agonists increased stimulated dopamine release (Gonzalez-Alvear and Werling, 1994), an effect mediated by protein kinase C (Nuwayhid and Werling, 2003). These and similar findings suggest the modulation of dopamine neurotransmission by the σR system (see Matsumoto et al., 2003, for a review).
The affinity of cocaine for σRs in radioligand-binding studies is approximately 50-fold less than that for the dopamine transporter (Cao et al., 2003), cocaine's primary target. Sharkey et al. (1988) suggested a mediation of cocaine's high-dose effects by σRs, which is consistent with the observed σR involvement in the acute toxic effects of cocaine. However, they also argue that a direct involvement of σRs in reinforcing effects of cocaine is unlikely because the reinforcing potencies of cocaine analogs do not correlate with their affinities for the σR. Consistent with that view are findings that σR agonists are ineffective in producing place conditioning (Romieu et al., 2002) and do not substitute for cocaine in subjects trained to discriminate cocaine from saline injections (Ukai et al., 1997). However, Maurice et al. (2002) suggested a mediation of reinforcing effects of cocaine by σRs, based on the antagonism of cocaine-induced place conditioning by σR antagonists.
In the course of studies on the role of the σR in the reinforcing effects of cocaine, we found σR agonists to produce a leftward shift in the cocaine self-administration dose-effect curve. Those findings led to the assessment of the reinforcing effects of σR agonists, and a further comparison of the effects of σR agonists and antagonists in rats self-administering cocaine or σR agonists. In the process we found reinforcing effects of the σR agonists, 1,3-di-(2-tolyl)guanidine (DTG) and PRE-084, and compared the pharmacology of cocaine and σR agonist self-administration. The findings support an important role of σRs in the reinforcing effects of drugs, and previous suggestions for the development of drugs targeting σRs as medications for cocaine dependence.
Materials and Methods
Subjects.
Twenty-seven male Sprague-Dawley rats (weighing approximately 300 g at the start of the study), obtained from Charles River Laboratories (Wilmington, MA), served as subjects after acclimation to the laboratory for at least one week. Subjects were used as the patency of catheters allowed, and replaced thereafter. Food (Scored Bacon Lover Treats; Bio-Serv, Frenchtown, NJ) and tap water were available in their home cages. After acclimation, weights of rats were maintained at approximately 320 g by adjusting their daily food ration. The animal-housing room was temperature- and humidity-controlled and maintained on a 12:12-h light/dark cycle with lights on at 7:00 AM. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by AAALAC International.
Apparatus.
Experimental sessions were conducted with animals placed in operant-conditioning chambers (modified ENV-203; MED Associates, St. Albans, VT) that measured 25.5 × 32.05 × 25.0 cm, and were enclosed within sound-attenuating cubicles equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two response levers, 5.0 cm from the midline and 4.0 cm above the grid floor. A downward displacement of a lever with a force approximating 20 g defined a response, which always activated a relay mounted behind the front wall of the chamber producing an audible “feedback” click. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of food pellets was mounted on the midline of the front wall between the two levers and 2.0 cm above the floor. A syringe infusion pump (model 22; Harvard Apparatus, Holliston, MA) placed above each chamber delivered injections of specified volumes and durations from a 10-ml syringe. The syringe was connected by Tygon tubing to a single-channel fluid swivel (375 Series Single Channel Swivels; Instech Laboratories, Inc., Plymouth Meeting, PA) that was mounted on a balance arm above the chamber. Tygon tubing from the swivel to the subject's catheter was protected by a surrounding metal spring and completed the connection to the subject.
Procedures.
Subjects were placed in chambers daily for experimental sessions, after which they were returned to their cages in the animal-housing room. During sessions subjects were initially trained with food reinforcement (45-mg food pellets; Bio-Serv) to press the right lever under a fixed-ratio (FR) 5-response schedule of reinforcement (each fifth response produced a food pellet). Food deliveries were followed by a 20-s time-out period during which responses had no scheduled consequences. During this training, sessions lasted for 20 min or until 30 food pellets were delivered.
After subjects were responding at a rate sufficiently high that they obtained 30 food pellets within each of three consecutive sessions, they were assigned to two groups. One group continued with food reinforcement, whereas subjects in the other group were surgically implanted in the right external jugular vein with a chronic indwelling catheter that exited at the mid-scapular region of the animal's back. Catheter implantation was performed under anesthesia (ketamine 60.0 mg/kg i.p. and xylazine 12.0 mg/kg i.p.). Catheters were infused daily with 0.1 ml of a sterile saline solution containing heparin (30.0 IU/ml) and penicillin G potassium (250,000 IU/ml) to minimize the likelihood of infection and the formation of clots or fibroids. All animals were allowed to recover from surgery for approximately 7 days before cocaine self-administration studies were initiated.
Cocaine self-administration sessions were conducted in 2-h daily sessions until cocaine self-administration was trained and stable. During these sessions, the LEDs above the right lever were illuminated when cocaine injections were available. Completion of five responses turned off the LEDs and activated the infusion pump, delivering a dose of 1.0 mg/kg. A 20-s time-out period, during which LEDs were off and responding had no scheduled consequences, started with the injection. After the time out, the LEDs were illuminated and responding again had scheduled consequences. Responses on the left lever at all times, and on the right lever during time-out periods, were recorded but (except as noted below) had no scheduled consequences; these data are not presented. Once rates of responding maintained by cocaine were relatively consistent from one session to the next, the session was divided into five 20-min components, each preceded by a 2-min time-out period. This arrangement allowed the assessment of a range of cocaine doses in a single session. By adjusting infusion volumes and durations, the cocaine dose per injection was incremented in the five sequential components in an ascending dose-order as follows: no injection (also referred to as extinction, or EXT, because responses had no scheduled consequences), 0.03, 0.10, 0.32, and 1.0 mg/kg/inj. Infusion volumes and durations were, respectively, 0, 5.6, 18.0, 56.0, and 180 μl and 0, 0.32, 1.0, 3.2, and 10.0 s, based on a body weight of 0.32 kg. A sample injection of cocaine at the corresponding dose occurred independently of responding at the end of the time-out period that preceded each component. Training continued until: 1) at least 5.0 mg/kg cocaine was self-administered within a session with less than 20% variation in the total number of cocaine injections compared with the previous session; 2) the dose of cocaine that maintained maximal response rates varied by no more than one-half log unit over two consecutive test sessions; and 3) maximum response rates were at least 5-fold higher than response rates maintained during EXT. The effects of substitution of other drugs for cocaine or presession treatments on cocaine self-administration were separated by a minimum of 72 h, and were conducted only if performances met the training criteria. All of the tests were conducted with a mixed order of drugs and doses.
The schedule of food reinforcement was also modified as in the present studies of cocaine self-administration. Under this modified procedure, the experimental session was separated into five sequential 20-min components, each preceded by a 2-min time-out period. The first of the five components was EXT (no food available), with an FR 5 schedule of food delivery in effect in the subsequent four components. Under these conditions, response rates maintained by food were higher than those maintained by cocaine. Because differences in control response rates can influence the effects of drug treatments, subjects were given their daily (∼15 g) ration of food (Harlan Rodent Chow; Harlan, Indianapolis, IN) 60 min before sessions, which decreased their response rates. As a result, none of control response rates maintained by injections of cocaine, DTG, PRE-084, or food presentation were significantly different (p = 0.255, one-way ANOVA).
Once performances were stable across successive sessions, as evidenced by a lack of significant trends in response rates, the effects of substitutions for cocaine of the σR agonists DTG (0.1–3.2 mg/kg/inj i.v.) or PRE-084 (0.032–1.0 mg/kg/inj i.v.) or the σR antagonists, BD 1008, BD 1047, and BD 1063 (0.1–3.2 mg/kg/inj i.v. each) were also assessed with a minimum of 72 h between treatments. In addition, the effects of presession intraperitoneal injections of the σR agonists or antagonists on the response rates maintained by cocaine injection or food presentation were assessed. Finally, the effects of presession intraperitoneal injections of the σR antagonists or the dopamine uptake inhibitor, WIN 35,428, on the response rates maintained by DTG and PRE-084 injection were also assessed. The dose ranges examined are shown in the figures.
Drugs.
The drugs used in the present study were as follows: (−)-cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO), DTG (Tocris Bioscience, Ballwin, MO), PRE-084 (Tocris Bioscience), BD 1008 (Tocris Bioscience), BD 1047 (Tocris Bioscience), BD 1063 (Tocris Bioscience), and WIN 35,428 (National Institute on Drug Abuse, Drug Supply Program). All drug solutions, with the exception of DTG (initially dissolved in 1 N HCl and neutralized with 1 N NaOH), were prepared fresh daily in 0.9% NaCl, and administered intravenously (self-administration). Self-administration of the test drugs was assessed with intravenous delivery of injections, whereas all drug pretreatments were administered intraperitoneally. DTG and PRE-084 were administered at 30 min before sessions. BD 1008, BD 1063, and WIN 35,428 were administered at 5 min before sessions. BD 1047 was administered at 15 min before sessions. Pretreatment times and doses of drugs used in the present study were chosen based on published reports (McCracken et al., 1999; Romieu et al., 2002) or preliminary data obtained in this laboratory.
Data Analysis.
Response rates were determined by dividing responses by elapsed time in each component, excluding time-outs after injections or food presentations. Average values across six subjects and S.E.M. are presented below. The significance of effects on response rates was assessed by ANOVA. A Dunnett's post hoc test was used to compare self-administered doses of cocaine, DTG, or PRE-084 with no injection. For studies of previous drug treatments on self-administration of cocaine or a σR agonist, a post hoc Bonferroni t test was used for all pairwise comparisons. To determine whether there was a difference in effects of cocaine compared with saline self-administration, a two-way, repeated-measures ANOVA was used (factors were component and substance injected: cocaine or saline). A one-way, repeated-measures ANOVA was used to assess the effects of successive components in the substitution for cocaine of the test drugs. A two-way ANOVA was used to assess the effects of presession treatments of the test drugs on cocaine self-administration, and for the comparison of effects of drug pretreatments on responding maintained by drug injection or food reinforcement.
For the comparison of the effects of drug pretreatments, the maximal response rates maintained by drug injection were compared with rates of responding maintained by food reinforcement (expressed as a percentage of control values). Effects on rates of responding maintained by food reinforcement during the fourth component of the session, corresponding to the component in which maximal response rates were maintained by drug injection, were used for this comparison. Dose-effect functions for the σR ligands were analyzed by use of ANOVA and linear regression techniques. From this analysis, ED50 values and their 95% confidence limits were derived from data by use of the linear portions of the dose-effect curves. For all analyses, the criterion for significance was set at p < 0.05.
Results
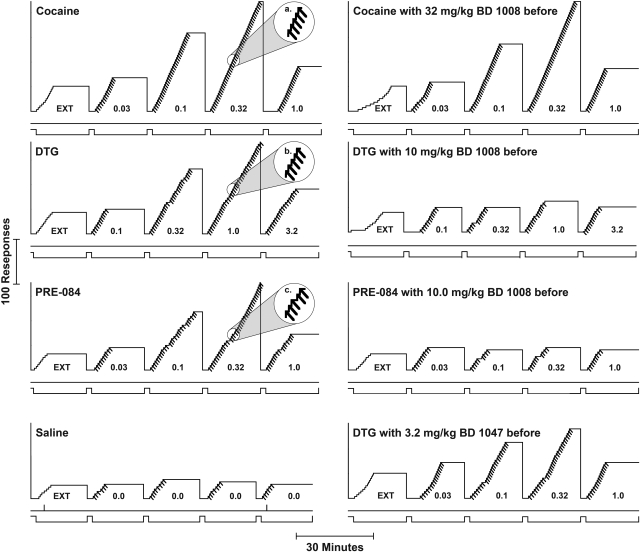
Performances maintained by cocaine were similar to those reported previously under FR schedules with various reinforcers; a brief pause was followed by a sequence of five responses made in rapid succession producing the injection (representative records of cumulative responding are shown in Fig. 1, top left, inset labeled a). Responses were rarely emitted on the inactive lever (vertical marks on the line below cumulative curve), or during the 2-min time-out periods between components with incrementing dose (lower event line displaced upward). During the EXT component, no injections were delivered and response rates were low and emitted in a pattern resembling the FR pattern. As dose of cocaine increased, response rates increased and pauses decreased. The highest rate of responding was obtained in the fourth component in which injections of 0.32 mg/kg/inj were available. When saline injections were available in the second through fifth components (Fig. 1, bottom left), responses were never emitted at rates greater than those maintained in EXT (first component).
Fig. 1.
Representative cumulative records of self-administration maintained by intravenous cocaine injection under the fixed-ratio five-response schedule and those obtained when σR agonists or saline were substituted for cocaine. Ordinates, cumulative responses. Abscissae, time. Each experimental session started with a 2-min time-out period during which all LEDs over the levers were off, and responses had no scheduled consequences (lower event line up). After the time-out (lower event line down), lights above the levers were illuminated, although responses had no scheduled consequences (extinction, EXT) for 20 min, followed by another 2-min time-out. When the LEDs were again illuminated, each fifth response turned off the LEDs and activated the infusion pump (diagonal marks on the cumulative record). Vertical marks on the line below the cumulative curve indicate responses on the left (inactive) lever. A 20-s time-out period during which lights were off and responding had no scheduled consequences followed each injection, after which the LEDs were again illuminated, and each fifth response produced an injection. During each 20-min period of drug availability, the injection volumes were adjusted to deliver doses (in mg/kg/inj as indicated on the figure) in an ascending order. The cumulative curve reset to the baseline at the end of the 20-min component.
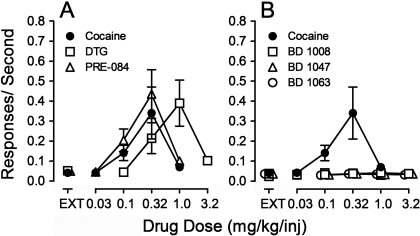
The average response rates maintained were a bell-shaped function of cocaine dose, with a maximum of 0.39 ± 0.11 responses/s at 0.32 mg/kg/inj, which was approximately 10-fold, and significantly greater than the 0.04 responses/s occurring in EXT (Fig. 2A, ●). One-way repeated-measures ANOVA indicated a significant effect of component (dose or no injection) on response rate (F4,44 = 11.5; p < 0.001). Post hoc comparisons indicated that the 0.32 mg/kg/inj maintained response rates greater than those obtained in EXT, indicative of the reinforcing effects of cocaine.
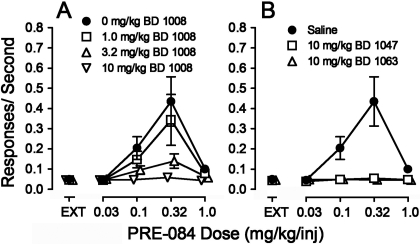
Fig. 2.
Substitution of σR ligands in rats trained to self-administer cocaine. Ordinates, responses per second. Abscissae, injection dose in mg/kg. Each point represents the mean ± S.E.M. (n = 6). A, cocaine (●), DTG (□), and PRE-084 (Δ). B, cocaine (●), BD 1008 (□), BD 1047 (Δ), and BD 1063 (○).
In rats trained to self-administer cocaine, DTG maintained responding that resembled that maintained by cocaine in all important aspects (Fig. 1, top middle left, inset labeled b). The highest rate of responding was maintained at a dose of 1.0 mg/kg/inj, with lower response rates at higher and lower doses (Fig. 2A, □). The maximal response rates maintained, and the shape of the DTG dose-effect curve were comparable with those for cocaine; DTG was approximately 3-fold less potent than cocaine (Fig. 2A). Response rates (F4,20 = 8.78, p < 0.001) were significantly affected by dose, and post hoc tests indicated that rates maintained by 1.0 mg/kg/inj were significantly greater than those in EXT.
Performances maintained by PRE-084 in rats trained to self-administer cocaine were also similar to those maintained by cocaine (Fig. 1, middle bottom left, inset labeled c). PRE-084 maintained maximal response rates comparable with those maintained by cocaine, and had a potency similar to that of cocaine (Fig. 2A, Δ). The highest rate of responding was maintained by 0.32 mg/kg/inj, and lower rates were maintained at higher and lower doses. Response rates (F4,20 = 11.0, p < 0.001) were significantly affected by dose, and post hoc tests indicated that rates maintained by 0.32 mg/kg/inj were significantly greater than those in EXT.
In contrast to results with the σR agonists, none of the substituted doses of any of the antagonists maintained response rates comparable with those maintained by cocaine (Fig. 2B). Statistically significant effects of component were obtained with BD 1008 (F4,20 = 20.0, p < 0.001), BD 1047 (F4,20 = 12.0, p < 0.001), and BD 1063 (F4,20 = 2.96, p = 0.045). Post hoc tests for BD 1063 and BD 1047 indicated that none of the response rates with the antagonists available were significantly greater than those obtained during the EXT component. For BD 1008, the only injection dose at which rates were greater than those in EXT was 1.0 mg/kg, and at this dose rates were less than 0.005 responses per second greater than those occurring during the EXT component.
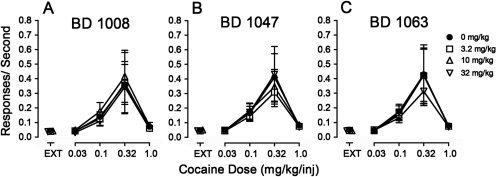
The σR antagonists generally had no significant effects on the self-administration of cocaine (e.g., Fig. 1, top right, and Fig. 3). Two-way ANOVA of the effects of BD 1008 on response rates indicated a significant effect of cocaine dose (F4,115 = 12.5, p < 0.001), a nonsignificant effect of presession BD 1008 treatment (F3,115 = 0.160, p = 0.923), and their interaction (F12,115 = 0.031, p = 1.0). Likewise, the two-way ANOVAs of the effects of BD 1047 or BD 1063 on response rates indicated a significant effect of cocaine dose (F4,100 = 15.9 or 13.8, respectively; p values <0.001), but effects of neither presession treatment (F3,100 = 0.198 or 0.188, respectively, p values ≥ 0.89) nor the interaction of σR antagonist dose and cocaine dose (F12,100 = 0.135 or 0.087; p values = 1.0).
Fig. 3.
Effects of presession treatments with σR antagonists on cocaine self-administration. Ordinates, responses per second. Abscissae, injection dose in mg/kg. Each point represents the mean ± S.E.M. (n = 6). BD 1008, BD 1047, and BD 1063 were administered intraperitoneally at 5, 15, and 5 min before sessions, respectively. A, effects of BD 1008 (3.2, 10, and 32 mg/kg) on cocaine self-administration. B, effects of BD 1047 (3.2, 10, and 32 mg/kg) on cocaine self-administration. C, effects of BD 1063 (3.2, 10, and 32 mg/kg) on cocaine self-administration.
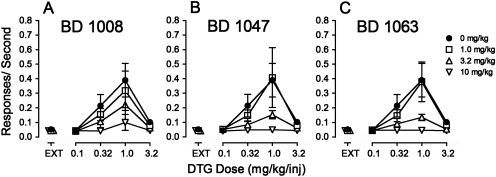
In contrast to their effects on cocaine self-administration, each of the σR antagonists dose-dependently decreased response rates maintained by DTG when it was substituted for cocaine (Fig. 4). The decreases were expressed as a flattening of the bell-shaped DTG dose-effect curve. At the highest doses of the antagonists, no dose of DTG maintained responding at levels above those maintained in EXT (Fig. 4; compare ▿ with ●). Patterns of responding maintained by DTG after pretreatment with 10 mg/kg BD 1008 resembled those obtained when saline was substituted for cocaine in all important aspects (Fig. 1; compare records in second row). An ANOVA of the effects on response rates of BD 1008 during DTG self-administration indicated a significant effect of DTG dose (F4,60 = 5.11, p = 0.005), presession BD 1008 treatment (F3,60 = 13.4; p < 0.001), and the interaction of the two (F12,60 = 7.73, p < 0.001). Post hoc tests indicated that the effects of 3.2 and 10 mg/kg BD 1008 significantly decreased response rates maintained by the 1.0 mg/kg/inj dose of DTG.
Fig. 4.
Effects of presession treatments with σR antagonists on self-administration of DTG in rats trained to self-administer cocaine. Ordinates, responses per second. Abscissae, injection dose in mg/kg. Each point represents the mean ± S.E.M. (n = 6). BD 1008, BD 1047, and BD 1063 were administered intraperitoneally at 5, 15, and 5 min before sessions, respectively. A, effects of BD 1008 (1.0, 3.2, and 10 mg/kg) on DTG self-administration. B, effects of BD 1047 (1.0, 3.2, and 10 mg/kg) on DTG self-administration. C, effects of BD 1063 (1.0, 3.2, and 10 mg/kg) on DTG self-administration.
The antagonists, BD 1047 and BD 1063, like BD 1008, dose-dependently decreased response rates maintained by DTG in rats trained to self-administer cocaine (Fig. 4, B and C, respectively, and Fig. 1, bottom right). At the 3.2 mg/kg dose of each antagonist, the maximal effect of DTG was significantly decreased, and at the highest doses of BD 1047 and BD 1063, rates of responding were not greater than those obtained in EXT (Fig. 4; compare ▿ with ● and with levels maintained in EXT). An ANOVA of the effects on response rates of each of these drugs indicated significant effects of DTG dose (F4,60 = 6.19 and 9.01, respectively; p values ≤ 0.002), of antagonist treatment (F3,60 = 5.82 and 8.37, respectively; p values ≤ 0.008), and the interaction of the two (F12,60 = 3.27 and 6.36, respectively; p values ≤ 0.001). Post hoc tests indicated that doses of 3.2 and 10 mg/kg BD 1047 significantly decreased response rates maintained by the 1.0 mg/kg/inj dose of DTG. The same doses of BD 1063 significantly decreased response rates maintained by the 1.0 mg/kg/inj dose of DTG.
The substitution of PRE-084 for cocaine was also blocked by BD 1008, which produced a dose-related flattening of the bell-shaped PRE-084 dose-effect curve (Fig. 5A). At the highest dose of BD 1008, no dose of PRE-084 maintained responding at levels above those maintained in EXT (Fig. 5; compare ▿ with ● above EXT). Patterns of responding maintained by PRE-084 after pretreatment with 10 mg/kg BD 1008 resembled those obtained when saline was substituted for cocaine in all important aspects (Fig. 1; compare records in third row). The ANOVA of effects of BD 1008 on response rates indicated a significant effect of PRE-084 dose (F4,60 = 9.09, p < 0.001), presession BD 1008 treatment (F3,60 = 10.3; p < 0.001), and their interaction (F12,60 = 8.07; p < 0.001). Post hoc tests indicated that the effects of 3.2 and 10 mg/kg BD 1008 significantly decreased response rates maintained by the 0.32 mg/kg/inj dose of PRE-084.
Fig. 5.
Effects of presession treatments with σR antagonists on self-administration of PRE-084 in rats trained to self-administer cocaine. Ordinates, responses per second. Abscissae, injection dose in mg/kg. Each point represents the mean ± S.E.M. (n = 6). BD 1008, BD 1047, and BD 1063 were administered intraperitoneally at 5, 15, and 5 min before sessions, respectively. A, effects of BD 1008 (1.0, 3.2, and 10 mg/kg) on PRE-084 self-administration. B, effects of BD 1047 or BD 1063 (each at 10 mg/kg) on PRE-084 self-administration.
Effects of pretreatments with BD 1047 or BD 1063 on PRE-084 self-administration when it was substituted for cocaine were only studied at the 10 mg/kg dose (Fig. 5B). Pretreatment with either drug decreased rates of responding to levels maintained in EXT. The ANOVAs for effects of BD 1047 and BD 1063 on response rates indicated significant effects of PRE-084 dose (F4,20 = 11.0 and 11.5, respectively; p values < 0.001), presession σR antagonist treatment (F1,20 = 13.4 and 12.6, respectively; p values ≤ 0.016), and significant interactions of the two (F4,20 = 11.0 and 10.5, respectively; p values < 0.001). Post hoc tests indicated that the effects of 10 mg/kg BD 1047 and BD 1063 significantly decreased response rates maintained by the 0.32 mg/kg/inj dose of PRE-084. In addition, post hoc tests indicated that the effects of 10.0 mg/kg BD 1047 and BD 1063 significantly decreased response rates maintained by the 0.1 mg/kg/inj dose of PRE-084.
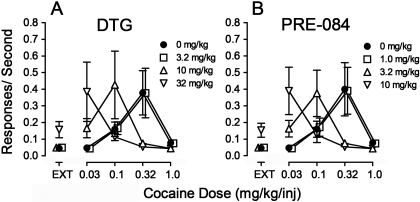
Presession treatments with DTG or PRE-084 produced an apparent leftward shift in the cocaine self-administration dose-effect curve, without affecting maximal response rate (Fig. 6A and 6B). The lowest doses were inactive, and doses of 10 and 3.2 mg/kg DTG and PRE-084, respectively, produced shifts that approximated 3-fold. The highest doses of DTG or PRE-084 seemed to shift the cocaine dose-effect curve further leftward, and to produce an increase in response rates during EXT. The ANOVA results of DTG and PRE-084 pretreatments indicated significant effects of cocaine dose (F4,60 = 6.05 and 5.77, respectively; p values ≤ 0.003); however, the effects of the presession treatments did not achieve statistical significance (F3,60 = 0.433 and 2.18, respectively; p values ≥ 0.133). There were, however, significant interactions of cocaine dose and σR agonist pretreatment (F12,60 = 4.00 and 6.15, respectively; p values < 0.001). Post hoc tests indicated that 32 mg/kg DTG increased response rates at a cocaine dose per injection of 0.03 mg/kg, and 10 mg/kg DTG increased rates at 0.1 mg/kg. In addition, at 0.32 mg/kg/inj cocaine both 10 and 32 mg/kg DTG decreased response rates. Likewise, post hoc tests indicated that 10 mg/kg PRE-084 increased response rates at the lowest cocaine dose per injection, and 3.2 mg/kg PRE-084 increased rates at 0.1 mg/kg/inj cocaine. Finally, at the injection dose of cocaine that maintained the highest response rates, both 3.2 and 10 mg/kg PRE-084 decreased those rates.
Fig. 6.
Effects of presession treatments with σR agonists on cocaine self-administration. Ordinates, responses per second. Abscissae, injection dose in mg/kg. Each point represents the mean ± S.E.M. (n = 6). Both σR agonists were administered intraperitoneally at 30 min before sessions. A, effects of DTG (3.2, 10, and 32 mg/kg) on cocaine self-administration. B, effects of PRE-084 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration.
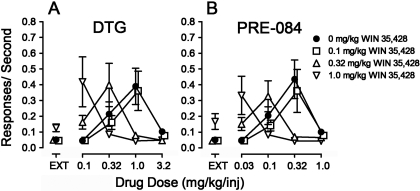
Presession treatments with the dopamine uptake inhibitor, WIN 35,428, in rats trained to self-administer cocaine shifted the DTG and PRE-084 self-administration dose-effect curves leftward, without affecting maximum response rates (Fig. 7, A and B). The leftward shifts were dose-dependent, with the lowest doses inactive; doses of 0.32 and 1.0 mg/kg WIN 35,428 shifted the dose-effect curve approximately 3- and 10-fold to the left, respectively (Fig. 7, A and B). In addition, at the highest dose, WIN 35,428 treatment increased response rates during EXT (Fig. 7, A and B, ▿). A two-way ANOVA indicated a significant effect of DTG (F4,60 = 7.07, p < 0.001) and PRE-084 dose (F4,60 = 8.35, p < 0.001), and presession treatment dose of WIN 35,428 (F3,60 = 6.84 and 10.4, respectively; p values ≤ 0.004), and a significant interaction of the two (F12,60 = 7.04 and 7.70, respectively; p values < 0.001).
Fig. 7.
Effects of presession treatments with WIN 35,428 on self-administration of σR agonists in rats trained to self-administer cocaine. Ordinates, responses per second. Abscissae, injection dose in mg/kg. Each point represents the mean ± S.E.M. (n = 6). WIN 35,428 was administered intraperitoneally at 5 min before sessions. A, effects of WIN 35,428 (0.1, 0.32, and 1.0 mg/kg) on DTG self-administration. B, effects of WIN 35,428 (0.1, 0.32, and 1.0 mg/kg) on PRE-084 self-administration.
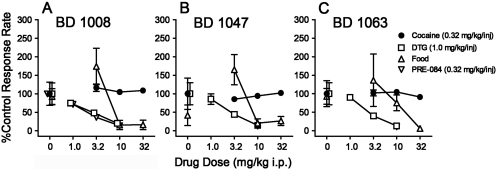
The effects of the σR antagonists on the highest rates of responding maintained by cocaine, DTG, or PRE-084 injection were compared with rates of responding in the corresponding component of food reinforcement sessions. BD 1008 decreased rates of responding maintained by DTG at doses lower than those necessary to decrease response rates maintained by food reinforcement (Fig. 8A, compare □ with Δ). Across this same range of doses, responding maintained by cocaine was not affected (Fig. 8A, ●). The self-administration of PRE-084 was affected at doses similar to those that decreased rates of responding maintained by DTG (Fig. 8A, ▿). ANOVA indicated a significant effect of dose (F3,65 = 9.38; p < 0.001) and reinforcer (F3,65 = 11.6; p < 0.001). Table 1 shows ED50 values for these effects indicating that BD 1008 was over 4-fold more potent against responding maintained by DTG and PRE-084 than against food-maintained responding.
Fig. 8.
Effects of presession treatments with σR antagonists on responding maintained by cocaine, PRE-084, or DTG injection or food presentation. Ordinates, response rates as percentage of control response rates (sessions before drug tests). Abscissae, mg/kg test compounds administered intraperitoneally, log scale. BD 1008, BD 1047, and BD 1063 were administered intraperitoneally at 5, 15, and 5 min before sessions, respectively. Responding was from the fourth 20-min component of the session (see Materials and Methods). Each point represents the mean ± S.E.M. (n = 6). Rates of responding maintained by food reinforcement averaged 0.58 ± 0.11 responses/s, whereas those maintained by cocaine, DTG, and PRE-084 averaged 0.39 ± 0.10, 0.39 ± 0.12, and 0.43 ± 0.12 responses/s, respectively. None of these control response rates were significantly different (p = 0.255, one-way ANOVA).
Table 1.
ED50 values (in milligrams per kilogram with 95% confidence limits) for σR antagonists and agonists in decreasing rates of responding maintained by DTG, PRE-084, cocaine, or food presentation
| Treatment | DTG | PRE-084 | Cocaine | Food Presentation |
|---|---|---|---|---|
| BD 1008 | 2.88 (2.46–3.36) | 2.19 (1.35–3.13) | NS | 13.2 (7.02–35.6) |
| BD 1047 | 9.47 (5.96–14.6) | NT | ND | 14.3 (7.46–46.2) |
| BD 1063 | 2.95 (2.46–3.52) | NT | NS | 15.0 (5.23–3590) |
NS, nonsignificant linear regression; ND, not determined; NT, not tested.
The σ antagonists, BD 1047 and BD 1063, also had selective effects on responding maintained by the σ agonist, DTG (Fig. 8, B and C, respectively), with decreases in rates of responding maintained by DTG (□) at lower doses than those that decreased rates maintained by food (Δ), and little if any effect on response rates maintained by cocaine (●). The ANOVAs for each of these treatments indicated a significant effect of dose for both BD 1047 and BD 1063 (F3,48 = 6.17; p = 0.001 and F3,48 = 4.09; p = 0.011, respectively). Significant effects of reinforcer were also obtained for both drugs (F2,48 = 7.18; p = 0.002 and F3,48 = 6.99; p = 0.002, respectively). BD 1063 (Fig. 8C), like BD 1008, was more potent in decreasing responding maintained by DTG injection than it was against responding maintained by food reinforcement. In contrast, BD 1047 was approximately equipotent in decreasing responding maintained by the two reinforcers (Fig. 8B and Table 1).
Discussion
The first main finding of the present study was that the σR agonists, DTG and PRE-084, dose-dependently shifted the cocaine self-administration dose-effect curve to the left, indicating that administration of these compounds potentiates the reinforcing effects of cocaine. Second, the σR agonists maintained self-administration when substituted for cocaine, indicating reinforcing effects of these compounds. Furthermore, similar to the effects of the σR agonists on cocaine self-administration, the DA uptake inhibitor, WIN 35,428, potentiated the reinforcing effects of the σR agonists, indicating reciprocity in the potentiation. However, the σR antagonists, BD 1008, BD 1047, and BD 1063, were without effect on cocaine-maintained self-administration behavior, but dose-dependently antagonized behavior maintained by the σR antagonists. These effects differ from previous results for σR ligands by use of place conditioning (see below). The self-administration of σR agonists in subjects trained with cocaine and the facilitation of that effect by dopamine uptake inhibition suggest unique interactions between the σR and dopaminergic system that may have important implications for the abuse-related effects of cocaine (Maurice et al., 2002; Matsumoto et al., 2003).
Reinforcing effects of σR agonists in subjects trained to self-administer cocaine are not without precedent. Slifer and Balster (1983) showed self-administration in rhesus monkeys of the benzomorphans, (+)-SKF 10047 and (+)-cyclazocine, which have well documented affinities for σRs. However, these benzomorphans also have affinities for phencyclidine binding sites on N-methyl-d-aspartate receptors, which are only 4- to 5-fold lower than their σR affinities (Su, 1993). The present reinforcing effects of the σR agonists, PRE-084 and DTG, in rats trained to self-administer cocaine can be more confidently attributed to their actions at the σR. Both compounds are selective for the σR over other binding sites, with more than 200- (DTG) or 2000-fold (PRE-084) greater affinity for σRs over N-methyl-d-aspartate receptors (Su, 1993). In addition, the self-administration of σR agonists was blocked by BD 1008, BD 1047, and BD 1063, which are selective σR antagonists (de Costa et al., 1992).
Subtypes of σRs have been identified, and most studies have concentrated on a role of the σ1R in altering the effects of cocaine (see reviews cited earlier). However, several studies have also suggested a role of the σ2R in modulating the dopamine system (Nuwayhid and Werling, 2006) and the effects of cocaine (Matsumoto and Mack, 2001). Thus, it is possible that either σR subtype is involved to some degree in the present effects. PRE-084 is selective for the σ1 over σ2 receptor (Maurice et al., 1999), and although the antagonist, BD 1008, is not selective, BD 1047 and BD 1063 are approximately 50-fold selective for σ1Rs over σ2Rs (Matsumoto et al., 1995; McCracken et al., 1999). Thus, the substitution of the selective agonist, PRE-084, and the antagonism of both agonists by each of the antagonists suggests that the self-administration of σR agonists was mediated by σ1Rs.
The antagonism of DTG and PRE-084 self-administration by σR antagonists was expressed as a downward rather than rightward shift in the agonist dose-effect curve. The absence of a rightward shift does not necessarily imply a noncompetitive interaction. When distinct mechanisms contribute to the ascending and descending limbs of a biphasic dose-effect curve, a downward shift is consistent with a competitive interaction in which only the low-dose effects are antagonized (Bergman et al., 2000; Collins et al., 2005).
A valid σR-mediated effect in behaving animals has been elusive (Su, 1993). Previous studies have examined the induction of a dystonic torticollis resulting from microinjection of σR ligands into the red nucleus of the rubrocerebellar system. Among σR ligands there was a correlation between σR-binding affinities and ED50 values for producing torticollis (Matsumoto et al., 1990). However, haloperidol, which in general is considered to have antagonist effects at σRs (Hayashi and Su, 2004), produced torticollis, and the σR agonist (+)-3-PPP was inactive, even though the latter compound often shows peculiar effects (Walker et al., 1988). Furthermore, the antagonism of torticollis by σR antagonists can be unreliable (Matsumoto et al., 1995, 1996). The present results indicate that substitution in subjects trained to self-administer cocaine could serve as a pharmacologically specific in vivo σR-agonist effect, which may facilitate the discovery of more selective behaviorally active σR agonists and antagonists. As such, the present results have implications for previously published findings. For example, the minimally effective antagonist dose of BD 1047 against DTG self-administration was 3.2 mg/kg. As mentioned above, Martin-Fardon et al. (2007) found an effect on reinstatement of cocaine self-administration at 20 mg/kg BD 1047, which is well above the in vivo σR antagonist dose identified in the present study. Thus, the effects observed by Martin-Fardon et al. (2007) are either relatively insensitive to σR antagonism or may not be σR mediated. However, a recent report by Sabino et al. (2009) showed decreases in ethanol self-administration produced by BD 1063 at doses similar to those shown to have in vivo σR antagonist effects in the present study.
Despite evidence for the involvement of the σR in behavioral effects of cocaine, the σR antagonists were inactive in altering the self-administration of cocaine, findings that extend those reported previously (Martin-Fardon et al., 2007). That lack of activity against cocaine self-administration contrasts with the dose-dependent blockade by σR antagonists of cocaine-induced place conditioning in mice (Romieu et al., 2000, 2002), another procedure used to assess (albeit indirectly) reinforcing effects. Furthermore, the σR-agonist substitution for cocaine in the present study contrasts with previous findings that the σR agonists, igmesine and PRE-084, did not produce significant place conditioning (Romieu et al., 2002). These seemingly disparate findings in procedures assessing reinforcing effects highlight the important consideration that different drug effects can be expressed depending on the environmental circumstances (the details of the behavioral procedures). The behaviors expressed in self-administration, place-conditioning, and other procedures are a function of incompletely overlapping sets of variables, and as such, drugs can have quite different effects that depend on the conditions of the study.
One possibly important condition of the present study is the history of the subject. Whether σR agonists have reinforcing effects in subjects not previously exposed to cocaine is currently under study in our laboratory. Several studies have indicated that a history of cocaine exposure changes parameters of the σR system. For example, Ujike and colleagues (Ujike et al., 1992a,b) showed that daily injections of cocaine or methamphetamine in rats produced an increase in the stereotyped behavioral response to the σR agonist (+)-3-PPP as well as sensitization to the stimulant drugs. That sensitization can be blocked by cotreatment with the σR antagonists, BMY 14802, rimcazole (Ujike et al., 1992a, 1996), or the flavone-derivative σR antagonist, NPC 16377 (Witkin et al., 1993). More recently, Liu et al. (2005) and Liu and Matsumoto (2008) reported that treatment with cocaine up-regulated several immediate early genes, among them fra-2 and σ1R genes, and protein levels. Those effects were prevented by coadministration of BD 1063. Furthermore, the changes in fra-2 and σ1R expression corresponded with increases in the behavioral sensitization resulting from repeated cocaine and were also blocked by BD 1063.
In the present study, a dopamine uptake inhibitor WIN 35,428 shifted the dose-effect curves for the σR agonists to the left, suggesting that elevated dopamine facilitates these σR agonist actions. In addition, the σR agonists similarly shifted the cocaine self-administration dose-effect curve leftward. Mechanisms for these reciprocal effects are not currently clear. Su and Hayashi (2001) have pointed out that the indirect agonist actions induced by dopamine uptake inhibitors mediated by dopamine D1-like, possibly D5, receptors (Undie et al., 2000; Sahu et al., 2009) will activate phospholipase C, which increases intracellular inositol 1,4,5-triphosphate and, in turn, increases Ca2+ efflux from the endoplasmic reticulum. Agonist actions at σ1Rs can produce a dissociation of the σR-ankyrin complex (Hayashi and Su, 2001) from inositol 1,4,5-triphosphate receptors enhancing binding of inositol 1,4,5-triphosphate to its receptor and further increasing calcium signaling. Thus, through actions mediated by dopamine D1-like receptors, indirect dopaminergic agonists and σR agonists may have synergistic effects on intracellular calcium signaling. These synergistic effects may contribute to the reciprocal facilitation of self-administration of σR agonists and DA uptake inhibitors.
The wide distribution of σR throughout the CNS, and the involvement of σRs in a variety of disease states have been detailed (Maurice and Su, 2009). That distribution and widespread involvement may portend a lack of specificity that may ultimately limit the therapeutic utility of σR ligands for a particular indication. However, although σR antagonists alone may not be effective in blocking the primary reinforcing effects of cocaine, they are effective in modulating other effects of cocaine, such as those involved in stimulus-drug conditioning that is fundamental to place conditioning. The present findings suggest further that, although σR antagonism alone will not appreciably blunt the direct reinforcing effects of cocaine, a treatment strategy directed at dopamine transport mechanisms along with intracellular calcium signaling may constitute a combined dual targeting that has greater efficacy than targeting either single mechanism alone.
Acknowledgments
We thank Patty Ballerstadt for administrative assistance, Dr. Tsung-Ping Su for generous gifts of compounds and invaluable advice, and Dr. Teruo Hayashi for his advice on these studies.
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse; and a Japan Society for the Promotion of Science Research Fellowship for Japanese Biomedical and Behavioral Researchers at the National Institutes of Health (to T.H.).
Parts of this work were presented previously: 2009 Annual Meeting of the Society for Neuroscience; 2009 Oct 17–21; Chicago, IL; and 2008 Meeting of the Behavioral Pharmacology Society; 2008 Apr 5–9; San Diego, CA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.159236
- σR
- σ-receptor
- σ1R
- σ1-receptor
- σ2R
- σ2-receptor
- inj
- injection
- BD 1008
- N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide
- BD 1047
- N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide; BD 1063, 1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride
- DTG
- 1,3-di-(2-tolyl)guanidine
- PRE-084
- 2-(4-morpholinethyl)1-phenylcyclohexane-1-carboxylate hydrochloride
- SKF 10,047
- N-allylnormetazocine hydrochloride
- WIN 35,428
- (-)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane tartrate
- BMY 14802
- α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazine-butanol
- NE-100
- N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]ethylamine
- NPC 16377
- 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone hydrochloride
- (+)-3-PPP
- (+)-3-(3-hydroxyphenyl-N-(1-propyl)piperidine
- ANOVA
- analysis of variance
- EXT
- extinction
- FR
- fixed ratio
- LED
- light-emitting diode.
References
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. (2000) Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology (Berl) 153:67–84 [DOI] [PubMed] [Google Scholar]
- Cao J, Kulkarni SS, Husbands SM, Bowen WD, Williams W, Kopajtic T, Katz JL, George C, Newman AH. (2003) Dual probes for the dopamine transporter and sigma1 receptors: novel piperazinyl alkyl-bis(4′-fluorophenyl)amine analogues as potential cocaine-abuse therapeutic agents. J Med Chem 46:2589–2598 [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. (2005) Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther 314:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Costa BR, Iadarola MJ, Rothman RB, Berman KF, George C, Newman AH, Mahboubi A, Jacobson AE, Rice KC. (1992) Probes for narcotic receptor mediated phenomena. 18. Epimeric 6 alpha- and 6 beta-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5 alpha-epoxymorphinans as potential ligands for opioid receptor single photon emission computed tomography: synthesis, evaluation, and radiochemistry of [125I]-6 beta-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5 alpha-epoxymorphinan. J Med Chem 35:2826–2835 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. (1994) Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther 271:212–219 [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2001) Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci U S A 98:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2004) Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs 18:269–284 [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. (2005) Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther 314:770–779 [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. (2008) Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther 327:187–195 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. (2007) Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology 32:1967–1973 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. (1995) Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol 280:301–310 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Walker JM, Patrick SL, Zambon AC, Vo VN, Truong DD, De Costa BR, Rice KC. (1996) Dissociation of the motor effects of (+)-pentazocine from binding to sigma 1 sites. Eur J Pharmacol 301:31–40 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Hemstreet MK, Lai NL, Thurkauf A, De Costa BR, Rice KC, Hellewell SB, Bowen WD, Walker JM. (1990) Drug specificity of pharmacological dystonia. Pharmacol Biochem Behav 36:151–155 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. (2003) Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol 469:1–12 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Mack AL. (2001) (+/−)-SM 21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice. Eur J Pharmacol 417:R1–2 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. (2001) Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol 419:163–174 [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. (2002) Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev 26:499–527 [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Noda Y, Yamada K, Privat A, Nabeshima T. (1999) The attenuation of learning impairments induced after exposure to CO or trimethyltin in mice by sigma (sigma) receptor ligands involves both sigma1 and sigma2 sites. Br J Pharmacol 127:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Su TP. (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, Matsumoto RR. (1999) Novel sigma receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur J Pharmacol 365:35–38 [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. (1991) Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol 201:251–252 [DOI] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. (2003) Sigma1 receptor agonist-mediated regulation of N-methyl-d-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J Pharmacol Exp Ther 304:364–369 [DOI] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. (2006) Sigma2 (sigma2) receptors as a target for cocaine action in the rat striatum. Eur J Pharmacol 535:98–103 [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. (2000) Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport 11:2885–2888 [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. (2002) Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology 26:444–455 [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr., Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. (2009) The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology 34:1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. (2009) D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol 75:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. (1988) Cocaine binding at sigma receptors. Eur J Pharmacol 149:171–174 [DOI] [PubMed] [Google Scholar]
- Slifer BL, Balster RL. (1983) Reinforcing properties of stereoisomers of the putative sigma agonists N-allylnormetazocine and cyclazocine in rhesus monkeys. J Pharmacol Exp Ther 225:522–528 [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. (2004) Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berl) 175:68–75 [DOI] [PubMed] [Google Scholar]
- Su TP. (1993) Delineating biochemical and functional properties of sigma receptors: emerging concepts. Crit Rev Neurobiol 7:187–203 [PubMed] [Google Scholar]
- Su TP, Hayashi T. (2001) Cocaine affects the dynamics of cytoskeletal proteins via sigma(1) receptors. Trends Pharmacol Sci 22:456–458 [DOI] [PubMed] [Google Scholar]
- Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. (1992a) Sigma (sigma) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci 50:PL129–PL134 [DOI] [PubMed] [Google Scholar]
- Ujike H, Kuroda S, Otsuki S. (1996) sigma Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol 296:123–128 [DOI] [PubMed] [Google Scholar]
- Ujike H, Tsuchida K, Akiyama K, Otsuki S. (1992b) Supersensitivity of sigma receptors after repeated administration of cocaine. Life Sci 51:PL31–PL36 [DOI] [PubMed] [Google Scholar]
- Ukai M, Mori E, Kameyama T. (1997) Modulatory effects of morphine, U-50488H and 1,3-di-(2-tolyl)guanidine on cocaine-like discriminative stimulus in the rat using two-choice discrete-trial avoidance paradigm. Methods Find Exp Clin Pharmacol 19:541–546 [PubMed] [Google Scholar]
- Undie AS, Berki AC, Beardsley K. (2000) Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology 39:75–87 [DOI] [PubMed] [Google Scholar]
- Wachtel SR, White FJ. (1988) Electrophysiological effects of BMY 14802, a new potential antipsychotic drug, on midbrain dopamine neurons in the rat: acute and chronic studies. J Pharmacol Exp Ther 244:410–416 [PubMed] [Google Scholar]
- Walker JM, Matsumoto RR, Bowen WD, Gans DL, Jones KD, Walker FO. (1988) Evidence for a role of haloperidol-sensitive sigma-‘opiate’ receptors in the motor effects of antipsychotic drugs. Neurology 38:961–965 [DOI] [PubMed] [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. (1993) Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther 266:473–482 [PubMed] [Google Scholar]