Abstract
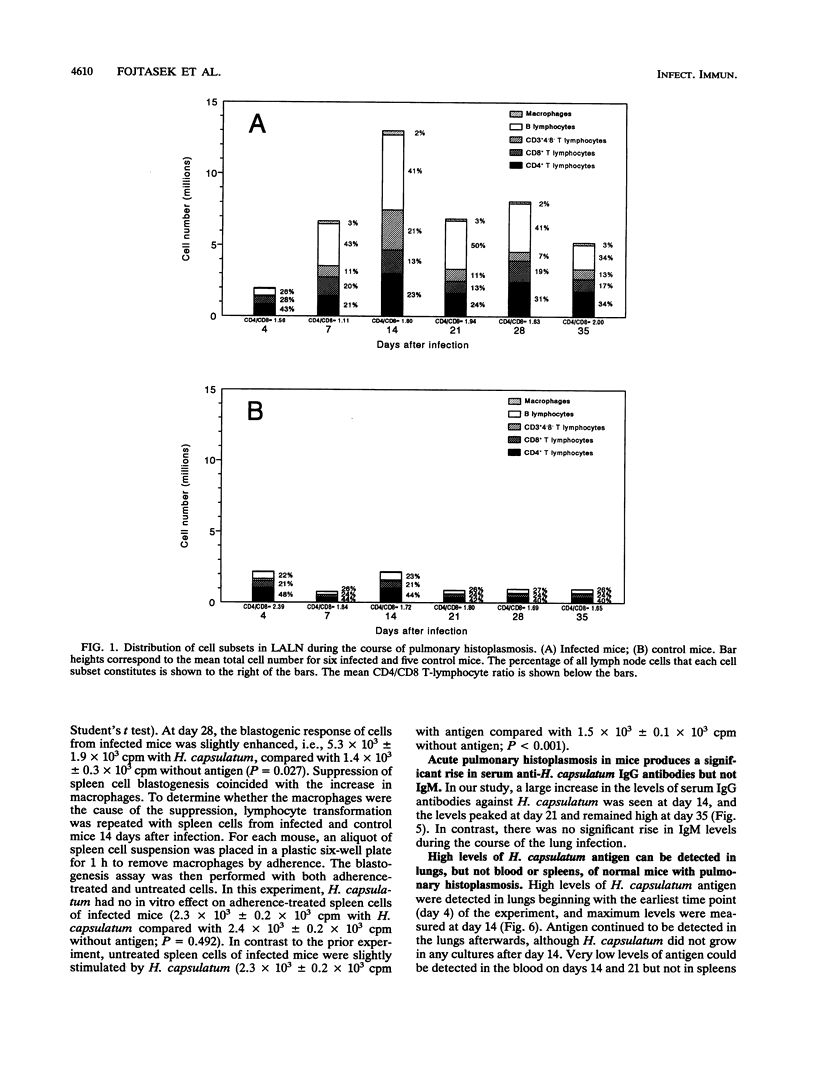
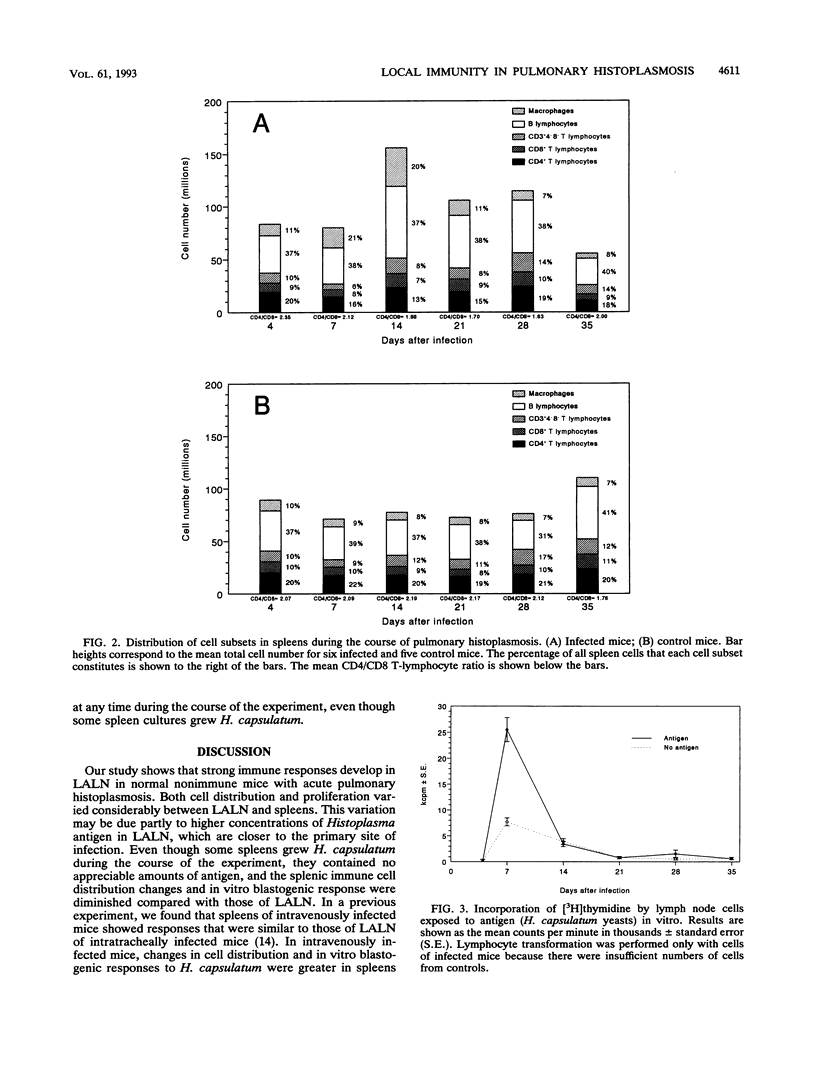
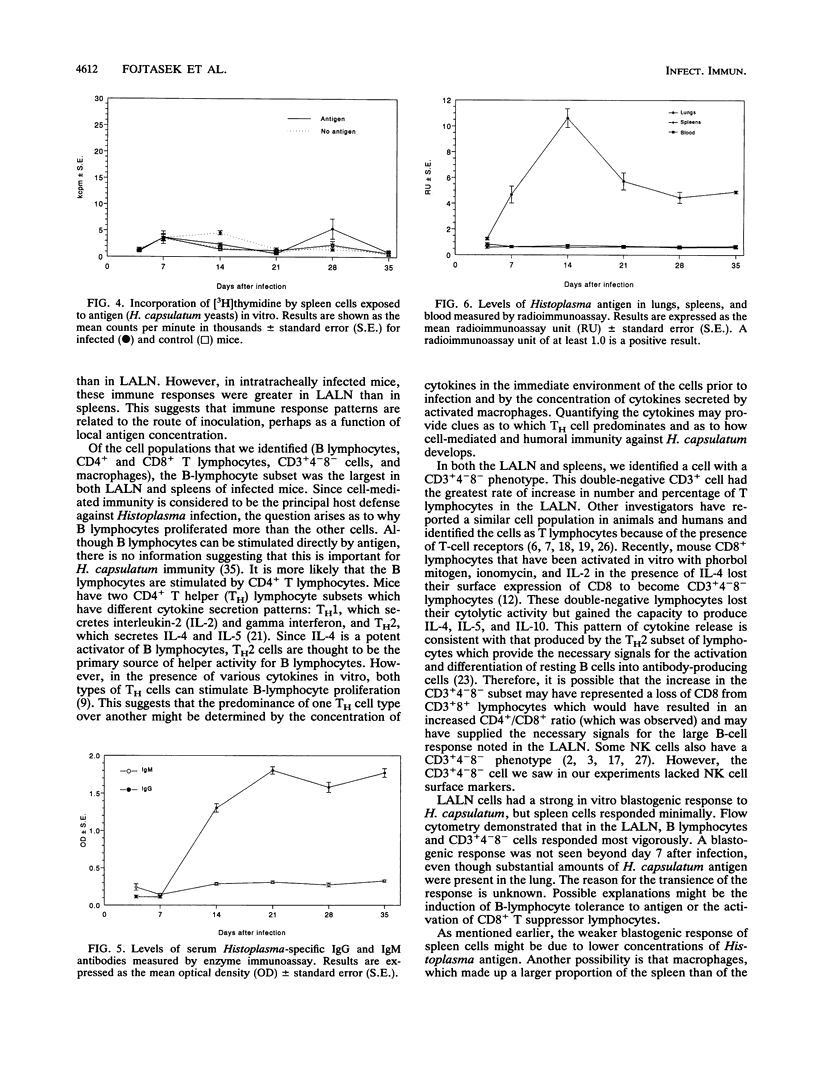
Local immunity against acute pulmonary histoplasmosis was studied in the lung-associated lymph nodes of normal nonimmune mice infected intratracheally with live Histoplasma capsulatum yeasts. The phenotypes and distribution of cells in lung-associated lymph nodes and spleens were determined by flow cytometry. In addition, the immune responsiveness of these cells was evaluated by in vitro blastogenesis. Anti-H. capsulatum antibodies in serum and H. capsulatum antigen in tissue were measured by immunoassays. Cellular immune responses were greater in the lymph nodes than in the spleens. In lymph nodes 7 days after infection, a marked increase in the number of B lymphocytes caused the percentage to rise to 43%, compared with 26% in controls, and it remained elevated throughout the course of infection. A CD3+ cell that did not express CD4 or CD8 increased in number until it constituted 21% of lymph node cells, compared with 5% in controls, by day 14. The numbers of CD4+ and CD8+ T lymphocytes were modestly increased from days 7 to 35, but their percentages dropped because of the greater numbers of B lymphocytes and CD3+4-8- cells. Macrophages consistently constituted 2 to 3% of lymph node cells during the study. In spleens 7 days after infection, the percentage of macrophages in infected mice rose to 21%, compared with 9% in controls, but the total spleen cell number did not increase until day 14, when all cell subsets were nearly double in number. The in vitro blastogenic response of lymph node cells to H. capsulatum peaked at day 7, but spleen cell response was minimal during the course of infection. Histoplasma-specific serum immunoglobulin G antibodies reached peak levels by day 21 and remained high to the end of the study. In contrast, levels of antigen-specific immunoglobulin M antibodies were very low. These data suggest that antigen-specific immune responses occur in lung-associated lymph nodes and that this draining lymph node response may be an important component in host defense against Histoplasma lung infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas Z. K., Rasmussen W. NK1.1+ thymocytes. Adult murine CD4-, CD8- thymocytes contain an NK1.1+, CD3+, CD5hi, CD44hi, TCR-V beta 8+ subset. J Immunol. 1990 Aug 15;145(4):1039–1045. [PubMed] [Google Scholar]
- Barton J. C., Prasthofer E. F., Egan M. L., Heck L. W., Jr, Koopman W. J., Grossi C. E. Rheumatoid arthritis associated with expanded populations of granular lymphocytes. Ann Intern Med. 1986 Mar;104(3):314–323. doi: 10.7326/0003-4819-104-3-314. [DOI] [PubMed] [Google Scholar]
- Baughman R. P., Kim C. K., Vinegar A., Hendricks D. E., Schmidt D. J., Bullock W. E. The pathogenesis of experimental pulmonary histoplasmosis. Correlative studies of histopathology, bronchoalveolar lavage, and respiratory function. Am Rev Respir Dis. 1986 Oct;134(4):771–776. doi: 10.1164/arrd.1986.134.4.771. [DOI] [PubMed] [Google Scholar]
- Baum G. L., Daniel T. M., Rice E. H. Immune globulin characterization of complement fixing antibodies in histoplasmic infection. Chest. 1973 Jul;64(1):16–20. doi: 10.1378/chest.64.1.16. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Cron R. Q., Cotterman M., Houlden B. A., Matis L. A. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4-, CD8- T lymphocytes. J Exp Med. 1988 Nov 1;168(5):1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Chandler J. W., Jr, Smith T. K., Newberry W. M., Jr, Chin T. D., Kirkpatrick C. H. Immology of the mycoses. II. Characterization of the immunoglobulin and antibody responses in histoplasmosis. J Infect Dis. 1969 Mar;119(3):247–254. doi: 10.1093/infdis/119.3.247. [DOI] [PubMed] [Google Scholar]
- Croft M., Swain S. L. B cell response to T helper cell subsets. II. Both the stage of T cell differentiation and the cytokines secreted determine the extent and nature of helper activity. J Immunol. 1991 Dec 1;147(11):3679–3689. [PubMed] [Google Scholar]
- Deepe G. S., Jr, Watson S. R., Bullock W. E. Cellular origins and target cells of immunoregulatory factors in mice with disseminated histoplasmosis. J Immunol. 1984 Apr;132(4):2064–2071. [PubMed] [Google Scholar]
- Defaveri J., Graybill J. R. Immunohistopathology of murine pulmonary histoplasmosis during normal and hypersensitive conditions. Am Rev Respir Dis. 1991 Dec;144(6):1366–1372. doi: 10.1164/ajrccm/144.6.1366. [DOI] [PubMed] [Google Scholar]
- Erard F., Wild M. T., Garcia-Sanz J. A., Le Gros G. Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science. 1993 Jun 18;260(5115):1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- Ettensohn D. B., Lalor P. A., Roberts N. J., Jr Human alveolar macrophage regulation of lymphocyte proliferation. Am Rev Respir Dis. 1986 Jun;133(6):1091–1096. doi: 10.1164/arrd.1986.133.6.1091. [DOI] [PubMed] [Google Scholar]
- Goodwin R. A., Jr, Des Prez R. M. State of the art: histoplasmosis. Am Rev Respir Dis. 1978 May;117(5):929–956. doi: 10.1164/arrd.1978.117.5.929. [DOI] [PubMed] [Google Scholar]
- Hayari Y., Kukulansky T., Globerson A. Regulation of thymocyte proliferative response by macrophage-derived prostaglandin E2 and interleukin 1. Eur J Immunol. 1985 Jan;15(1):43–47. doi: 10.1002/eji.1830150109. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Ruitenberg J. J., Phillips J. H. Human CD3+ T lymphocytes that express neither CD4 nor CD8 antigens. J Exp Med. 1986 Jul 1;164(1):339–344. doi: 10.1084/jem.164.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Weiss A. Presence of Ti (WT31) negative T lymphocytes in normal blood and thymus. Nature. 1986 Nov 20;324(6094):268–270. doi: 10.1038/324268a0. [DOI] [PubMed] [Google Scholar]
- Lehmann P. F., Sawyer T., Donabedian H. Novel abnormality in subpopulations of circulating lymphocytes. T gamma delta and CD2-, 3+, 4-, 8- lymphocytes in histoplasmosis-associated immunodeficiency. Int Arch Allergy Appl Immunol. 1989;90(3):213–218. doi: 10.1159/000235027. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S., Painter A., Travis S. Morphogenesis and pathogenicity of Histoplasma capsulatum. Infect Immun. 1987 Jun;55(6):1355–1358. doi: 10.1128/iai.55.6.1355-1358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nickerson D. A., Fairclough P. Immune responsiveness following intratracheal inoculation with Histoplasma capsulatum yeast cells. Clin Exp Immunol. 1984 May;56(2):337–344. [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Picardi J. L., Kauffman C. A., Schwarz J., Phair J. P. Detection of precipitating antibodies to Histoplasma capsulatum by counterimmunoelectrophoresis. Am Rev Respir Dis. 1976 Jul;114(1):171–176. doi: 10.1164/arrd.1976.114.1.171. [DOI] [PubMed] [Google Scholar]
- Reimann J. Double-negative (CD4-CD8-), TCR alpha beta-expressing, peripheral T cells. Scand J Immunol. 1991 Dec;34(6):679–688. doi: 10.1111/j.1365-3083.1991.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Murray C., Daley J. F., Schlossman S. F., Ritz J. A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. J Exp Med. 1986 Jul 1;164(1):351–356. doi: 10.1084/jem.164.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizlein C. T., Munson A. E., Rhoades R. A. Immunomodulation of local and systemic immunity after subchronic pulmonary exposure of mice to benzo(a)pyrene. Int J Immunopharmacol. 1987;9(1):99–106. doi: 10.1016/0192-0561(87)90115-9. [DOI] [PubMed] [Google Scholar]
- Sweet G. H., Dulohery S. M. Experimental histoplasmosis in the mouse. Immunoglobulin class response, total immunoglobulin A levels, immune complex formation, and occurrence of antigen in serum and urine. Am Rev Respir Dis. 1989 Oct;140(4):967–973. doi: 10.1164/ajrccm/140.4.967. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Miller T. B., Redington T. J., Bullock W. E. Immunoregulation in experimental disseminated histoplasmosis: flow microfluorometry (FMF) studies of the Thy and Lyt phenotypes of T lymphocytes from infected mice. J Immunol. 1983 Aug;131(2):984–990. [PubMed] [Google Scholar]
- Wheat L. J., Kohler R. B., French M. L., Garten M., Kleiman M., Zimmerman S. E., Schlech W., Ho J., White A., Brahmi Z. Immunoglobulin M and G histoplasmal antibody response in histoplasmosis. Am Rev Respir Dis. 1983 Jul;128(1):65–70. doi: 10.1164/arrd.1983.128.1.65. [DOI] [PubMed] [Google Scholar]
- Wheat L. J., Kohler R. B., Tewari R. P. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N Engl J Med. 1986 Jan 9;314(2):83–88. doi: 10.1056/NEJM198601093140205. [DOI] [PubMed] [Google Scholar]
- Williams B. J., Connolly-Stringfield P., Bartlett M., Durkin M., Garringer T., Blair R., Connolly K., Tewari R. P., Wheat L. J. Correlation of Histoplasma capsulatum polysaccharide antigen with the severity of infection in murine histoplasmosis. J Clin Lab Anal. 1991;5(2):121–126. doi: 10.1002/jcla.1860050209. [DOI] [PubMed] [Google Scholar]


