Abstract
Pulmonary hypertension (PH) is a life-threatening disease with unclear vascular mechanisms. We tested whether PH involves abnormal pulmonary vasoconstriction and impaired vasodilation. Male Sprague-Dawley rats were exposed to hypoxia (9% O2) for 2 weeks or injected with single dose of monocrotaline (MCT, 60 mg/kg s.c.). Control rats were normoxic or injected with saline. After the hemodynamic measurements were performed, pulmonary and mesenteric arteries were isolated for measurement of vascular function. Hematocrit was elevated in hypoxic rats. Right ventricular systolic pressure and Fulton's Index [right/(left + septum) ventricular weight] were greater in hypoxic and MCT-treated rats than in normoxic rats. Pulmonary artery contraction by phenylephrine and 96 mM KCl was less in hypoxic and MCT-treated rats than in normoxic rats. Acetylcholine-induced relaxation was less in the pulmonary arteries of hypoxic and MCT-treated rats than of normoxic rats, suggesting reduced effects of endothelium-derived vasodilators. The nitric oxide synthase inhibitor, Nω-nitro-l-arginine methyl ester, and the guanylate cyclase inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, inhibited acetylcholine relaxation, suggesting that it was mediated by nitric oxide (NO)-cGMP. The NO donor sodium nitroprusside caused less relaxation in the pulmonary arteries of hypoxic and MCT-treated than of normoxic rats, suggesting decreased responsiveness of vascular smooth muscle cells (VSMCs) to vasodilators. Phenylephrine and KCl contraction and acetylcholine and sodium nitroprusside relaxation were not different in the mesenteric arteries from all groups. In lung tissue sections, the wall thickness of pulmonary arterioles was greater in hypoxic and MCT-treated rats than in normoxic rats. The specific reductions in pulmonary, but not systemic, arterial vasoconstriction and vasodilation in hypoxia- and MCT-induced PH are consistent with the possibility of de-differentiation of pulmonary VSMCs to a more proliferative/synthetic and less contractile phenotype in PH.
Pulmonary hypertension (PH) is a devastating disease characterized by increased pulmonary arterial blood pressure and right ventricular hypertrophy (Budhiraja et al., 2004; Farber and Loscalzo, 2004). The prognosis of PH is poor, and early detection is critical (Runo and Loyd, 2003). The etiology of PH is not completely understood, but idiopathic and familial factors have been implicated. PH often occurs in children with congenital heart disease and after cardiac surgery (Hopkins et al., 1991), and it affects adults, in particular, with HIV infection, chronic obstructive pulmonary disease, and other cardiopulmonary disease (Fattouch et al., 2005). The course of PH progresses rapidly and ultimately leads to right ventricular failure and premature death (D'Alonzo et al., 1991). Understanding the vascular mechanisms involved in PH should help design specific and efficient therapy for this life-threatening disease.
Histological evidence has suggested that PH is associated with vascular remodeling of the small pulmonary arteries, vascular cell proliferation, and obliteration of pulmonary microvasculature, leading to progressive increase in pulmonary vascular resistance and right ventricular failure (Pietra et al., 1989; Budhiraja et al., 2004; Farber and Loscalzo, 2004). Because vasodilators such as prostacyclin, nitrates, and phosphodiesterase-5 inhibitors are commonly used as the first line of treatment in severe PH (Burger, 2009; Stehlik and Movsesian, 2009; Yin et al., 2009), it is important to determine whether the responsiveness of the pulmonary circulation to vasodilators is affected in the setting of PH.
Previous studies have examined pulmonary vascular function in animal models of PH with variable results (Adnot et al., 1991; Fullerton et al., 1996; Gillespie et al., 1986; Shimoda et al., 2000; Barman, 2007). The variable results could be due to differences in the pulmonary hypertensive animal model, the experimental preparation (isolated perfused lung versus vascular rings), or the vasoactive mediators used. In addition, the specificity of the effects of PH on the pulmonary circulation compared with other systemic vascular beds has not been clearly established (Toporsian and Ward, 1997; Auer and Ward, 1998).
The present study was designed to test the hypothesis that PH involves alterations in the vasoconstriction and vasodilator responses that are specific to the pulmonary arteries. We measured the hemodynamics and examined the vascular function in two separate vascular beds, the pulmonary and mesenteric arteries, isolated from two different rat models of PH, the hypoxia and monocrotaline (MCT)-induced models, to determine: 1) whether the responsiveness of the pulmonary arteries to vasoconstrictors is altered in PH, 2) whether pulmonary arterial relaxation to endothelium-dependent nitric oxide (NO)-cGMP pathway is impaired in PH, and 3) whether the pulmonary vascular smooth muscle cell (VSMC) responsiveness to vasodilators is impaired in PH.
Materials and Methods
Animals and Exposures.
Twelve-week-old (250–300 g) male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were housed in the animal facility in 12 h/12 h light/dark cycle, at 22 ± 1°C ambient temperature and maintained on ad libitum normal Purina Rodent Chow (Purina, St. Louis, MO) and tap water. After 3 days of acclimatization, animals were exposed to hypoxia at 9% O2 inside a chamber where O2 is controlled to within a 0.2% range by an OxyCycler controller (BioSpherix, Redfield, NY) (Vitali et al., 2009). The controllers injected nitrogen and O2 into the chamber to maintain the appropriate FiO2, and ventilation was adjusted to remove CO2 so that it did not exceed 5000 ppm (0.5%). Ammonia was removed by ventilation and activated charcoal filtration with use of an electric air purifier. The duration of hypoxic exposure was 2 weeks. Normoxic rats breathed air under otherwise identical conditions. Age-matched rats were injected with a single dose of MCT (60 mg/kg/day s.c.) and examined 4 weeks later. Control rats were injected with saline. All experimental procedures followed the guidelines of, and were approved by, the Harvard Institutional Animal Care and Use Committee.
Right and Left Ventricular Systolic Pressure Measurements.
Animals were anesthetized with 3% isoflurane inhalation and continued to breathe spontaneously. A small transverse incision was made in the abdominal wall, and the transparent diaphragm was exposed. A 23-gauge butterfly needle with tubing attached to a pressure transducer was inserted through the diaphragm, first into the right ventricle and then into the left ventricle, and pressure measurements were recorded with PowerLab monitoring hardware and software (ADInstruments, Colorado Springs, CO). Animals with heart rates less than 300 beats/min were considered overanesthetized, and their right and left ventricular systolic pressure (RVSP and LVSP) measurements were excluded. Mean RVSP and LVSP over the first 10 stable heartbeats were recorded.
Hematocrit.
After hemodynamic measurements were completed, a 0.2-ml sample of blood was collected from the cardiac chambers for hematocrit determination in a blood gas analyzer.
Tissue Preparation.
In euthanized rats, the thoracic cavity was opened, and the heart, lung, and pulmonary arteries were rapidly excised. The abdominal cavity was then opened and the mesentery and mesenteric arterial arcade were excised and placed in oxygenated Krebs' solution. The right and left pulmonary artery, and second-order mesenteric arteries were carefully dissected and cleaned of connective tissue under microscopic visualization, and cut into 3-mm-wide rings.
Right Ventricular Weight Measurement and Determination of Fulton's Index.
After the heart was excised, both ventricles were weighed, and then the right ventricular wall was dissected, and the remaining left ventricular wall and ventricular septum were weighed. Fulton's Index was calculated as the ratio of right ventricular weight/(left ventricular + septum weight).
Isometric Contraction.
Vascular segments were suspended between two tungsten wire hooks; one hook was fixed at the bottom of a tissue bath and the other hook was connected to a Grass force transducer (FT03, Astro-Med, West Warwick, RI). Pulmonary artery and mesenteric artery segments from the same rat were stretched under 1 or 0.5 g of resting tension, respectively (as determined by preliminary tension-contraction curves to KCl), and allowed to equilibrate for 45 min in a temperature-controlled, water-jacketed tissue bath, filled with 50 ml of Krebs' solution continuously bubbled with 95% O2/5% CO2 at 37°C. The changes in isometric contraction were recorded on a Grass polygraph (model 7D; Astro-Med).
After tissue equilibration, a control contraction in response to 96 mM KCl was elicited. Once maximal KCl contraction was reached, the tissue was rinsed with Krebs' 3 times, 10 min each. The control KCl-induced contraction followed by rinsing in Krebs' was repeated twice. Vascular segments were stimulated with increasing concentrations of phenylephrine (PHE, 10−9 to 10−5 M), concentration-contraction curves were constructed, and the maximal PHE contraction was measured. The individual PHE concentration-response curves were analyzed further by use of a nonlinear regression curve (best-fit sigmoidal dose-response curve; Sigmaplot), and the effective concentration that produced half the maximal contraction (ED50) was measured and presented as pED50 (−log M). In all experiments, the viability of the endothelium was verified by demonstrating acetylcholine (ACh)-induced relaxation in vascular segments precontracted with PHE. The tissues were precontracted with PHE (10−5 M), increasing concentrations (10−9 to 0−5 M) of ACh were added, and the percentage of relaxation of the PHE contraction was measured. Parallel contraction and relaxation experiments were performed in endothelium-intact vascular rings pretreated with the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M) or the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10−5 M) for 10 min. In other experiments the relaxation to increasing concentrations (10−9 to 0−5 M) of the exogenous NO donor sodium nitroprusside (SNP) was measured in vascular rings precontracted with PHE.
Lung Histology and Morphometric Analysis.
Lung sections (6 μm) were stained with hematoxylin and eosin and examined with light microscopy by two independent investigators (E.A. and H.A.C.) in a blinded fashion. Images of the arterioles were captured with a microscope digital camera and analyzed by use of an image analysis program (NIH Image). At least 15 arterioles of comparable size (50–100 μm in diameter) per rat from the lungs of five different rats from each experimental group were evaluated. The percentage of wall thickness was determined by dividing the area occupied by the vessel wall by the total cross-sectional area of the arteriole as reported previously (Christou et al., 2000). This method accounts for uneven media thickness and areas that have obliquely sectioned pulmonary arterioles.
Solutions and Drugs.
Krebs' solution contained 120 mM NaCl, 5.9 mM KCl, 25 mM NaHCO3, 1.2 mM NaH2PO4, 11.5 mM dextrose, 2.5 mM CaCl2, 1.2 mM MgCl2, at pH 7.4, and bubbled with 95% O2 and 5% CO2. The 96 mM KCl was prepared as Krebs' solution with equimolar substitution of NaCl with KCl. Stock solutions of PHE, ACh, and l-NAME (10−1 M; Sigma-Aldrich, St. Louis, MO) were prepared in distilled water. Stock solution of ODQ (10−1 M) was prepared in dimethyl sulfoxide. Final concentration of dimethyl sulfoxide in experimental solution was <0.1%. All other chemicals were of reagent grade or better.
Statistical Analysis.
The data were analyzed and presented as means± S.E.M. Vascular contraction and relaxation data were analyzed by use of Student's t test for unpaired data. Concentration-contraction curves were further analyzed with use of nonlinear regression best-fit sigmoidal curve (Sigmaplot). Histology and morphometric data were compared by use of a nonparametric Mann-Whitney test. Differences were considered statistically significant if P was <0.05.
Results
Measurements of hemodynamics revealed signs of PH in hypoxic- and MCT-treated rats. Right ventricular systolic pressure, an indicator of the blood pressure in the pulmonary circulation, was significantly greater in hypoxic and MCT-treated rats than in normoxic rats (Table 1). In contrast, the left ventricular systolic pressure, an indicator of the blood pressure in the systemic circulation, was not significantly increased in the hypoxic rats, and was actually reduced in the MCT-treated rats compared with the control normoxic rats (Table 1).
Table 1.
Hemodynamics, hematocrit, Fulton's Index, PHE contraction, and ACh and SNP relaxation in pulmonary and mesenteric arteries of normoxic, hypoxic, and MCT-treated rats
| Parameter | Control | Hypoxia | MCT-Treated |
|---|---|---|---|
| RVSP (mm Hg) | 26.3 ± 4.9 (4) | 60.2 ± 2.1 (10)* | 56.2 ± 6.8 (5)* |
| LVSP (mm Hg) | 117.5 ± 4.3 (3) | 124.6 ± 5.7 (6) | 98.2 ± 4.7 (5)* |
| Hematocrit | 38.5 ± 1.2 (14) | 63.3 ± 2.3 (8)* | 41 ± 1 (7) |
| Fulton's Indexa | 0.28 ± 0.01 (17) | 0.57 ± 0.02 (12)* | 0.51 ± 0.03 (8)* |
| Pulmonary Artery | |||
| PHE | |||
| Max (10−5 M) contraction (g/mg) | 1.03 ± 0.11 (16) | 0.28 ± 0.05 (12)* | 0.56 ± 0.15 (12)* |
| + l-NAME (3 × 10−4 M) | 1.15 ± 0.15 (8) | 0.36 ± 0.11 (6)* | 0.65 ± 0.26 (6) |
| +ODQ (10−5 M) | 1.45 ± 0.27 (8) | 0.38 ± 0.11 (6)* | 0.81 ± 0.24 (6) |
| pED50 (−log M) | 7.64 ± 0.13 (16) | 7.57 ± 0.08 (12) | 7.43 ± 0.07 (12) |
| + l-NAME (3 × 10−4 M) | 7.93 ± 0.07 (8) | 7.71 ± 0.24 (6) | 7.53 ± 0.15 (6)* |
| +ODQ (10−5 M) | 7.86 ± 0.11 (8) | 7.94 ± 0.17 (6)** | 8.07 ± 0.06 (6)** |
| ACh (10−5 M) % relaxation | 51.2 ± 2.9 (16) | 19.5 ± 5.4% (12)* | 9.3 ± 2.8% (12)* |
| SNP (10−5 M) % relaxation | 95.2 ± 2.1 (8) | 78.2 ± 7.7 (6)* | 50.4 ± 5.5 (8)* |
| Mesenteric artery | |||
| PHE | |||
| Max (10−5 M) contraction (g) | 0.89 ± 0.09 (8) | 0.70 ± 0.12 (6) | 0.76 ± 0.11 (6) |
| pED50 (−log M) | 6.29 ± 0.15 (8) | 6.36 ± 0.16 (6) | 6.42 ± 0.20 (6) |
| ACh (10−5 M) % relaxation | 100.0 ± 0.0 (8) | 97.3 ± 1.8 (6) | 97.9 ± 1.1 (6) |
| SNP (10−5 M) % relaxation | 100.0 ± 0.0 (3) | 100.0 ± 0.0 (3) | 100.0 ± 0.0 (4) |
Data represent means ± S.E.M. (n).
Fulton's Index = right/(left + septum) ventricle weight.
, measurements in hypoxia or MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in normoxic rats.
, measurements in L-NAME- or ODQ-treated arteries are significantly different from corresponding measurement in nontreated arteries.
Hematocrit level, an indicator of hypoxia, was significantly greater in hypoxic than in normoxic rats, and as expected, was not significantly different between MCT-treated and normoxic rats (Table 1). The Fulton's Index [right/(left + septum) ventricular weight] was significantly greater in hypoxic and MCT-treated rats (Table 1), indicating right ventricular hypertrophy compared with normoxic rats.
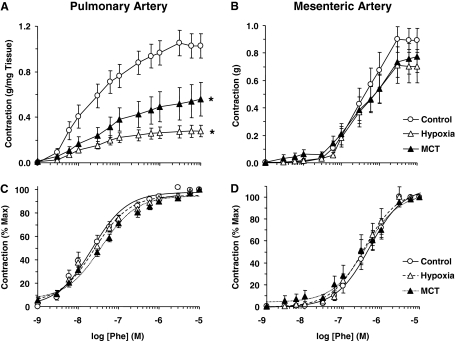
In pulmonary artery rings of normoxic rats, the α-adrenergic agonist PHE caused concentration-dependent contraction that reached a maximum at 10−5 M concentration (Fig. 1A). The PHE-induced contraction was significantly reduced in pulmonary artery rings from hypoxic and MCT-treated rats compared with normoxic rats (Fig. 1A, Table 1). When the PHE contraction was presented as percentage of maximal contraction, and the ED50 was calculated, PHE seemed to be equally potent in the pulmonary arteries of the various groups of rats (Fig. 1C, Table 1). In contrast, in the mesenteric arteries, the PHE-induced maximal contraction did not seem to be different among the various groups of rats (Fig. 1B, Table 1). In addition, the PHE ED50 was not different in the mesenteric arteries of the different experimental groups (Fig. 1D, Table 1).
Fig. 1.
PHE-induced contraction in pulmonary and mesenteric artery segments of normoxic, hypoxic, and MCT-treated rats. Pulmonary artery (A and C) and mesenteric artery rings (B and D) were stimulated with increasing concentrations of PHE. The contractile response was measured and presented in grams per milligram tissue weight for pulmonary vessels (A), in grams for mesenteric vessels (B), or as percentage of maximal PHE contraction (C and D). Data represent means ± S.E.M. (n = 6–16). *, measurements in hypoxic and MCT-treated rats are significantly different (p < 0.05) from corresponding measurements in normoxic rats.
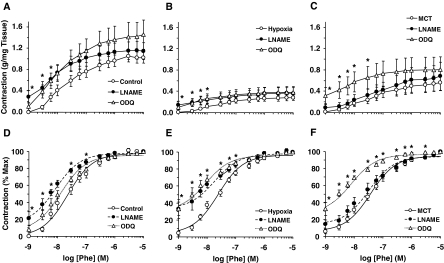
Pretreatment of pulmonary artery segments with the NOS inhibitor l-NAME (3 × 10−4 M) for 10 min caused an increase in basal tension (0.29 ± 0.12 g/mg tissue) and enhanced the magnitude of PHE contraction in normoxic rats (Fig. 2A, Table 1). Treatment of the pulmonary artery with l-NAME caused a small increase in basal tension in hypoxic (0.15 ± 0.07 g/mg tissue) and MCT-treated rats (0.09 ± 0.06 g/mg tissue) and minimally enhanced the magnitude of PHE contraction (Fig. 2, B and C, Table 1), and the PHE responses were still less than those in the control normoxic rats. Presenting the PHE contraction as percentage of maximum and the measurement of PHE ED50 indicated that PHE was more potent in l-NAME-treated than nontreated normoxic rats (Fig. 2D, Table 1). The PHE contractile response as percentage of maximum was shifted to the left in l-NAME-treated compared with nontreated pulmonary artery of hypoxic rats (Fig. 2E, Table 1). In contrast, the PHE contractile response as percentage of maximum and the PHE ED50 were not significantly different between l-NAME-treated and nontreated pulmonary arteries of MCT-treated rats (Fig. 2F, Table 1).
Fig. 2.
Effect of blockade of the NO-cGMP pathway on PHE-induced contraction in pulmonary artery segments of normoxic, hypoxic, and MCT-treated rats. Pulmonary artery segments of normoxic (A and D), hypoxic (B and E), and MCT-treated rats (C and F) were either nontreated (○) or pretreated with the NOS inhibitor l-NAME (3 × 10−4 M) (●), or the guanylate cyclase inhibitor ODQ (10−5 M) (Δ) for 10 min. The tissues were stimulated with increasing concentrations of PHE, and the contractile response was measured and presented as grams per milligram tissue weight (A, B, and C) or as percentage of maximal PHE contraction (D, E, and F). Data represent means ± S.E.M. (n = 6–16). *, measurements in l-NAME- or ODQ-treated pulmonary artery segments are significantly different (p < 0.05) from corresponding measurements in nontreated segments.
Pretreatment of pulmonary artery segments with the guanylate cyclase inhibitor ODQ (10−5 M) for 10 min caused an increase in basal tension (0.11 ± 0.07 g/mg tissue) and enhanced the magnitude of PHE contraction in normoxic rats (Fig. 2A, Table 1). ODQ caused an increase in basal tension in hypoxic (0.10 ± 0.05 g/mg tissue) and MCT-treated rats (0.32 ± 0.15 g/mg tissue), and minimally enhanced PHE contraction, and the PHE responses were still less than those in the normoxic rats (Fig. 2, B and C, Table 1). Presenting the PHE contraction as percentage of maximum and the measurement of PHE ED50 indicated that PHE was equally as potent in ODQ-treated as in nontreated normoxic rats (Fig. 2D), but was more potent in ODQ-treated than in nontreated hypoxic and MCT-treated rats (Fig. 2, E and F, Table 1).
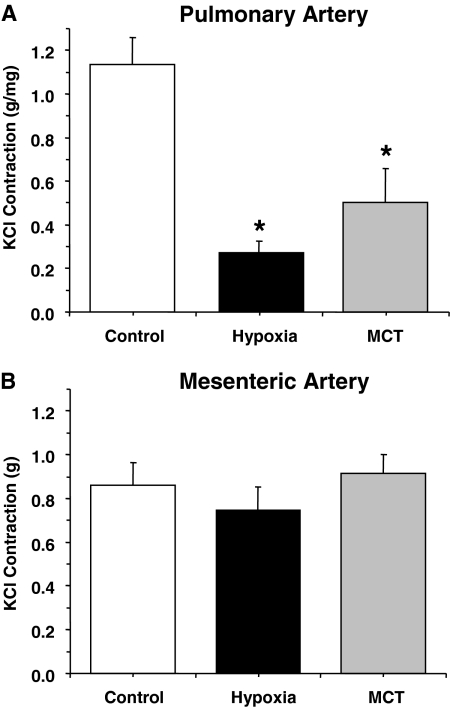
Membrane depolarization by 96 mM KCl caused significant contraction in the pulmonary artery of control normoxic rats. The KCl-induced contraction was significantly reduced in pulmonary arteries of hypoxic and MCT-treated rats (Fig. 3A). In contrast, KCl-induced contraction was not significantly different in mesenteric arteries of normoxic, hypoxic, and MCT-treated rats (Fig. 3B).
Fig. 3.
KCl-induced contraction in pulmonary and mesenteric artery segments of normoxic, hypoxic, and MCT-treated rats. Pulmonary artery (A) and mesenteric artery segments (B) were stimulated with KCl (96 mM) and the contractile response was presented in grams per milligram tissue weight for pulmonary vessels (A) or in grams for mesenteric vessels (B). Data represent means ± S.E.M. (n = 6–16). *, measurements in hypoxic and MCT-treated rats are significantly different (p < 0.05) from corresponding measurements in normoxic rats.
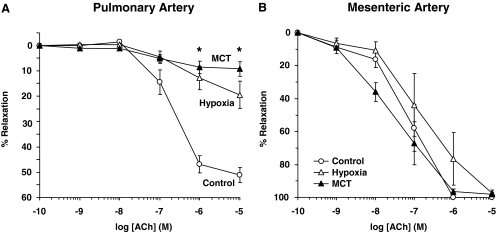
ACh caused concentration-dependent relaxation in PHE-precontracted pulmonary artery rings of normoxic rats that reached a maximum of 51.16 ± 2.92% at 10−5 M concentration. ACh-induced pulmonary artery relaxation was significantly reduced in hypoxic and MCT-treated rats compared with normoxic rats (Fig. 4A, Table 1), suggesting either reduced production of, or decreased responsiveness to, endothelium-derived vasodilators such as NO in the setting of experimental PH. In contrast, ACh relaxation was not significantly different in PHE-precontracted mesenteric artery rings of normoxic, hypoxic, and MCT-treated rats (Fig. 4B, Table 1).
Fig. 4.
ACh-induced relaxation in pulmonary and mesenteric artery segments of normoxic, hypoxic, and MCT-treated rats. Pulmonary artery (A) and mesenteric artery segments (B) were precontracted with PHE (10−5 M), increasing concentrations of ACh were added and the percentage of relaxation of PHE contraction was measured. Data represent means ± S.E.M. (n = 6–16). *, measurements in hypoxic and MCT-treated rats are significantly different (p < 0.05) from corresponding measurements in normoxic rats.
In pulmonary arteries of normoxic rats, the NOS inhibitor l-NAME and the guanylate cyclase inhibitor ODQ abolished ACh relaxation, suggesting the involvement of the NO-cGMP pathway (Fig. 5A). Pretreatment with l-NAME or ODQ also abolished the small ACh-induced relaxation in pulmonary artery of hypoxic and MCT-treated rats (Fig. 5, B and C), suggesting that the residual vasorelaxation response to ACh in experimental PH is mediated by the NO-cGMP pathway.
Fig. 5.
Effect of blockade of the NO-cGMP pathway on ACh-induced relaxation in pulmonary artery segments of normoxic, hypoxic, and MCT-treated rats. Pulmonary artery segments of normoxic (A), hypoxic (B), and MCT-treated rats (C) were either nontreated (○) or pretreated with the NOS inhibitor l-NAME (3 × 10−4 M) (●), or the guanylate cyclase inhibitor ODQ (10−5 M) (Δ) for 10 min. The tissues were precontracted with PHE (10−5 M), increasing concentrations of ACh were added, and the percentage of relaxation of PHE contraction was measured. Data represent means± S.E.M. (n = 6–16). *, measurements in l-NAME- or ODQ-treated pulmonary artery segments are significantly different (p < 0.05) from corresponding measurements in nontreated segments.
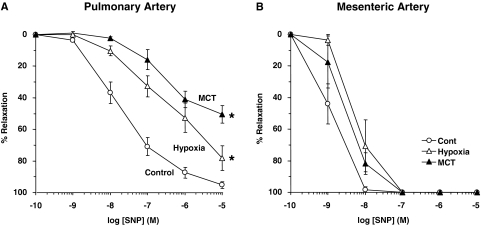
In pulmonary artery segments precontracted with PHE (10−5 M), the exogenous NO donor SNP caused concentration-dependent relaxation that was significantly reduced in hypoxic and MCT-treated rats compared with normoxic rats (Fig. 6A, Table 1), suggesting decreased responsiveness of VSMCs to vasodilators. In contrast, SNP-induced relaxation was not significantly different in the mesenteric arteries of normoxic, hypoxic, and MCT-treated rats (Fig. 6B, Table 1).
Fig. 6.
SNP-induced relaxation in pulmonary and mesenteric artery segments of normoxic, hypoxic, and MCT-treated rats. Pulmonary artery (A) and mesenteric artery segments (B) were contracted with PHE (10−5 M), increasing concentrations of SNP were added, and the percentage of relaxation of PHE contraction was measured. Data represent means± S.E.M. (n = 3–8). *, measurements in hypoxic and MCT-treated rats are significantly different (p < 0.05) from corresponding measurements in normoxic rats.
To correlate the observations of vascular function in the pulmonary vessels with structural remodeling of the pulmonary arterioles, lung histology and morphometric analysis were performed on lung tissue sections from control normoxic rats and hypoxic and MCT-treated rat models of PH. In lung tissue sections stained with hematoxylin and eosin, the percentage of wall thickness of the pulmonary arterioles was significantly greater (p < 0.001) in hypoxic and MCT-treated PH rats compared with control normoxic rats (Fig. 7). These data suggest significant pulmonary vascular remodeling in the hypoxic and MCT-treated rat models of PH.
Fig. 7.
Pulmonary vascular remodeling in hypoxic and MCT-treated models of PH. A, representative hematoxylin and eosin-stained peripheral lung sections from control normoxic rat, and hypoxic (2 weeks) and MCT-treated rats. Pulmonary arterioles are indicated with arrows. Total magnification, 400×. Scale bar = 50 μm. B, quantitative morphometric analysis of percentage of wall thickness of pulmonary arterioles defined as the area occupied by the vessel wall divided by the total cross-sectional area of the arteriole. Graph bars represent the means ± S.E.M. of measurements in 15 vessels per rat, five rats from each experimental group. *, p < 0.001 compared with the normoxic group.
Discussion
An imbalance of the pulmonary vasoconstrictor and vasodilator stimuli has been implicated in the pathogenesis of PH (Hoshikawa et al., 2001; Ivy et al., 2002; Fagan et al., 2004; Ambalavanan et al., 2005; Schermuly et al., 2005; Homma et al., 2007), and vasodilators aiming to restore the pulmonary vascular balance constitute the mainstay of current pharmacologic therapy (Burger, 2009; Stehlik and Movsesian, 2009; Yin et al., 2009). However, many patients do not respond to vasodilators, possibly because of the “fixed ” component of PH caused by excessive pulmonary vascular remodeling. Understanding the mechanisms of pulmonary vascular remodeling would help design novel therapies to reverse the established pathology (Arciniegas et al., 2007; Nozik-Grayck and Stenmark, 2007; Majka et al., 2008). However, until those therapies are developed, a useful approach is to improve the responsiveness of the remodeled pulmonary circulation to vasodilators. We therefore sought to examine pulmonary vascular responsiveness to vasoactive stimuli in the hypoxic and MCT models of PH, which have significant pulmonary vascular remodeling (Christou et al., 2000; Schermuly et al., 2004).
The present study demonstrates that chronic hypoxia and MCT treatment in rats are associated with 1) reduced pulmonary artery contraction by vasoconstrictor stimuli, 2) decreased endothelium-dependent NO-cGMP mediated pulmonary artery relaxation, and 3) decreased pulmonary artery responsiveness to nitrovasodilators. The hypoxia- and MCT-induced changes are specific to the pulmonary and not the mesenteric vessels.
We found that the pulmonary artery contraction by the α-adrenergic agonist PHE was reduced in hypoxic and MCT-treated rats. This probably does not result from changes in the amount/sensitivity of α-adrenergic receptors, because the PHE ED50 was not different between normoxic, hypoxic, and MCT-treated rats. In addition, membrane depolarization by high KCl, a receptor-independent response, was reduced in PH rats, suggesting a reduction in a common postreceptor contraction pathway in pulmonary VSMCs.
Previous studies have shown enhanced endothelin-1 (ET-1)-induced pulmonary vasoconstriction in the Fawn-Hooded rat model of spontaneous PH (Barman, 2007) and increased vasomotor tone in hypoxic PH (Shimoda et al., 2000; Oka et al., 2007). Chronic hypoxia is associated with increased ET-1 and angiotensin II in the lung and changes in the receptor population, K+ current, membrane depolarization, cytosolic Ca2+, and Rho kinase in pulmonary VSMCs, leading to increased vascular contraction, pulmonary vascular resistance, and PH (Shimoda et al., 2000; Oka et al., 2007). Our observed decrease in pulmonary artery contraction in hypoxic rats is different from these reports, but consistent with other reports that ET-1-induced pulmonary vasoconstriction was reduced in the hypoxic rat model of PH (Itoh et al., 1999). The different results could be related to the vasoconstrictive agonist or the vascular preparation used.
In the isolated perfused lung, Gillespie et al. (1986) demonstrated that pulmonary vascular responsiveness to angiotensin II, but not high KCl, was augmented in MCT-treated rats at days 4 and 7, but not at day 14. Our findings of decreased PHE-induced pulmonary artery contraction on day 28 after MCT treatment are consistent with this report, and suggest that, although some enhanced responsiveness may occur before development of PH, this early alteration may not contribute to the sustained elevation in pulmonary arterial pressure.
We examined whether the impaired pulmonary vasoconstriction in experimental PH is due to enhanced release of endothelium-derived relaxing factors such as NO. Blockade of NO production by l-NAME enhanced PHE potency in hypoxic rats and, to a lesser extent, in MCT-treated rats. In addition, inhibition of guanylate cyclase and cGMP production by ODQ enhanced PHE potency in hypoxic and MCT-treated rats, suggesting a potential increase in the NO-cGMP vasodilator pathway as a compensatory mechanism to PH. However, even with NOS or guanylate cyclase inhibition, PHE contraction remained less in hypoxic and MCT-treated rats than in normoxic rats, suggesting that the α-adrenergic postreceptor signaling mechanisms or the pulmonary artery contractile machinery are not responsive to PHE. In addition, the reduced ACh-induced relaxation in the pulmonary arteries of hypoxic and MCT-treated rats probably is not caused by decreased endothelial cholinergic receptors or NO synthase, because the pulmonary artery relaxation by the NO donor SNP was also reduced in PH rats. Although the reduced ACh relaxation in the PH rats could be due to increased oxidative stress and decreased NO bioavailability in hypoxic rats (Cowan et al., 2003), it cannot account for the reduced ACh relaxation in the MCT-treated rats. In addition, the enhanced PHE contraction in l-NAME- or ODQ-treated pulmonary arteries of hypoxic rats suggests that NO production/bioavailability was not significantly compromised. A more plausible explanation for the reduced ACh relaxation in PH rats is the development of structural changes in the pulmonary vascular wall that decrease the responsiveness of pulmonary VSMCs to vasodilators. This is supported by the reduced pulmonary artery relaxation by the NO donor SNP in PH rats. However, the decreased responsiveness to SNP could also results from increased phosphodiesterase-5 or decreased protein kinase G activity.
Studies in isolated perfused lung suggested reduced synthesis/responsiveness to EDRF in hypoxic models of PH. Adnot et al. (1991) demonstrated that ACh-induced relaxation was reduced in rats exposed to 1-week hypoxia and abolished after 3-weeks hypoxia. They also found enhanced pressor response to ET-1, no potentiation of the pressor response by the EDRF antagonists hydroquinone and methylene blue, and fully active endothelium-independent vasodilation by SNP in hypoxic rats, and concluded that hypoxia-induced PH is associated with a loss of EDRF activity in pulmonary vessels. We observed potentiation of PHE contraction in pulmonary arteries of hypoxic rats by l-NAME or ODQ, suggesting that the NO-cGMP activity is preserved. In addition, SNP-induced relaxation was reduced in pulmonary arteries of hypoxic rats. The different results could be related to the vascular preparation (pulmonary artery versus isolated perfused lung), or the NO-cGMP blocker used (l-NAME and ODQ versus hydroquinone and methylene blue). The reduced ACh- and SNP-induced relaxation in pulmonary arteries of MCT-treated rats is consistent with the report that both endothelium-dependent and -independent relaxation are reduced in the rat model of MCT-induced progressive lung injury (Fullerton et al., 1996).
The cause of the decreased contraction by PHE and KCl, and the impaired ACh and SNP relaxation in the pulmonary artery of PH rats is unclear, but could be related to extensive vascular remodeling and pulmonary VSMC growth and proliferation. This possibility is supported by reports that PH is associated with remodeling of the small pulmonary arteries, vascular cell proliferation, and obliteration of the pulmonary microvasculature (Pietra et al., 1989; Mitani et al., 2001; Budhiraja et al., 2004; Farber and Loscalzo, 2004). In addition, hypoxia is associated with increased proliferation and migration of VSMCs, and a synthetic pulmonary VSMC phenotype (Cooper and Beasley, 1999; Schultz et al., 2006; Chen et al., 2008). The observed increase in wall thickness in pulmonary arterioles of PH rats supports the contention that the decreased pulmonary artery responsiveness to both vasoconstrictor and vasodilator stimuli is related to pulmonary vascular remodeling and potential change in pulmonary VSMCs from a contractile to proliferative phenotype.
Hypoxia has diverse effects on the pulmonary and systemic vascular tone. Acute hypoxia elicits vascular adaptations that redistribute blood flow to metabolically active tissues and improve the capacity for O2 extraction (Doyle and Walker, 1991; Kuwahira et al., 1993). However, hypoxia for 16 to 48 h may cause reduction in contraction of systemic arteries in response to agonist stimulation or transmural pressure. Large vessels such as the aorta, skeletal muscle, and diaphragmatic and mesenteric arterioles could be affected (Doyle and Walker, 1991; Kuwahira et al., 1993; Toporsian and Ward, 1997; Auer and Ward, 1998). Although MCT treatment may cause both pulmonary and systemic vascular inflammation and endothelial damage, MCT-treated rats have been used as a model of progressive lung injury and PH (Gillespie et al., 1986; Fullerton et al., 1996). In our hypoxia and MCT models of PH, we found no changes in the responsiveness of the mesenteric vessels to vasoconstrictor or vasodilator stimuli, indicating specific changes in the pulmonary but not the systemic circulation in experimental PH.
The results observed in extralobar first-branch pulmonary arteries may not reflect the entire pulmonary circulation. Future studies should examine whether the reduced contraction/relaxation in extralobar pulmonary arteries also occur in the intralobar resistance, which play a major role in the increased vascular resistance associated with PH. However, given that there is increasing appreciation of the contribution of proximal vascular stiffness to pulmonary vascular impedance in the setting of PH (Lammers et al., 2008, Sanz et al., 2009), it is equally important to examine these responses in experimental PH.
Our results may have important experimental and clinical implications for PH. The results raise the possibility that unless approaches to improve the responsiveness of the pulmonary circulation to vasoactive substances are developed, therapeutic interventions targeting vasoconstriction may not produce the desired effects.
In conclusion, hypoxia- and MCT-induced PH is associated with reduced responsiveness of the pulmonary arterial circulation to both vasoconstrictor and vasodilator stimuli. The specific reduction in the pulmonary vascular responses in the setting of hypoxia- and MCT-induced PH may be explained by a vascular bed-specific switch of VSMCs from the contractile to the synthetic phenotype, and this needs to be examined further in future studies.
Acknowledgments
We thank Drs. S. Kourembanas and S. A. Mitsialis for generous support in the development of the hypoxic rat model.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL-65998 and HL-70659] (to R.A.K.); and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD-60702] (to R.A.K.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160119
- PH
- pulmonary hypertension
- ACh
- acetylcholine
- EDRF
- endothelium-derived relaxing factor
- ET-1
- endothelin-1
- l-NAME
- Nω-nitro-l-arginine methyl ester
- LVSP
- left ventricular systolic pressure
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PHE
- phenylephrine
- RVSP
- right ventricular systolic pressure
- SNP
- sodium nitroprusside
- VSMC
- vascular smooth muscle cell
- MCT
- monocrotaline.
References
- Adnot S, Raffestin B, Eddahibi S, Braquet P, Chabrier PE. (1991) Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest 87:155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. (2005) Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas E, Frid MG, Douglas IS, Stenmark KR. (2007) Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293:L1–L8 [DOI] [PubMed] [Google Scholar]
- Auer G, Ward ME. (1998) Impaired reactivity of rat aorta to phenylephrine and KCl after prolonged hypoxia: role of the endothelium. J Appl Physiol 85:411–417 [DOI] [PubMed] [Google Scholar]
- Barman SA. (2007) Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol 293:L472–L479 [DOI] [PubMed] [Google Scholar]
- Budhiraja R, Tuder RM, Hassoun PM. (2004) Endothelial dysfunction in pulmonary hypertension. Circulation 109:159–165 [DOI] [PubMed] [Google Scholar]
- Burger CD. (2009) Pulmonary hypertension in COPD: a review and consideration of the role of arterial vasodilators. COPD 6:137–144 [DOI] [PubMed] [Google Scholar]
- Chen SC, Wang BW, Wang DL, Shyu KG. (2008) Hypoxia induces discoidin domain receptor-2 expression via the p38 pathway in vascular smooth muscle cells to increase their migration. Biochem Biophys Res Commun 374:662–667 [DOI] [PubMed] [Google Scholar]
- Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. (2000) Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res 86:1224–1229 [DOI] [PubMed] [Google Scholar]
- Cooper AL, Beasley D. (1999) Hypoxia stimulates proliferation and interleukin-1alpha production in human vascular smooth muscle cells. Am J Physiol 277:H1326–H1337 [DOI] [PubMed] [Google Scholar]
- Cowan DB, Jones M, Garcia LM, Noria S, del Nido PJ, McGowan FX., Jr (2003) Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler Thromb Vasc Biol 23:1754–1760 [DOI] [PubMed] [Google Scholar]
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115:343–349 [DOI] [PubMed] [Google Scholar]
- Doyle MP, Walker BR. (1991) Attenuation of systemic vasoreactivity in chronically hypoxic rats. Am J Physiol 260:R1114–R1122 [DOI] [PubMed] [Google Scholar]
- Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. (2004) Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287:L656–L664 [DOI] [PubMed] [Google Scholar]
- Farber HW, Loscalzo J. (2004) Pulmonary arterial hypertension. N Engl J Med 351:1655–1665 [DOI] [PubMed] [Google Scholar]
- Fattouch K, Sbraga F, Bianco G, Speziale G, Gucciardo M, Sampognaro R, Ruvolo G. (2005) Inhaled prostacyclin, nitric oxide, and nitroprusside in pulmonary hypertension after mitral valve replacement. J Card Surg 20:171–176 [DOI] [PubMed] [Google Scholar]
- Fullerton DA, Hahn AR, McIntyre RC., Jr (1996) Mechanistic imbalance of pulmonary vasomotor control in progressive lung injury. Surgery 119:98–103 [DOI] [PubMed] [Google Scholar]
- Gillespie MN, Olson JW, Reinsel CN, O'Connor WN, Altiere RJ. (1986) Vascular hyperresponsiveness in perfused lungs from monocrotaline-treated rats. Am J Physiol 251:H109–H114 [DOI] [PubMed] [Google Scholar]
- Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. (2007) Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50:697–702 [DOI] [PubMed] [Google Scholar]
- Hopkins RA, Bull C, Haworth SG, de Leval MR, Stark J. (1991) Pulmonary hypertensive crises following surgery for congenital heart defects in young children. Eur J Cardiothorac Surg 5:628–634 [DOI] [PubMed] [Google Scholar]
- Hoshikawa Y, Voelkel NF, Gesell TL, Moore MD, Morris KG, Alger LA, Narumiya S, Geraci MW. (2001) Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med 164:314–318 [DOI] [PubMed] [Google Scholar]
- Itoh H, Yokochi A, Yamauchi-Kohno R, Maruyama K. (1999) Effects of the endothelin ET(A) receptor antagonist, TA-0201, on pulmonary arteries isolated from hypoxic rats. Eur J Pharmacol 37:233–238 [DOI] [PubMed] [Google Scholar]
- Ivy DD, Yanagisawa M, Gariepy CE, Gebb SA, Colvin KL, McMurtry IF. (2002) Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol 282:L703–L712 [DOI] [PubMed] [Google Scholar]
- Kuwahira I, Gonzalez NC, Heisler N, Piiper J. (1993) Changes in regional blood flow distribution and oxygen supply during hypoxia in conscious rats. J Appl Physiol 74:211–214 [DOI] [PubMed] [Google Scholar]
- Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. (2008) Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart and Circ Physiol 295:H1451–H1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Skokan M, Wheeler L, Harral J, Gladson S, Burnham E, Loyd JE, Stenmark KR, Varella-Garcia M, West J. (2008) Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 295:L1028–L1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani Y, Ueda M, Komatsu R, Maruyama K, Nagai R, Matsumura M, Sakurai M. (2001) Vascular smooth muscle cell phenotypes in primary pulmonary hypertension. Eur Respir J 17:316–320 [DOI] [PubMed] [Google Scholar]
- Nozik-Grayck E, Stenmark KR. (2007) Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol 618:101–112 [DOI] [PubMed] [Google Scholar]
- Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. (2007) Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100:923–929 [DOI] [PubMed] [Google Scholar]
- Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM. (1989) Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80:1198–1206 [DOI] [PubMed] [Google Scholar]
- Runo JR, Loyd JE. (2003) Primary pulmonary hypertension. Lancet 361:1533–1544 [DOI] [PubMed] [Google Scholar]
- Sanz J, Kariisa M, Dellegrottaglie S, Prat-Gonzalez S, Garcia MJ, Fuster V, Rajagopalan S. (2009) Evaluation of Pulmonary Artery Stiffness in Pulmonary Hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging 2:286–295 [DOI] [PubMed] [Google Scholar]
- Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. (2005) Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115:2811–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, Ermert M, Weissmann N, Rose F, Guenther A, et al. (2004) Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 169:39–45 [DOI] [PubMed] [Google Scholar]
- Schultz K, Fanburg BL, Beasley D. (2006) Hypoxia and hypoxia-inducible factor-1alpha promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290:H2528–H2534 [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sham JS, Sylvester JT. (2000) Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 49:549–560 [PubMed] [Google Scholar]
- Stehlik J, Movsesian MA. (2009) Combined use of PDE5 inhibitors and nitrates in the treatment of pulmonary arterial hypertension in patients with heart failure. J Card Fail 15:31–34 [DOI] [PubMed] [Google Scholar]
- Toporsian M, Ward ME. (1997) Hyporeactivity of rat diaphragmatic arterioles after exposure to hypoxia in vivo. Role of the endothelium. Am J Respir Crit Care Med 156:1572–1578 [DOI] [PubMed] [Google Scholar]
- Vitali SH, Mitsialis SA, Liang OD, Liu X, Fernandez-Gonzalez A, Christou H, Wu X, McGowan FX, Kourembanas S. (2009) Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLoS ONE 4:e5978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin N, Kaestle S, Yin J, Hentschel T, Pries AR, Kuppe H, Kuebler WM. (2009) Inhaled nitric oxide versus aerosolized iloprost for the treatment of pulmonary hypertension with left heart disease. Crit Care Med 37:980–986 [DOI] [PubMed] [Google Scholar]