Abstract
We have previously shown that nicotine, the addictive component of tobacco products, alters the blood-brain barrier (BBB) Na+,K+,2Cl− cotransporter (NKCC) during in vitro hypoxia-aglycemia exposure. Attenuation of abluminal NKCC suggests that accumulation of ions in the brain extracellular fluid would result in an increase of fluid or cytotoxic edema in the brain during hypoxia-aglycemia or stroke conditions. To further investigate whether nicotine products have the potential to worsen stroke outcome by increasing edema formation, two separate models to mimic stroke conditions were utilized to decipher the effects of short-term and long-term administrations of nicotine products on brain edema following stroke. Oxygen glucose deprivation (OGD) was studied in rat hippocampal slices with short-term or long-term exposure to nicotine and cigarette smoke constituents. During short-term exposure, the presence of nicotine at a concentration mimicking heavy smokers increased water content of hippocampal slices during OGD. Furthermore, long-term 1-week administration of nicotine increased water content in hippocampal slices that could be attenuated with nicotine acetylcholine receptor (nAChR) antagonists, suggesting nicotine increase edema during OGD via nAChRs. A second model of focal ischemia, middle cerebral artery occlusion, showed an increase of infarct size during short-term exposure to nicotine and an increase of edema during both short-term and long-term administration of nicotine, compared with saline controls. These findings support the paradigm that nicotine products not only increase the incidence of stroke but also have the potential to worsen stroke outcome by increased edema formation.
Stroke has the third highest mortality rate of 160,000 per year and is a leading cause of neurological disease resulting in long-term disability, with 3.5 million survivors in the United States (Hankey, 1999; Carandang et al., 2006). Cigarette smoking and even second-hand smoke have been associated with increased incidence of stroke in both men and women (Bonita et al., 1999). Smoking cigarettes clearly have been shown to be a risk factor for stroke (Bonita et al., 1999; Ueshima et al., 2004). Twenty-five percent of strokes is associated with smoking (Hankey, 1999). The risk of stroke is dose-dependent and increases from 3.57 to 4.65 with 5 to 15 cigarettes per day (Bonita et al., 1999). In addition to enhanced risk factors for brain ischemia, there is a growing body of evidence that nicotine alters BBB permeability characteristics that have a direct influence on stroke outcome and the pathophysiology of brain ischemia (Abbruscato et al., 2002, 2004; Hawkins et al., 2002, 2004). More importantly, stroke outcome has been shown to be worsened by increased edema observed in a 2-week exposure to nicotine after focal ischemia in rats (Wang et al., 1997).
The controlled water movement and maintenance of ion balance in the brain extracellular fluid are imperative for the reduction of edema and neuronal survival during stroke conditions. Other diseases also have brain water balance as a central point of damage, including head trauma, brain cancer, and certain types of epilepsy (Agre et al., 2004). Edema is thought to play a central role at all levels of the neurovascular unit during neuronal damage associated with ischemia. Cellular swelling can be seen at the endothelial cell, pericytes, and astrocytic foot processes within the first 90 min of focal occlusion (Liu et al., 2001). Edema formation contributes to stroke morbidity and mortality, and Na+ accumulation in the brain occurs during ischemic assault before BBB breakdown (Dzialowski et al., 2004).
Two types of edema are associated with stroke, cytotoxic cellular edema and vasogenic edema (Unterberg et al., 2004). Cytotoxic edema, experienced at the beginning of an ischemic incident, is associated with increased extracellular ion accumulation resulting in increased water content in cells, including glia and neurons (Unterberg et al., 2004). Vasogenic edema later amplifies the damage done by cellular edema and is contributed by blood-brain barrier breakdown (Unterberg et al., 2004). Brain water content begins to immediately accumulate before BBB breakdown and has been monitored with computed tomography scan in a rat middle cerebral artery occlusion (MCAO) model (Dzialowski et al., 2004). Accumulation of water in astrocytes has been suggested to produce the most damage due to the volume of astrocytes versus neurons in the brain (20:1 in humans and 10:1 in rats) (Kimelberg et al., 1995; Unterberg et al., 2004).
Previously, we have shown that nicotine, the addictive component of tobacco products, is responsible for alteration of the Na+,K+,2Cl− cotransporter 1 (NKCC1) on the abluminal (brain facing) surface of the BBB during in vitro hypoxia/aglycemia conditions used to model stroke. This could result in the accumulation of ions, including Na+, K+, and Cl− in the brain extracellular fluid, resulting in increased fluid or brain edema during hypoxic or stroke conditions with or without reperfusion (Abbruscato et al., 2004; Paulson et al., 2006). However, there is no further information on how cigarette smoke constituents, not only nicotine, will affect edema formation in animal models of stroke, especially under long-term exposure.
To further investigate the possibility that nicotine products have the potential to worsen stroke outcome by increased edema formation, two separate models to mimic stroke conditions were utilized to decipher the effects of short-term and long-term administration of cigarette smoke constituents or nicotine on brain edema following stroke. The first model, oxygen glucose deprivation (OGD), was studied in rat hippocampal slices with short-term exposure to nicotine or long-term exposure to cigarette smoke constituents. A second model of focal ischemia, MCAO, was used to evaluate infarct size and edema during short-term and long-term exposure to nicotine. Knowledge gained from these studies could lead to better therapeutic approaches to protect the central nervous system from neurological damage associated with nicotine and/or stroke insults.
Materials and Methods
Preparation of Smoke Condensates.
Cigarettes (Marlboro filter cigarettes; Philip Morris Inc., Richmond, VA; and Quest 3; Vector Tobacco Inc., Research Triangle Park, NC) were obtained through commercial sources. Marlboro filtered cigarettes contain 1.1 mg of nicotine per cigarette, and Quest 3 contains no more than 0.05 mg of nicotine per cigarette and is therefore regarded to be nicotine-free for these studies. Mainstream smoke was bubbled through 50 ml of acetone by a slight vacuum drawn repetitiously through a custom manufactured apparatus. A cigarette smoke extract-acetone solution was generated, which was concentrated under vacuum. The residue was subsequently redissolved in 1.626-ml propylene glycol/dimethyl sulfoxide (PG/DMSO, 1:1). The products of the Marlboro and Quest cigarette extraction were designated as N-CSE (nicotine-containing cigarette smoke extract) and NF-CSE (nicotine-free cigarette smoke extract), respectively. These methods have successfully been used in vitro to model exposure to cigarette smoke chemicals (Sherratt et al., 1988; Paulson et al., 2006).
Hippocampal Slice Oxygen Glucose Deprivation.
Experimental design was formed by varying a method of hippocampal brain slicing and subsequent exposure to oxygen glucose-deprived artificial CSF (MacGregor et al., 2003). Female Sprague Dawley rats were anesthetized and euthanized through cervical dislocation. The brain was extracted, and the hippocampus identified by stereotaxic coordinator (Franklin and Paxinos, 2001) was sliced with a McIlwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd., Guilford, Surrey, UK) at 400 μm. Hippocampal slices were pre-equilibrated in a solution of ice-cold artificial cerebrospinal fluid (aCSF; 137 mM NaCl, 2.7 mM KCl, 0.2 mM CaCl2, 1.2 mM MgCl2, 0.2 mM NaH2PO4, 11.9 mM NaHCO3, and 5.6 mM glucose), with the addition of 1 mM ascorbic acid. Preincubation with ascorbic acid prevents artificial edematous conditions produced by in vitro conditions (Brahma et al., 2000). To ensure cellular survival, the time period from brain extraction to placement in artificial CSF was consistently kept under 3 min.
Hippocampal slices were treated under normoxic or OGD conditions in a PermeGear permeation chamber (AMIE Systems, Riegelsville, PA), with the slices placed between thin nylon 200 mesh screen (Ted Pella Inc., Redding, CA). Artificial CSF flowed through the chamber at a constant rate of 0.7 ml/min at a temperature of 37°C. Normoxic conditions were maintained through control aCSF, with the presence of glucose bubbled with oxygen (137 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 0.2 mM NaH2PO4, 11.9 mM NaHCO3, and 5.6 mM glucose). OGD was maintained through aCSF with the absence of glucose and bubbled with 95% nitrogen and 5% CO2 and kept at a temperature of 37°C.
Short-term and long-term treatments were tested in this study. Short-term treatment of nicotine/cotinine was administered through the aCSF at varying concentrations ranging from 1/10, 10/100, 100/1000, and 1000/10,000 ng/ml nicotine and cotinine, respectively. Long-term 1-week and 3-week administration of vehicle control, nicotine, N-CSE, or NF-CSE was given to rats using Alzet 2ML4 osmotic pumps (Alzet, Cupertino, CA) at a concentration to mimic heavy smokers (4.5 mg/kg/day) in accordance with previous studies (Murrin et al., 1987; Wang et al., 1997). Nicotine and cotinine plasma levels were verified by RIA and HPLC to confirm comparable plasma levels within groups. In addition, hippocampal slices subjected to OGD with N/C and nAChR antagonists (25, 125, and 250 μM mecamylamine or 10 nM α-bungarotoxin) were tested to evaluate the edematous effects involved with nAChRs.
Edema formation was evaluated by determining the percentage of water content of each slice. Hippocampal slices were initially weighed on a Cahn microbalance immediately following the treatment incubation on preweighed aluminum foil and considered “wet weight.” After a 24-h period of desiccation at 95°C, the slices were again weighed and recorded as “dry weight.” Water content was then calculated as [(wet weight − dry weight)/wet weight] × 100.
In Vivo MCAO Model.
The methods used here were a slight modification of previously published procedures (Mdzinarishvili et al., 2005). CD-1 female mice were pretreated with nicotine, N-CSE, or NF-CSE in short-term dosages of 1, 3, or 6 h or long-term dosages of 1 or 3 weeks (nicotine in 0.9% NaCl; N-CSE and NF-CSE in PG/DMSO). Short-term injections of nicotine or CSEs were equivalent to 4.5 mg/kg/day calculated from the known nicotine yield. For short-term nicotine dosages of 1, 3, and 6 h, intraperitoneal injections of 187.5, 562.5, and 1125 μg/kg nicotine, respectively, were given. The doses were calculated as done for 1 h, 4.5 mg/kg divided by 24 h = 187.5 μg/kg and so on for 3- and 6-h doses. This dose was selected because it did not result in gross signs of toxicity; the resulting nicotine and cotinine plasma concentrations reached were similar to those found in heavy smokers (80–100 ng/ml) (Murrin et al., 1987; Lockman et al., 2005). Data from our previous studies (Lockman et al., 2005a,b) demonstrate that this dosage of nicotine does not alter regional BBB integrity or regional cerebral perfusion flow. Long-term administration of nicotine was delivered through subcutaneously placed Alzet 2001 mini-osmotic pumps at a concentration to mimic heavy smokers (4.5 mg/kg/day) in accordance with previous studies (Murrin et al., 1987; Wang et al., 1997). Nicotine and cotinine plasma levels were verified by RIA and HPLC to confirm comparable plasma levels within groups.
Animals were anesthetized with 4% isoflurane and maintained with 1% isoflurane in 30% O2/70% N2O using a face mask and a SurgiVet vaporizer (Smiths Medical North America, Waukesha, WI). Surgery was performed using a Zeiss OPMI pico surgical microscope (Carl Zeiss GmbH, Jena, Germany). Local cortical blood flow in the left middle cerebral artery territory was monitored with laser-Doppler flowmetry and observed online in real time on computer using a software program (Moor Instruments, Ltd., Axminster, Devon, UK). After occlusion of the common carotid artery by a microclip, the left external carotid artery was ligated, coagulated, and cut distally to the cranial thyroid artery. A small incision in the external carotid was made, and a 20-mm monofilament nylon suture (5-0; Harvard Apparatus Inc., Holliston, MA), which had been rounded at the tip by heat (final diameter: 0.2–0.3 mm), was inserted into the external carotid and gently advanced through the internal carotid artery until its tip occluded the origin of the MCA. Correct placement of the suture was indicated by a sudden drop of the local cortical blood flow in the left MCA territory to 10 to 15% baseline as seen by laser-Doppler flowmetry. After successful occlusion, the monofilament was secured in place with ligature, and the incision was closed by microsurgical clips.
MCAO was sustained for a period of 24 h, after which the animals were deeply anesthetized with isoflurane and euthanized by decapitation. It has been reported that permanent MCAO (24 h) creates predictable neuronal injury that is caused by both cytotoxic and vasogenic edema (Kumar et al., 2006; Mdzinarishvili et al., 2007). The brains were quickly removed and sectioned coronally into slices of 1 mm thickness using McIlwain Tissue Chopper. Slices were incubated in a 1% solution of 2,3,5-triphenyltetrazolium chloride in phosphate-buffered saline at 37°C for 15 min and fixed by immersion in 4% buffered formaldehyde solution. 2,3,5-Triphenyltetrazolium chloride stains viable brain tissue dark red based on intact mitochondrial function, whereas infarcted tissue areas remain unstained (white) (Gorgulu et al., 2000). Images were acquired by digital camera (Nikon, Melville, NY), and areas of both hemispheres and the infarcted regions were quantified for each slice using image analysis software (Image J 1.30 and Scion Image version Beta 4.0.2; National Institutes of Health, Bethesda, MD). We elected to measure the areas of damage and brain swelling by area increase in the ipsilateral (ischemic) hemisphere and compare these with the contralateral (nonischemic) hemisphere (Elliot and Jasper, 1949; Sydserff et al., 1996). This allowed direct comparison of areas of damage with brain swelling and allowed us to use each animal as its own control in addition to interexperimental comparisons (Sydserff et al., 1996).
Five animals were used for each experiment, and three measurements were made on each slice to calculate the size of the lesion and to correct for overestimation due to the effects of brain swelling. The calculations are: a = area of infarct (mm2), b = area of the infarcted (ipsilateral) hemisphere slice (mm2), c = area of the noninfarcted (contralateral) hemisphere slice (mm2), d = brain swelling (mm2) = b − c (Park and Kang, 2000). The area (Al) of the lesion (mm2), corrected for swelling, was derived from the equation Al = a − d. The swelling area was designated Ae and quantified by determining the ratio between the areas of the infarcted and noninfarcted hemisphere slices, thus: Ae = b − c. Infarct and edema ratios of hemispheric areas were expressed as the mean ± S.E.M. and compared using Student's t test. Values of P < 0.05 were considered statistically significant. All of the animal studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications 80-23) revised 1996 and approved by the Animal Care and Use Committee at Texas Tech University Health Sciences Center.
Locomotor Activity Measurements.
Six groups of female CD-1 mice (including naive mice control) were included in this study: 1) mice with 1-h exposure of nicotine 4.5 mg/kg/day; 2) mice with MCAO for 6 h; 3) mice with MCAO for 6 h plus 1 h before exposure to nicotine 4.5 mg/kg/day; 4) mice with exposure to nicotine 4.5 mg/kg/day for 3 weeks; and 5) mice with MCAO for 6 h plus 3 weeks before exposure to nicotine 4.5 mg/kg/day. Long-term administration of nicotine (4.5 mg/kg/day for 3 weeks) was delivered through subcutaneously placed Alzet 2001 mini-osmotic pumps at a concentration to mimic heavy smokers (4.5 mg/kg/day) in accordance with previous studies (Murrin et al., 1987; Wang et al., 1997). Nicotine (45 ng/ml) and cotinine (262.2 ng/ml) plasma levels were verified by RIA and HPLC to confirm comparable plasma levels within groups.
After the treatments, behavioral data were collected using VersaMax animal monitors (Accuscan Instruments Inc., Columbus, OH), which has been used to monitor stroke outcome using several locomotor parameters and behavioral deficits that are predictive of stroke injury (Vendrame et al., 2004). The chamber cage was 42 × 42 × 30 cm, made of clear Plexiglas glass, and covered with a Plexiglas lid with holes for ventilation. Infrared monitoring sensors were located every 2.5 cm along the perimeter (16 infrared beams along each side) and 2.5 cm above the floor. Two additional sets of 16 sensors were located 8.0 cm above the floor on opposite sides. Data were collected and analyzed by a VersaMax analyzer (Accuscan Instruments Inc.), which in turn sent information to a computer that ran the VersaMax and Versadat programs. These programs tabulated and processed a number of variables related to locomotor behavior. For the present experiments, four different locomotor parameters were measured every 4 min for 20 min, which has been shown previously to reflect behavioral changes associated with neurological damage induced by MCAO (Vendrame et al., 2004). Before the experiments, mice were acclimatized to the behavioral procedure. All testings occurred between 12:00 PM and 5:00 PM. Behavioral tests were performed in all six groups of animals.
Statistical Analysis.
All data are expressed as the mean ± S.D., and values were compared by ANOVA. When the differences in the means were significant, post hoc pairwise comparisons were conducted using Newman-Keuls multiple comparison (Prism, version 3.03; GraphPad Software, Inc., San Diego, CA). Differences in p values less than 0.05 were considered statistically significant.
Results
Effects of Nicotine on Blood Gases and Temperature.
Studies were carried out to determine whether the nicotine administration altered physiologic parameters (temperature and blood gas) during MCAO. Arterial blood samples were drawn (100 μl/sample) under anesthesia from groups of mice that were exposed to 24 h of MCAO and or 3 h of nicotine dosage (0.56525 mg/kg). Blood samples were drawn 1 h after induction of MCAO as described above. Blood gases and serum electrolytes were analyzed on a RapidLab 348 blood gas analyzer (Bayer Diagnostics, Tarrytown, NY). We found nicotine exposure did not change pO2, pCO2, or pH parameters when compared with control. It is noteworthy that we also found that nicotine exposure before 24-h MCAO did not change these parameters when compared with the 24-h MCAO alone. Higher pO2 values are explained by the slightly higher oxygen content of the gas mixture (30% compared with room air) used for MCAO (Table 1).
TABLE 1.
Blood gas values and temperature
Studies were carried out to determine whether the nicotine administration altered physiologic parameters (temperature and blood gas) during MCAO. Arterial blood samples were drawn (100 μl/sample) under anesthesia from groups of mice that were exposed to 24 h of MCAO and or 3 h of nicotine (0.56525 mg/kg). Blood samples were drawn 1 h after induction of MCAO as described above. Blood gases and serum electrolytes were analyzed on a RapidLab 348 blood gas analyzer (Bayer Diagnostics). Higher pO2 values are explained by the slightly higher oxygen content of the gas mixture (30% compared to room air) that is used for MCAO.
| Control | 3-h Nicotine | 24-h MCAO | 24-h MCAO + 3-h Nicotine | |
|---|---|---|---|---|
| pO2 (mm Hg) | 86.3 ± 4.3 | 85.4 ± 3.8 | 92.1 ± 2.9 | 95.6 ± 3.1 |
| pCO2 (mm Hg) | 32.1 ± 3.8 | 32.7 ± 3.9 | 29.8 ± 2.6 | 28.3 ± 2.1 |
| PH | 7.41 ± 0.18 | 7.43 ± 0.25 | 7.39 ± 2.0 | 7.41 ± 0.8 |
| Temp (°C) | 37.1 | 37.0 | 37.3 | 37.1 |
Hippocampal Slices Subjected to Short-Term Nicotine Exposure.
Each cigarette smoking results in absorption of 1 to 1.5 mg of nicotine by the smoker (Hukkanen et al., 2005). Nicotine is metabolized in the liver resulting in six metabolites of which cotinine is the primary metabolite averaging 72% (Benowitz and Jacob, 1994). Plasma levels of nicotine in smokers range from 10 to 50 ng/ml, and cotinine levels predominantly range from 250 to 300 ng/ml yet can be variable up to 900 ng/ml (Hukkanen et al., 2005). We used levels of nicotine/cotinine, respectively, as 1/10, 10/100, 100/1000, and 1000/10,000 ng/ml for the dosing paradigm in these studies.
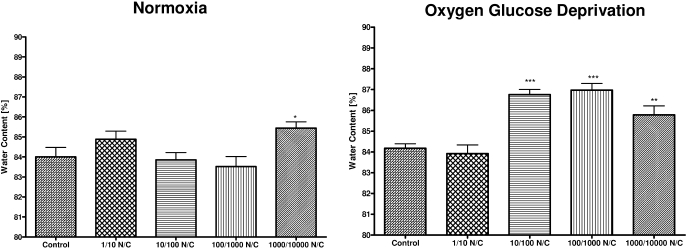
During normoxic conditions, nicotine exposure at the highest dose of 1000 ng/ml nicotine and 10,000 ng/ml cotinine showed an increase in water content compared with control (p < 0.05) (Fig. 1). OGD exposed slices showed greater sensitivity to nicotine/cotinine exposure with significant increase in water content at doses of nicotine/cotinine: 10/100 (p < 0.01), 100/1000 (p < 0.01), and 1000/10,000 (p < 0.01) (Fig. 1).
Fig. 1.
Water content of hippocampal slices subjected to normoxia or oxygen glucose deprivation with short-term nicotine/cotinine exposure. Effect of short-term 1-h exposure to aCSF control, nicotine (1 ng/ml)/cotinine (10 ng/ml) (1/10 NC); nicotine (10 ng/ml)/cotinine (100 ng/ml) (10/100 NC); nicotine (100 ng/ml)/cotinine (1000 ng/ml) (100/1000 NC); and nicotine (1000 ng/ml)/cotinine (10,000 ng/ml) (1000/10,000 NC) on water content of brain hippocampal slices following normoxic or oxygen glucose deprivation conditions. Data represent mean ± S.E.M. of 9 to 17 independent determinations. * denotes significance of p < 0.05; ** denotes significance of p < 0.01, and *** denotes significance of p < 0.001 using one-way ANOVA with Newman-Keuls post hoc analysis.
Hippocampal Slices Subjected to Long-Term Cigarette Smoke Constituent Exposure.
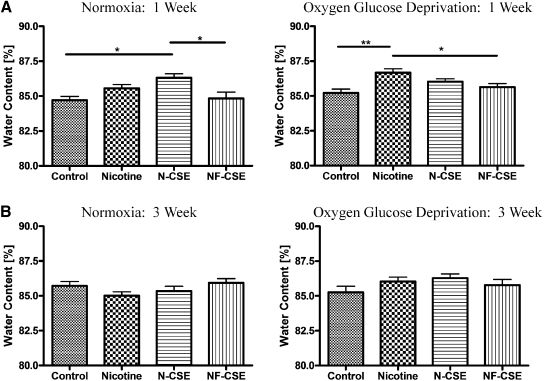
Our long-term nicotine exposure through an Alzet minipump delivery system for 1 week (4.5 mg/kg/day) revealed that, during normoxic conditions, N-CSE significantly increased water content of hippocampal slices compared with the vehicle control PG/DMSO and NF-CSE (p < 0.05) (Fig. 2A). There is no significant difference in water content of hippocampal slices between vehicle control (PG/DMSO) and saline control (data not shown). It is noteworthy that nicotine and N-CSE at 4.5 mg/kg/day did not show a significant difference in water content. During OGD conditions, a 1-week exposure to nicotine 4.5 mg/kg/day increased water content compared with control (p < 0.01) and NF-CSE (p < 0.05) (Fig. 2A). Normoxic and OGD exposure following 3-week minipump delivery of all cigarette smoke constituents resulted in no significant alteration of hippocampal slice water content (Fig. 2B).
Fig. 2.
Water content of hippocampal slices subjected to normoxia or oxygen glucose deprivation with long-term 1- and 3-week cigarette smoke constituent exposure. Effect of 1-week exposure to vehicle control, nicotine (4.5 mg/kg), N-CSE, and NF-CSE on water content of brain hippocampal slices following normoxic or oxygen glucose deprivation conditions. Data represent mean ± S.E.M. of 9 to 20 independent determinations. * denotes significance of p < 0.05, and ** denotes significance of p < 0.01 using one-way ANOVA with Newman-Keuls post hoc analysis.
Hippocampal Slices Subjected to OGD with N/C and nAChR Antagonists.
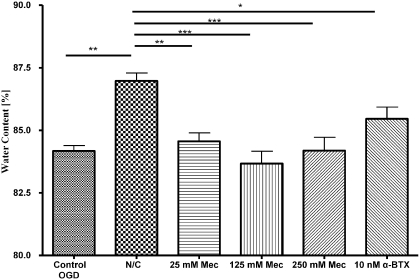
In OGD-exposed hippocampal brain slices, N/C exposure at 100/1000 ng/ml significantly increased water content (p < 0.01) compared with control OGD conditions (Fig. 3). In addition, nAChR antagonists abolished the findings of N/C exposure by significantly decreasing water content in hippocampal slices, even in the presence of nicotine/cotinine. The increase of water content with N/C (100/1000) exposure is reduced with various concentrations of nAChR antagonists (i.e., 25–250 μM mecamylamine present in the aCSF): 25 μM mecamylamine (p < 0.01), 125 μM mecamylamine (p < 0.001), and 250 μM mecamylamine (p < 0.001) (Fig. 3). In addition, the presence of 10 μM α-bungarotoxin in the presence of N/C (100/1000) inhibits the water gain induced by N/C (p < 0.05) but remained higher than 125 μM mecamylamine exposure (p < 0.05) (Fig. 3). All nAChR antagonist exposures alone did not significantly differ in water gain compared with OGD control (data not shown), suggesting that these antagonists are acting through a mechanism involving the nAChR.
Fig. 3.
Water content of hippocampal slices subjected to nicotine/cotinine and nicotinic acetylcholine receptor antagonists during oxygen glucose deprivation. Effects of short-term exposure of 100 ng/ml nicotine and 1000 ng/ml cotinine with or without nAChR antagonists on water content of hippocampal slices subjected to OGD. Control, N/C, or N/C along with 25, 125, 250 μM mecamylamine, or 10 nM α-bungarotoxin in aCSF. Data represent the mean ± S.E.M. of 9 to 20 independent determinations. * denotes significance of p < 0.05, and ** denotes significance of p < 0.01 using one-way ANOVA with Newman-Keuls post hoc analysis.
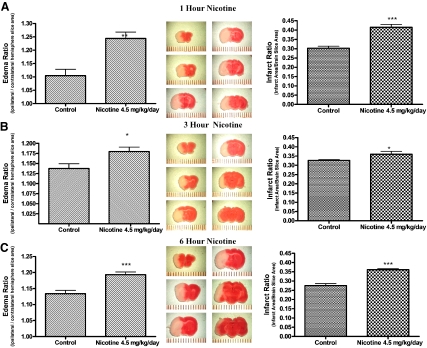
Short-Term 1-h Exposure to Cigarette Smoke Constituents in MCAO Model.
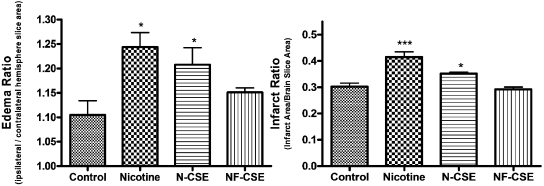
Infarct ratio increases with a 1-h dosage of 187.5 μg/kg nicotine (4.5 mg/kg/day, 4.5 mg/24 h = 187.5 μg/kg/1 h) (p < 0.001) and N-CSE (p < 0.05) at concentrations that mimic plasma levels of a heavy smoker (Fig. 4). Exposure to NF-CSE did not significantly differentiate infarct ratio from vehicle control. Edema ratio also increased for 1-h exposure to nicotine (p < 0.05) and N-CSE (p < 0.05) compared with control (Fig. 4). Again, NF-CSE did not differ from control in edema ratio same as with infarct ratio.
Fig. 4.
Effect of 1-h exposure to cigarette smoke constituents on infarct and edema ratios following MCAO. Effect of parenteral injection 1 h before 24-h MCAO of vehicle control, nicotine (4.5 mg/kg/day), N-CSE, or NF-CSE on infarct ratio (infarct area/brain slice area) and edema ratio (ipsilateral/contralateral hemisphere slice area). Data represent the mean ± S.E.M. of five independent determinations containing five to six slices each. * denotes significance of p < 0.05, and *** denotes significance of p < 0.001 using one-way ANOVA with Newman-Keuls post hoc analysis.
Short-Term 1-, 3-, and 6-h Exposure to Nicotine in MCAO Model.
All short-term intraperitoneal injections of nicotine equivalent to a total dosage of 4.5 mg/kg/day [1 h (4.5 mg/24 h = 187.5 μg/kg) (p < 0.01), 3 h (4.5 mg/8 h = 562.5 μg/kg) (p < 0.05), and 6 h (4.5 mg/4 h = 1125 μg/kg) (p < 0.001)] dosage before MCAO resulted in a significant increase in edema. Furthermore, infarct ratio was significantly increased for all three short-term nicotine 4.5 mg/kg/day injections: 1 h (p < 0.001) (Fig. 5A), 3 h (p < 0.05) (Fig. 5B), and 6 h (p < 0.001) (Fig. 5C).
Fig. 5.
Effect of 1-, 3-, and 6-h intraperitoneal injection of nicotine on edema and infarct ratios following MCAO. Effect of parenteral injection 1, 3, and 6 h of nicotine (4.5 mg/kg/day) on infarct ratio (infarct area/brain slice area) and edema ratio (ipsilateral/contralateral hemisphere slice area) following 24-h MCAO. Data represent the mean ± S.E.M. of five independent determinations containing five to six slices each. * denotes significance of p < 0.05, ** denotes significance of p < 0.01, and *** denotes significance of p < 0.001 using one-way ANOVA with Newman-Keuls post hoc analysis.
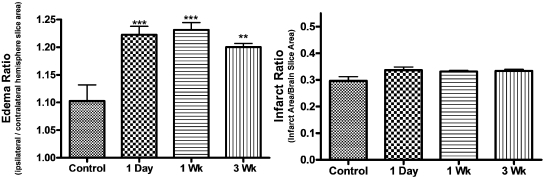
Long-Term Exposure to Nicotine in MCAO Model.
Long-term administration of nicotine was delivered through subcutaneously placed Alzet 2001 mini-osmotic pumps at a concentration to mimic heavy smokers (4.5 mg/kg/day) in accordance with previous studies (Murrin et al., 1987; Wang et al., 1997). Nicotine and cotinine plasma levels were verified by RIA and HPLC to confirm comparable plasma levels within groups. Long-term exposure to 4.5 mg/kg/day nicotine did not alter infarct ratio for 1-day, 1-week, or 3-week minipump administration (Fig. 6), whereas edema ratio was significantly altered compared with control: 1 day (p < 0.001), 1 week (p < 0.001), and 3 week (p < 0.01) (Fig. 6).
Fig. 6.
Effect of long-term exposure to nicotine on infarct and edema ratios following MCAO. Effect of long-term administration of vehicle control, nicotine (4.5 mg/kg) 1 day, nicotine (4.5 mg/kg) 1 week, and nicotine (4.5 mg/kg) 3 weeks on infarct ratio (infarct area/brain slice area) and edema ratio (ipsilateral/contralateral hemisphere slice area) following 24-h MCAO. Data represent the mean ± S.E.M. of five independent determinations containing five to six slices each. ** denotes significance of p < 0.01, and *** denotes significance of p < 0.001 using one-way ANOVA with Newman-Keuls post hoc analysis.
Locomotor Activity Measurements.
A series of locomotor activity parameters were monitored in this set of experiments, including vertical activity, total distance, movement time, and stereotypy time. The results showed that for parameters, such as vertical activity, total distance, movement time, and stereotypy time, there was no significant difference between control group and nicotine (1 h)-treated group (Table 2). Vehicle control had no effect on these locomotor parameters (data not shown). However, these locomotor parameters were significantly decreased with animals subjected to MCAO, compared with the control (p < 0.05). Combining treatment of nicotine (1 h) worsened the stroke outcome by decreasing these locomotor activity parameters compared with the MCAO group (Table 2). For long-term nicotine use (3 weeks), nicotine itself did not affect these selected locomotor activity parameters compared with the control. Animals subjected to both nicotine (3 weeks) and MCAO significantly decreased the locomotor parameters compared with the control (Table 2) (p < 0.05). However, a statistically significant difference could not be detected between MCAO group and MCAO/nicotine 3-week group (p > 0.05). It is noteworthy that there was no significant difference in those parameters between nicotine (1-h exposure)/MCAO group and nicotine (3-week exposure)/MCAO group (Table 2).
TABLE 2.
Effects of stroke and nicotine treatment on the mouse locomotor activity
Data represent the mean ± S.D. of three to four independent determinations.
| Parameters | Control | Nicotine 1 h | MCAO | MCAO + Nicotine 1 h | Nicotine 3 Weeks | MCAO + Nicotine 3 Weeks |
|---|---|---|---|---|---|---|
| Vertical activity | 4859 ± 872 | 4098 ± 1018 | 640 ± 209* | 140 ± 52# | 4414 ± 1214 | 578 ± 262* |
| Total distance (cm) | 3558 ± 934 | 2993 ± 817 | 397 ± 247* | 244 ± 82# | 4337 ± 627 | 216 ± 225* |
| Movement time (s) | 278 ± 79 | 217 ± 69 | 40 ± 16* | 22 ± 10# | 309 ± 32 | 34 ± 16* |
| Stereotypy time (s) | 178 ± 24 | 181 ± 38 | 25 ± 3* | 16 ± 6# | 167 ± 98 | 24 ± 16* |
P < 0.05 compared to the control group;
P < 0.05 compared to the MCAO group.
Discussion
Studies have shown that nicotine has a detrimental effect at the cerebral microcirculation involving tissue plasminogen activator, plasminogen activator inhibitor 1, nitric-oxide synthase, leukocyte migration, and BBB dysfunction of tight junctional proteins (Hawkins et al., 2002). In our studies using in situ and in vivo models, we have demonstrated that nicotine can increase cytotoxic and vasogenic edema after stroke conditions and therefore worsen the stroke outcome.
Hippocampal slice OGD is a model that represents cellular edema associated with the accumulation of water content and has previously been utilized to measure the neuroprotective effects of bilobalide (Mdzinarishvili et al., 2007). Therefore, an in situ 1-h incubation of hippocampal slices in either normoxic or OGD media with or without the presence of nicotine was used to investigate cytotoxic cellular edema, and in vivo 24-h murine MCAO pretreated with nicotine or vehicle control was used to investigate both cellular and vasogenic edema. With short-term exposure of nicotine/cotinine at concentrations ranging from 10/100 to 1000/10,000 ng/ml N/C, there is a significant increase of water content during OGD conditions compared with OGD control (Fig. 1). This shows that the concentration of 10/100 ng/ml N/C, similar to plasma levels found in smokers, can increase cellular edema during stroke conditions, which is consistent with previous studies (Hukkanen et al., 2005). During normoxic conditions, only the highest concentration of 1000/10,000 ng/ml N/C, 100-fold higher than concentrations applicable to heavy smokers, showed a significant effect of increasing water content compared with control (Fig. 1). Exposure to nicotine and its metabolite cotinine in this model at concentrations similar to cigarette smokers seems to be detrimental only during OGD and does not produce edematous conditions during normoxia. This could be explained by the fact that this hippocampal brain slice model mimics cellular edema only during OGD conditions (Mdzinarishvili et al., 2007).
Effective administration in the 1- and 3-week long-term dosage of nicotine through osmotic minipumps was verified via an RIA kit (Cozart Bioscience UK, Abingdon, Oxfordshire, UK) for detection of cotinine in plasma and detected as a positive or negative reading (Table 3). Further HPLC analysis of plasma from plasma of rats administered 4.5 mg/kg/day nicotine for 1 day, 1 week, and 2 weeks through Alzet minipumps detected levels of nicotine and the major metabolite cotinine at levels similar to a heavy smoker (Lockman et al., 2005b) (Table 4). All values correlate well with values reported in the literature (Benowitz and Jacob, 1994; Hukkanen et al., 2005). In addition, brain uptake of nicotine and cotinine in rats delivered through nicotine-loaded Alzet minipumps at 4.5 mg/kg/day for 28 days demonstrated a unidirectional influx across the BBB for nicotine at a rate of 114 to 143 μg/g/day and cotinine at a rate of 43 to 61 μg/g/day (Lockman et al., 2005a).
TABLE 3.
A plasma cotinine presence in 1- and 3-week osmotic minipump administration via RIA cotinine analysis
| Sample | 1 Week | 3 Weeks |
|---|---|---|
| Vehicle | N.D. | N.D. |
| Nicotine | + | + |
| NF-CSE | N.D. | N.D. |
| N-CSE | + | + |
N.D., not determined.
TABLE 4.
Nicotine and cotinine plasma concentrations via HPLC analysis
| 1 Day | 1 Week | 2 Weeks | |
|---|---|---|---|
| ng/ml | |||
| Plasma nicotine | 45.0 | 71.7 | 60.42 |
| Plasma cotinine | 269.2 | 261.6 | 240.8 |
Water content representing cytotoxic edema increased after a 1-week exposure to nicotine when hippocampal slices were exposed to OGD compared with control and NF-CSE exposures. It is interesting to note that NF-CSE exposure resulted in significantly less water gain compared with administration of nicotine alone, suggesting that the remaining components of cigarette smoke are not responsible for increased cytotoxic edema during a 1-week administration (Fig. 2A). Furthermore, when hippocampal slices were exposed to normoxic conditions, only a 1-week N-CSE exposure resulted in an increase of water content (Fig. 2A). This suggests that nicotine is the main component of cigarette smoke, which is detrimental both during normal conditions and also during OGD conditions exacerbating water gain in the hippocampal slice.
The edematous effects of 100 ng/ml/1000 ng/ml N/C during OGD were returned back to control levels with nAChR antagonists mecamylamine (25, 125, and 250 μM) and α-bungarotoxin (10 nM) (Fig. 3). Mecamylamine inhibits the ability of nicotine to induce the influx of calcium through the open position at the ion channel site of the nAChR (Zevin et al., 2000). The ion channel properties of nAChR of inward Ca+ and Na+ movements could facilitate edema formation when the agonist nicotine is present, and this action is abolished in the presence of nAChR antagonist mecamylamine and a more selective α7-antagonist, α-bungarotoxin (Colquhoun and Patrick, 1997). Our studies suggest that nicotine increases edema of the hippocampus during OGD via nAChR as proven by the ability of mecamylamine and α-bungarotoxin to reduce water content.
All cellular components of the neurovascular unit have nAChR subunit expression, including neurons, astrocytes, and endothelial cells (Abbruscato et al., 2002; Xiu et al., 2005; Brody et al., 2006). Long-term smoking has been associated with an alteration of nAChR expression in the brain with α4 subunit expression increased in neurons and dendritic processes (Teaktong et al., 2004) and decreased in α7 subunit expression in astrocytes and hippocampal regions (Teaktong et al., 2004). We previously have demonstrated that nicotine decreases NKCC activity in bovine brain microvessel cells, which also express α-3, α-5, α-7, β-2, and β-3 nAChR subunit proteins (Abbruscato et al., 2004). The decrease of α7 subunit expression in astrocytes of the hippocampus and a possible modulation of NKCC activity and movement of ions may be responsible for the lack of edema observed in the 3-week long-term administration of nicotine in the hippocampal OGD model. Therefore, we investigated these results using a second model of focal ischemia, MCAO, to evaluate infarct size and both forms of vasogenic and cellular edema during short- and long-term exposure to nicotine.
MCAO (24 h) creates predictable neuronal injury that is caused by both cytotoxic and vasogenic edema due to the ability of this model to replicate damage to the neurovascular unit (Mdzinarishvili et al., 2007). Cytotoxic edema has been observed within 12 h of occlusion, and water content and ionic disturbances have been observed to increase for 72 h following rat brain MCAO (Gotoh et al., 1985). Vasogenic edema has been observed to peak at 6 h following photochemical MCAO and measured through contrast-enhanced T1-weighted imaging (Chen et al., 2007). This combination of edema gives a more predictable model of damage from a chronic focal stroke. Short-term 1-h exposure to nicotine and N-CSE resulted in a significant increase in infarct ratio and edema (p < 0.001 and p < 0.05), respectively, which predicts the size of unrecoverable brain cell loss in the ischemic core (Fig. 4). It is noteworthy that exposure to NF-CSE did not result in a change of infarct or edema ratio compared with control, which is similar to the hippocampal 1-week OGD experiments suggesting that nicotine alone is responsible for the infarct volume changes. We then investigated the effects of nicotine alone on infarct and edema ratio with short-term administration (1, 3, and 6 h) before 24-h MCAO (Fig. 5). All short-term intraperitoneal injections of nicotine equivalent to 4.5 mg/kg/day before MCAO resulted in a significant increase of edema at various time points, such as 1 h (p < 0.01), 3 h (p < 0.05), and 6 h (p < 0.001), and also a significant increase in infarct ratio, such as 1 h (p < 0.001) (Fig. 5A), 3 h (p < 0.05) (Fig. 5B), and 6 h (p < 0.001) (Fig. 5C). We did not observe any increase in infarct area 24 h after MCAO with long-term dosages of nicotine (4.5 mg/kg/day s.c.: 1 day, 1 week, and 3 weeks). Yet, edema ratio significantly increases with nicotine exposure for long-term (1 day, 1 week, and 3 weeks) exposures (Fig. 6).
To further explore the nicotine effect of the functional outcome of the stroke, locomotor studies were designed. This study shows that activity parameter data were decreased significantly after MCAO surgery compared with those of control. Parameters from nicotine plus MCAO treatment group were statistically lower compared with the MCAO group, which supports our conclusion that nicotine worsens stroke outcome (Table 2). It is noteworthy that we did not observe any differences in locomotor activity parameters between MCAO group and MCAO + nicotine 3-week group (Table 2). It is apparent that some level of tolerance develops to the worsened infarct size effects of nicotine with long-term exposure. This may be due to nAChR receptor desensitization in the hippocampus slice. Desensitization of α4β2 nAChR, the predominant receptor in the brain, has recently been elucidated in cigarette smokers through positron emission tomography scanning (Brody et al., 2006). Plasma levels at 4% of the level of typical smokers resulted in 50% occupancy of α4β2 nAChR, and tobacco-dependent smokers with plasma levels ranging from 10 to 50 ng/ml nicotine maintained 96 to 98% receptor occupancy/desensitization throughout the day. Future studies will investigate nAChR expressions in brain regions susceptible to stroke damage.
In conclusion, this study shows that the nicotine produces an increase of both cytotoxic and vasogenic edema as seen in the hippocampal slice OGD and MCAO models, which simulate brain ischemia. Increased edema in the ischemic hemisphere may negatively affect penumbral region recovery and eventually lead to increased neuronal damage that may otherwise have been recoverable or responsive to neuron-protective therapy. Prevention of increased edematous conditions during stroke could be afforded through the avoidance of nicotine products in stroke prone individuals. Furthermore, clinicians should be aware of the propensity for edema in nicotine using or cigarette-smoking patients experiencing cerebrovascular accidents.
This work was supported by the National Institutes of Health [Grant R01-NS046526] (to T.J.A.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.157776
- BBB
- blood-brain barrier
- N-CSE
- nicotine-containing cigarette smoke extract
- NF-CSE
- nicotine-free cigarette smoke extract
- NKCC
- Na,K,2Cl− cotransporter
- aCSF
- artificial cerebrospinal fluid
- MCAO
- middle cerebral artery occlusion
- OGD
- oxygen glucose deprivation
- nAChR
- nicotinic acetylcholine receptor
- PG/DMSO
- propylene glycol/dimethyl sulfoxide
- RIA
- radioimmunoassay
- HPLC
- high-performance liquid chromatography
- N/C
- nicotine/cotinine
- ANOVA
- analysis of variance.
References
- Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. (2002) Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci 91:2525–2538 [DOI] [PubMed] [Google Scholar]
- Abbruscato TJ, Lopez SP, Roder K, Paulson JR. (2004) Regulation of blood-brain barrier Na,K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J Pharmacol Exp Ther 310:459–468 [DOI] [PubMed] [Google Scholar]
- Agre P, Nielsen S, Ottersen OP. (2004) Towards a molecular understanding of water homeostasis in the brain. Neuroscience 129:849–850 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd (1994) Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 56:483–493 [DOI] [PubMed] [Google Scholar]
- Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. (1999) Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control 8:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma B, Forman RE, Stewart EE, Nicholson C, Rice ME. (2000) Ascorbate inhibits edema in brain slices. J Neurochem 74:1263–1270 [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, et al. (2006) Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. (2006) Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. J Am Med Assoc 296:2939–2946 [DOI] [PubMed] [Google Scholar]
- Chen F, Suzuki Y, Nagai N, Jin L, Yu J, Wang H, Marchal G, Ni Y. (2007) Rodent stroke induced by photochemical occlusion of proximal middle cerebral artery: evolution monitored with MR imaging and histopathology. Eur J Radiol 63:68–75 [DOI] [PubMed] [Google Scholar]
- Colquhoun LM, Patrick JW. (1997) Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv Pharmacol 39:191–220 [DOI] [PubMed] [Google Scholar]
- Dzialowski I, Weber J, Doerfler A, Forsting M, von Kummer R. (2004) Brain tissue water uptake after middle cerebral artery occlusion assessed with CT. J Neuroimaging 14:42–48 [PubMed] [Google Scholar]
- Elliott KA, Jasper H. (1949) Measurement of experimentally induced brain swelling and shrinkage. Am J Physiol 157:122–129 [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. (2001) The Mouse Brain in Stereotaxic Coordinates Academic Press Inc., San Diego, CA: [Google Scholar]
- Gorgulu A, Kins T, Cobanoglu S, Unal F, Izgi NI, Yanik B, Kucuk M. (2000) Reduction of edema and infarction by Memantine and MK-801 after focal cerebral ischemia and reperfusion in rat. Acta Neurochir (Wien) 142:1287–1292 [DOI] [PubMed] [Google Scholar]
- Gotoh O, Asano T, Koide T, Takakura K. (1985) Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: the time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke 16:101–109 [DOI] [PubMed] [Google Scholar]
- Hankey GJ. (1999) Smoking and risk of stroke. J Cardiovasc Risk 6:207–211 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. (2004) Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res 1027:48–58 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. (2002) Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol Sci 23:78–82 [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57:79–115 [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Rutledge E, Goderie S, Charniga C. (1995) Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab 15:409–416 [DOI] [PubMed] [Google Scholar]
- Kumar V, Naik RS, Hillert M, Klein J. (2006) Effects of chloride flux modulators in an in vitro model of brain edema formation. Brain Res 1122:222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KF, Li F, Tatlisumak T, Garcia JH, Sotak CH, Fisher M, Fenstermacher JD. (2001) Regional variations in the apparent diffusion coefficient and the intracellular distribution of water in rat brain during acute focal ischemia. Stroke 32:1897–1905 [DOI] [PubMed] [Google Scholar]
- Lockman PR, McAfee G, Geldenhuys WJ, Van der Schyf CJ, Abbruscato TJ, Allen DD. (2005a) Brain uptake kinetics of nicotine and cotinine after chronic nicotine exposure. J Pharmacol Exp Ther 314:636–642 [DOI] [PubMed] [Google Scholar]
- Lockman PR, Van der Schyf CJ, Abbruscato TJ, Allen DD. (2005b) Chronic nicotine exposure alters blood-brain barrier permeability and diminishes brain uptake of methyllycaconitine. J Neurochem 94:37–44 [DOI] [PubMed] [Google Scholar]
- MacGregor DG, Avshalumov MV, Rice ME. (2003) Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J Neurochem 85:1402–1411 [DOI] [PubMed] [Google Scholar]
- Mdzinarishvili A, Geldenhuys WJ, Abbruscato TJ, Bickel U, Klein J, Van der Schyf CJ. (2005) NGP1–01, a lipophilic polycyclic cage amine, is neuroprotective in focal ischemia. Neurosci Lett 383:49–53 [DOI] [PubMed] [Google Scholar]
- Mdzinarishvili A, Kiewert C, Kumar V, Hillert M, Klein J. (2007) Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience 144:217–222 [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Zeng WY, Haley NJ. (1987) Nicotine administration to rats: methodological considerations. Life Sci 40:1699–1708 [DOI] [PubMed] [Google Scholar]
- Park CK, Kang SG. (2000) Effects of brain oedema in the measurement of ischaemic brain damage in focal cerebral infarction. Acta Neurochir Suppl 76:269–271 [DOI] [PubMed] [Google Scholar]
- Paulson JR, Roder KE, McAfee G, Allen DD, Van der Schyf CJ, Abbruscato TJ. (2006) Tobacco smoke chemicals attenuate brain-to-blood potassium transport mediated by the Na,K,2Cl-cotransporter during hypoxia-reoxygenation. J Pharmacol Exp Ther 316:248–254 [DOI] [PubMed] [Google Scholar]
- Sherratt AJ, Culpepper BT, Lubawy WC. (1988) Prostacyclin and thromboxane formation following chronic exposure to cigarette smoke condensate administered via osmotic pumps in rats. A method for chronic administration of particulates of whole smoke. J Pharmacol Methods 20:47–56 [DOI] [PubMed] [Google Scholar]
- Sydserff SG, Green AR, Cross AJ. (1996) The effect of oedema and tissue swelling on the measurement of neuroprotection; a study using chlormethiazole and permanent middle cerebral artery occlusion in rats. Neurodegeneration 5:81–85 [DOI] [PubMed] [Google Scholar]
- Teaktong T, Graham AJ, Johnson M, Court JA, Perry EK. (2004) Selective changes in nicotinic acetylcholine receptor subtypes related to tobacco smoking: an immunohistochemical study. Neuropathol Appl Neurobiol 30:243–254 [DOI] [PubMed] [Google Scholar]
- Ueshima H, Choudhury SR, Okayama A, Hayakawa T, Kita Y, Kadowaki T, Okamura T, Minowa M, Iimura O. (2004) Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke 35:1836–1841 [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. (2004) Edema and brain trauma. Neuroscience 129:1021–1029 [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. (2004) Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 35:2390–2395 [DOI] [PubMed] [Google Scholar]
- Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. (1997) Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab 17:136–146 [DOI] [PubMed] [Google Scholar]
- Xiu J, Nordberg A, Zhang JT, Guan ZZ. (2005) Expression of nicotinic receptors on primary cultures of rat astrocytes and up-regulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide(1–42). Neurochem Int 47:281–290 [DOI] [PubMed] [Google Scholar]
- Zevin S, Jacob P, 3rd, Benowitz NL. (2000) Nicotine-mecamylamine interactions. Clin Pharmacol Ther 68:58–66 [DOI] [PubMed] [Google Scholar]