Abstract
This study assessed the pharmacokinetic profiles for intramuscular and intravenous ceftaroline treatment for rats, rabbits, and monkeys. Ceftaroline, a novel cephalosporin with broad-spectrum activity against Gram-positive and Gram-negative pathogens, demonstrated favorable pharmacokinetic profiles following intramuscular administration in all 3 animal species, comparable to the levels for intravenous dosing. The areas under the plasma concentration-time curve obtained after intramuscular administration were increased in rats and similar in rabbits and monkeys, compared with the levels obtained after intravenous dosing (129%, 7.29%, and 12.7% greater in rats, rabbits, and monkeys, respectively). The data reported here support the development of an intramuscular formulation for ceftaroline.
Ceftaroline is a novel, parenteral, broad-spectrum cephalosporin exhibiting bactericidal activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Streptococcus pneumoniae, as well as common Gram-negative pathogens (1, 13). Ceftaroline has also demonstrated in vitro bactericidal activity against etiologic pathogens frequently associated with complicated skin and skin structure infections (cSSSIs), community- and hospital-associated pneumonia (CAP and HAP), and other serious infections (4, 10). In vivo, the water-soluble prodrug ceftaroline fosamil is rapidly dephosphorylated in the plasma into the bioactive metabolite (ceftaroline), which exhibits antibacterial properties. To date, phase 3 trials utilizing intravenous (i.v.) administration of ceftaroline fosamil have been completed for cSSSI and are in progress for CAP (8). Like other cephalosporins, ceftaroline may be administered by both intramuscular (i.m.) and i.v. routes (2, 6). The availability of ceftaroline in an i.m. formulation would allow for more-convenient dosing, particularly in an outpatient setting. As established for other broad-spectrum antibiotics, it is desirable to determine the safety, tolerability, and pharmacokinetic (PK) profile of ceftaroline after i.m. administration (2, 6). This study assessed the PK profile of a single dose of ceftaroline fosamil administered via the i.m. route, compared with that of a single dose administered by the i.v. route, for 3 different animal species.
(A preliminary report of these results was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy in Washington, DC, 25 to 28 October 2008.)
This study was approved by the Institutional Animal Care and Use Committee. Totals of 18 male Sprague-Dawley rats, 4 female New Zealand White rabbits, and 4 cynomolgus monkeys (3 male and 1 female) were included in this study, which was conducted at LAB Research, Inc. (Laval, Quebec, Canada). Rats were assigned to either i.m. or i.v. dosing with ceftaroline by block randomization based on body weight. For rabbits and monkeys, a crossover design with a 3-day washout period between i.m. and i.v. dosing was used. Ceftaroline fosamil powder was dissolved in 1.9% l-arginine solution. The amount of weighed powder was adjusted using a conversion factor of 1.115 for ceftaroline acetate-free base to salt.
For all 3 animal species, ceftaroline was administered at a dose of 20 mg/kg of body weight, calculated based on the most recent body weight of the animal. i.m. doses were administered in the thighs of the animals, whereas i.v. doses were administered over a period of approximately 1 min through the tail vein, marginal ear vein, and saphenous vein in rats, rabbits, and monkeys, respectively. Mortality and clinical signs were evaluated for all 3 animal species. Blood samples of approximately 0.5 ml were collected from the animals prior to dosing and at 5, 15, 30, 60, and 90 min and 2, 4, and 8 h postdose for PK analysis. For rats, blood samples were collected from 3 rats per time point. Samples were placed in prechilled tubes containing sodium heparin as an anticoagulant and stored on wet ice until processing. The blood samples were centrifuged within 1 h of collection at approximately 1,500 × g for 10 min in a refrigerated centrifuge. An aliquot of 200 μl of plasma was transferred into a prelabeled 0.5-ml polypropylene tube containing 2.0 μl of phosphatase inhibitor cocktail 2 (Sigma-Aldrich, St. Louis, MO), placed on dry ice, and stored at approximately −70°C within 30 min after processing. The samples were shipped on dry ice for determination of ceftaroline plasma concentrations (MicroConstants, Inc., San Diego, CA). Plasma samples were analyzed by validated liquid chromatography-tandem mass spectrometry (LC-MS-MS) methods having lower limits of quantitation of 10 ng/ml in rabbits and monkeys and 50 ng/ml in rats for ceftaroline. PK parameter values were calculated using noncompartmental analysis with the software program WinNonlin (Pharsight Corporation, Mountain View, CA). The area under the plasma concentration curve from time zero to infinity (AUC0-∞), terminal half-life (t1/2), highest plasma concentration observed (Cmax), and time to Cmax (Tmax) for ceftaroline were determined. Plasma clearance and volume of distribution at steady state were also determined after i.v. administration but are not reported here.
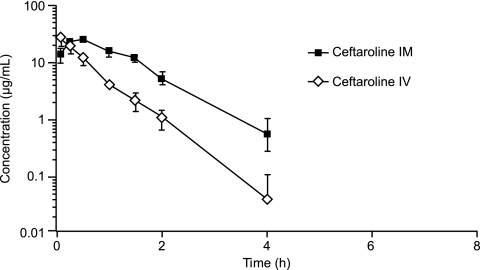
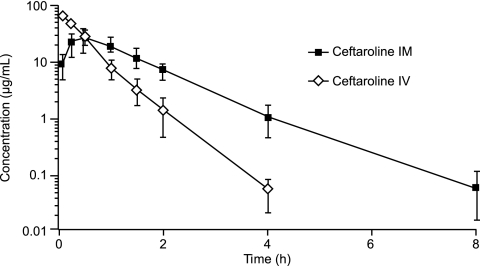
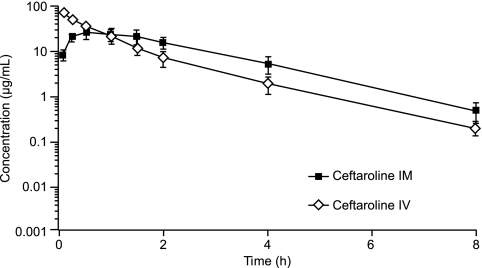
Injections of ceftaroline by the 2 routes of administration were well tolerated among all 3 animal species. There were no mortalities in the study. Various clinical signs were observed (e.g., skin and fur discoloration, fur loss, decreased appetite, and soft feces), but they were considered incidental or commonly observed in normal populations of the 3 animal species. These signs were also comparable between the i.m. and i.v. dosing groups for all species. The mean plasma concentrations of ceftaroline observed after i.m. and i.v. administration in rats, rabbits, and monkeys are presented in Fig. 1, 2, and 3, respectively. For all 3 animal species, the plasma concentrations of ceftaroline observed after i.m. dosing were sustained and were slightly higher than the mean plasma concentrations observed after i.v. administration. The PK parameter values for ceftaroline observed after i.m. and i.v. administration are presented in Table 1. The AUCs for i.m. dosing were similar to those for i.v. dosing for rabbits and monkeys and greater than those for i.v. dosing for rats, indicating excellent bioavailability. For rabbits and monkeys, the mean AUCs were 7.3% and 12.7% greater, respectively, following i.m. administration than following i.v. administration. For rats, the mean AUC was 129% greater following i.m. administration than following i.v. dosing. It is not clear why there was a significant difference in AUC for rats versus rabbits and monkeys between the i.m. and i.v. administration groups. The metabolism and elimination of ceftaroline were faster for rats than for rabbits or monkeys. The AUC values observed after i.m. dosing for rabbits and monkeys were likely within the experimental error of the AUC values observed after i.v. dosing. The time required to reach Cmax was longer following i.m. administration than following i.v. administration, and the ceftaroline t1/2s were longer for rats and rabbits following i.m. administration. These findings may be explained by a slow release from the injection site and/or a limited conversion of the prodrug in the i.m. space, which may contribute to a long and sustainable drug concentration in the plasma, resulting in a higher percentage of dosing interval during which the antibiotic concentration is above the MIC (%T>MIC) than that obtained with i.v. administration of the drug.
FIG. 1.
Mean ± standard deviation (SD) plasma concentrations of ceftaroline in rats after i.m. and i.v. administration.
FIG. 2.
Mean ± SD plasma concentrations of ceftaroline in rabbits after i.m. and i.v. administration.
FIG. 3.
Mean ± SD plasma concentrations of ceftaroline in monkeys after i.m. and i.v. administration.
TABLE 1.
PK parameter values for ceftaroline observed after i.m. and i.v. administrationa
| Route and animal group | AUC0-∞(h·μg/ml) | Cmax (μg/ml) | t1/2 (h) | Tmax (h) |
|---|---|---|---|---|
| i.m. | ||||
| Rat (n = 9)b | 37.4 | 25.5 | 0.621 | 0.500 |
| Rabbit (n = 4) | 39.7 ± 11.4 | 25.8 ± 11.6 | 0.833 ± 0.181 | 0.438 ± 0.125 |
| Monkey (n = 4) | 72.1 ± 21.4 | 25.6 ± 7.82 | 1.17 ± 0.152 | 0.500 |
| i.v.c | ||||
| Rat (n = 9)b | 16.3 | 27.1 | 0.426 | 0.0830 |
| Rabbit (n = 4) | 37.0 ± 7.84 | 67.8 ± 3.77 | 0.410 ± 0.00701 | 0.0830 |
| Monkey (n = 4) | 64.0 ± 12.1 | 69.1 ± 12.8 | 1.16 ± 0.0676 | 0.0830 |
Values shown are means ± SD. AUC0-∞, area under the plasma concentration-time curve from time zero to infinity; Cmax, highest plasma concentration observed; t1/2, terminal half-life; Tmax, time at which Cmax occurred.
The PK parameter values for rats are composite values.
The first measured time point following the i.v. bolus was 0.083 h.
The excellent PK profile observed for ceftaroline following i.m. administration in these animal models predicts an outcome better than or comparable to that provided by i.v. administration in terms of the pharmacodynamic (PD) parameter %T>MIC. As previous studies have demonstrated, %T>MIC is an important parameter for predicting efficacy for cephalosporins, including ceftaroline (1, 5, 9, 12, 14). The PK/PD index of ceftaroline has been established in a neutropenic mouse thigh infection model; the ceftaroline mean free-drug %T>MICs associated with the static effect end point for S. pneumoniae, S. aureus, and Gram-negative isolates were approximately 39%, 26%, and 32%, respectively (1). One study, conducted in a rabbit endocarditis model using a simulated human therapeutic dosing regimen (600 mg every 12 h [q12h] over 1 h of infusion), demonstrated a correlation between %T>MIC attained after i.m. administration and bactericidal activity against MRSA observed after a 4-day treatment regimen of ceftaroline (7). The %T>MICs achieved after a single i.m. dose of ceftaroline at 20 mg/kg were 46.3% ± 2.0% and 30.9% ± 1.3% at 8 h and 12 h postdose, respectively. In addition, the values for this parameter have been found to be similar in animal infection models and human infections (1, 3). Ceftaroline has been developed for the treatment of cSSSIs and CAP. The MIC90s of ceftaroline for the main target pathogens (staphylococci and pneumococci) from those infections were 2 μg/ml or less (4). For humans, preliminary data from a phase 1 study suggest that a dosing regimen of ceftaroline at 600 mg i.m. administered every 12 h results in favorable %T>MICs (assuming 20% binding to human plasma proteins), ranging from 99.82% ± 0.63% to 64.65% ± 12.39% for MICs of 0.12 to 2.0 μg/ml, respectively, following multiple i.m. doses (11). An i.m. formulation could provide flexibility and convenience in outpatient or institutional settings, in which a route of administration other than the i.v. route might be preferred.
In conclusion, for rats, rabbits, and monkeys, the favorable pharmacokinetic profile for ceftaroline seen after i.m. administration was comparable to that seen after i.v. dosing. It appears that a longer %T>MIC was achieved with i.m. dosing than with i.v. dosing. Cmax was lower for i.m. dosing than for i.v. dosing, but AUC was similar or greater for i.m. dosing. The time required to reach Cmax was longer for i.m. dosing but should have a minimal impact on the efficacy and pharmacokinetics/pharmacodynamics of ceftaroline because its efficacy is correlated with %T>MIC and not Cmax. The data fully support clinical development of ceftaroline by the i.m. route of administration.
Acknowledgments
Scientific Therapeutics Information, Inc., Springfield, NJ, provided editorial assistance with the manuscript.
Funding for this study and for editorial assistance was provided by Forest Laboratories, Inc.
Footnotes
Published ahead of print on 30 November 2009.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbhaiya, R. H., C. A. Knupp, J. Tenney, R. R. Martin, D. J. Weidler, and K. A. Pittman. 1990. Safety, tolerance, and pharmacokinetics of cefepime administered intramuscularly to healthy subjects. J. Clin. Pharmacol. 30:900-910. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 4.Ge, Y., D. Biek, G. H. Talbot, and D. F. Sahm. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber, A. U., W. A. Craig, H. P. Brugger, C. Feller, A. P. Vastola, and J. Brandel. 1983. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J. Infect. Dis. 147:910-917. [DOI] [PubMed] [Google Scholar]
- 6.Goonetilleke, A. K. E., D. Dev, I. Aziz, C. Hughes, M. J. Smith, and G. S. Basran. 1996. A comparative analysis of pharmacokinetics of ceftriaxone in serum and pleural fluid in humans: a study of once daily administration by intramuscular and intravenous routes. J. Antimicrob. Chemother. 38:969-976. [DOI] [PubMed] [Google Scholar]
- 7.Jacqueline, C., J. Caillon, V. Le Mabecque, E. Batard, A. F. Miegeville, D. Biek, Y. Ge, and G. Potel. 2008. Evaluation of the efficacy of intramuscular (IM) administration of ceftaroline (CPT) against a methicillin-resistant Staphylococcus aureus (MRSA) strain in a rabbit endocarditis model (REM), abstr. B-1003. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (ISDA) 46th Annu. Meet., Washington, DC.
- 8.Kanafani, Z. A., and G. R. Corey. 2009. Ceftaroline: a cephalosporin with expanded gram-positive activity. Future Microbiol. 4:25-33. [DOI] [PubMed] [Google Scholar]
- 9.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 10.Morrissey, I., Y. Ge, and R. Janes. 2009. Activity of the new cephalosporin ceftaroline against bacteraemia isolates from patients with community-acquired pneumonia. Int. J. Antimicrob. Agents 33:515-519. [DOI] [PubMed] [Google Scholar]
- 11.Riccobene, T., E. Fang, and D. Thye. 2008. A single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics (PK) of ceftaroline (CPT) administered by intramuscular (IM) injection to healthy subjects, abstr. A-1888. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet., Washington, DC.
- 12.Roosendaal, R., I. A. Bakker-Woudenberg, J. C. van den Berg, and M. F. Michel. 1985. Therapeutic efficacy of continuous versus intermittent administration of ceftazidime in an experimental Klebsiella pneumoniae pneumonia in rats. J. Infect. Dis. 152:373-378. [DOI] [PubMed] [Google Scholar]
- 13.Sader, H. S., T. R. Fritsche, K. Kaniga, Y. Ge, and R. N. Jones. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]