Abstract
With the current high prevalence of infection caused by methicillin-resistant Staphylococcus aureus (MRSA) strains but in light of the general belief that β-lactam antibiotics are more effective than vancomycin against infections caused by methicillin-susceptible S. aureus (MSSA) isolates, clinicians may utilize antistaphylococcal penicillins in combination with vancomycin for the empirical treatment of S. aureus infections. Vancomycin is considered to kill MSSA more slowly than oxacillin. Thus, we sought to evaluate the interaction of the combination of oxacillin and vancomycin on bacterial killing in vitro. Ten clinical isolates of MSSA isolated in the year 2000 were investigated. The killing observed at 24 h by vancomycin at 20 μg/ml, oxacillin at 16 μg/ml, or the combination did not differ (approximately 2.5 to 3.5 log10 CFU/ml). In a separate experiment, we assessed bacterial killing in a dynamic model simulating the free plasma concentration profiles expected following the administration of a combination of vancomycin at 1 g every 12 h and oxacillin at 1 g every 6 h. The time-kill profiles of these regimens against S. aureus ATCC 29213 were comparable to those observed in the fixed-concentration experiments. Using these methods, we found no evidence that vancomycin antagonized the bactericidal effect of oxacillin or that there was any benefit from use of the combination.
In many clinical settings, methicillin-resistant Staphylococcus aureus (MRSA) strains comprise such a high proportion of the S. aureus isolates that empirical treatment with vancomycin is begun when a staphylococcal infection is suspected (10, 18). However, several studies have reported that for infections due to methicillin-susceptible S. aureus (MSSA) strains, treatment with β-lactam antibiotics results in clinical outcomes better than those achieved with vancomycin (15, 18). For this reason, some clinicians use vancomycin and a β-lactam together as empirical treatment, both to provide adequate coverage for MRSA and in the belief that the β-lactam would provide superior antimicrobial activity should the organism prove to be methicillin susceptible.
Small and Chambers (17) reported that vancomycin killed MSSA more slowly than nafcillin when they used time-kill methods. This observation suggests that when the antibiotics are used together, the more slowly acting bactericidal agent, vancomycin, may have the potential to antagonize the more rapid bactericidal effect of oxacillin, negating the anticipated benefit of the β-lactam. Such in vitro antagonism has been demonstrated when ß-lactams are combined with bacteriostatic agents such as erythromycin (8). In the present study, we sought to evaluate the interactions between vancomycin and oxacillin against clinical isolates of MSSA.
(Part of the data presented here were presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12 to 15 September 2009 [abstr. E-1452].)
MATERIALS AND METHODS
Bacterial strains.
The clinical MSSA isolates tested in part A of the present study were A8115, A8117, A8561, A8562, A8564, A8565, A8566, A8568, A8569, and A8570. Thus, 10 clinical isolates of MSSA were used in our study and were collected in the year 2000. All isolates of MSSA had been isolated from different individuals and were obtained from our frozen collection. We included S. aureus ATCC 29213 as an additional test strain.
Antimicrobials.
Vancomycin (lot no. 106K1407) and oxacillin (lot no. 016K0489) were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Stock solutions of each antibiotic were freshly prepared or were frozen at −80°C and thawed immediately before use.
Susceptibility testing.
MICs were determined by Etest (AB Biodisk, Solna, Sweden), according to the manufacturer's instructions. Oxacillin Etests were therefore performed on Mueller-Hinton II agar plates with 2% NaCl to enhance the detection of resistance.
Time-kill studies.
Time-kill assays were performed in flasks containing brain heart infusion broth (BHI; Becton Dickinson and Company, Sparks, MD) inoculated with the test strains. The bacteria were grown to a density of approximately 9 log10 CFU/ml, and 1 ml of bacterial suspension was inoculated into 19 ml of fresh BHI, which contained no antibiotic, oxacillin at 16 μg/ml, vancomycin at 20 μg/ml, or the combination of both antibiotics. A 200-μl aliquot was obtained from each flask at 0, 4, and 24 h; serially diluted in saline; and plated on antibiotic-free agar (tryptic soy agar plus 5% sheep blood [TSA]; Northeast Laboratory Services, Waterville, ME) in duplicate to determine the colony counts, as described previously (4). The lower limit of detection was 200 CFU/ml.
Pharmacodynamic model.
The pharmacokinetic (PK) profiles of vancomycin and oxacillin in plasma were calculated from the values for the key parameters available from the literature and from the drug package inserts provided by the manufacturers (13, 16). On the basis of the mean values for plasma protein binding of 55% (vancomycin) and 95.5% (oxacillin), half-lives of 4 h (vancomycin) and 0.5 h (oxacillin), and total maximum concentrations of drug in plasma of 90 μg/ml (vancomycin) and 60 μg/ml (oxacillin) following the administration of intravenous doses of 2 g of vancomycin (1-h infusion) and 1 g of oxacillin (0.5-h infusion) we calculated the PK profile over 24 h. The trough levels of unbound vancomycin were between 5 and 10 μg/ml (Table 1). In this calculation, we simulated an intravenous loading dose of 2 g of vancomycin followed by a maintenance dose of 1 g twice a day. The pharmacokinetic profile of oxacillin in plasma was calculated for a dosage of 1 g four times a day (see Fig. 2a). The concentrations used were corrected for their respective plasma protein binding values.
TABLE 1.
Calculated values of key PK parameters for oxacillin and vancomycina
| Study and antibiotic | fAUC0-24 (μg·h/ml) | fAUC0-∞ (μg·h/ml) | t1/2ß (h) | fCmax (μg/ml) | ftrough (μg/ml) | PK-PDb index |
|---|---|---|---|---|---|---|
| Static (part A) | ||||||
| Oxacillin | 384.0 | NA | NA | 16 | 16 | 100% |
| Vancomycin | 480.0 | NA | NA | 20 | 20 | 240-480 |
| Dynamic (part B) | ||||||
| Oxacillin | 19.5 | 19.5 | 0.5 | 4.5 | 0 | 40-65% |
| Vancomycin | 514.9 | 544.2 | 4 | 60/34* | 10/5* | 258-515 |
As the PK profiles for the dynamic simulation were calculated from the values for the key PK parameters derived from the literature, they must be considered theoretical but are representative and therapeutically achievable in plasma after a loading dose of 2 g of vancomycin followed by a maintenance dose of 1 g every 12 h (13, 16). The PK profile of oxacillin was calculated for a 1-g dosage given every 6 h. Abbreviations: fAUC0-24, area under the free concentration-time curve from time zero to 24 h; fAUC0-∞, area under the free concentration-time curve from time zero to infinity; t1/2β, elimination half-life; fCmax, maximum concentration of free drug in plasma; ftrough, trough free concentration; NA, not applicable; *, result obtained with a dose of 1 g.
Taking the appropriate PK-PD parameter into account, the data represent fT > MIC for free oxacillin (expressed as a percentage of the dosing interval) and the ratio of the free area under the concentration-time curve from time zero to 24 h to MIC for vancomycin. The respective MICs used for these calculations were 0.25 to 1.0 μg/ml for oxacillin and 1.0 to 2.0 μg/ml for vancomycin.
FIG. 2.
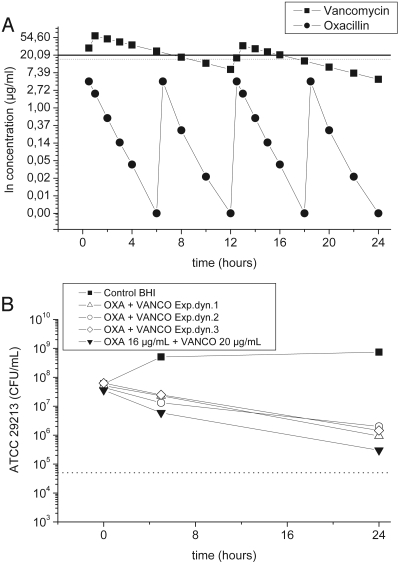
(a) Virtual plasma pharmacokinetic profile for free vancomycin (squares) after a loading dose of 2 g, followed by a maintenance dose of 1 g every 12 h. The calculated plasma pharmacokinetic profile of free oxacillin (circles) is depicted for a dosage of 1 g every 6 h. Every single data plot (dot or square) represents a time point when the drug concentrations in the flask were adjusted. The horizontal lines indicate the concentrations of vancomycin (20 μg/ml; solid line) and oxacillin (16 μg/ml; dotted line) used in the static experiments. (b) Time-kill curves of S. aureus ATCC 29213 following exposure to the free concentration-versus-time profiles of vancomycin and oxacillin, as depicted in panel a. The results from the fixed-concentration experiment (Fig. 1c) are shown for comparison. OXA, oxacillin; VANCO, vancomycin.
On the basis of the results derived from the calculation of key pharmacokinetic parameters for plasma, we performed time-kill curve analyses using a dynamic model simulating the concentrations of oxacillin, vancomycin, and their combination in plasma over 24 h. S. aureus ATCC 29213 was used as the test strain and was exposed to the dynamically changing drug concentration profiles of vancomycin and oxacillin in plasma over 24 h.
BHI was inoculated from a static culture at an approximate starting inoculum of 8 log10 CFU/ml. The flasks were incubated at 35°C without agitation. Increasing antibiotic concentrations were simulated by adding antibiotic in appropriate amounts; decreasing plasma concentrations were simulated by centrifuging the bacteria at 3,000 × g and 37°C for 15 min, withdrawal of the supernatant at appropriate volumes, and subsequent replacement of identical volumes of fresh broth. Approximately 99.9% of the bacteria were recovered by this procedure. Separate studies (data not shown) demonstrated that the procedure of spinning and washing of the cells had no effect on the bacterial growth profiles even after several repetitions, as determined by comparison with the growth of the untreated controls. For the combination regimen experiments, the elimination rate was set for the drug with the shorter half-life, and the drug with the longer half-life was supplemented (2). Bacteria were counted at the baseline and 4 h and 24 h after incubation.
Immediately before 200-μl samples were withdrawn, the culture flasks were gently swirled and the samples withdrawn were subsequently serially diluted with 0.9% sodium chloride. Twenty-five-microliter volumes from each dilution tube were plated onto TSA plates. These plates were incubated for 24 h at 35°C, after which the colonies were counted to calculate the numbers of CFU/ml. Each simulation was performed in triplicate. Controls represent bacterial growth in the absence of antibiotic. The lower limit of detection was 200 CFU/ml.
Background PK calculations and analysis for the simulation of the dynamic experiment.
The main PK data were derived from the literature. Missing data points were calculated by use of the formula 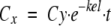 , where kel is the elimination rate constant (h−1), Cx is the unknown concentration at time point x, and Cy is the last known concentration at time point x − t. The time that the concentration (C) in plasma exceeded the MIC (T > MIC; in hours) was calculated by use of the equation ln(CD/MIC)/kel + D, where D is the duration of infusion (in hours) and CD is the concentration of oxacillin at time point D. The cumulating factor (R) for vancomycin was calculated by the equation
, where kel is the elimination rate constant (h−1), Cx is the unknown concentration at time point x, and Cy is the last known concentration at time point x − t. The time that the concentration (C) in plasma exceeded the MIC (T > MIC; in hours) was calculated by use of the equation ln(CD/MIC)/kel + D, where D is the duration of infusion (in hours) and CD is the concentration of oxacillin at time point D. The cumulating factor (R) for vancomycin was calculated by the equation 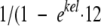 ). The areas under the concentration-time curves from time zero to 24 h (AUC0-24s) were calculated by use of the linear trapezoidal rule and commercially available computer software (Kinetica, version 3.0; Innaphase, Philadelphia, PA).
). The areas under the concentration-time curves from time zero to 24 h (AUC0-24s) were calculated by use of the linear trapezoidal rule and commercially available computer software (Kinetica, version 3.0; Innaphase, Philadelphia, PA).
RESULTS
The objective of the present study was to explore the antimicrobial effects of vancomycin, oxacillin, and the combination of both on MSSA strains by employing established fixed-concentration and dynamic time-kill models.
Fixed-concentration model.
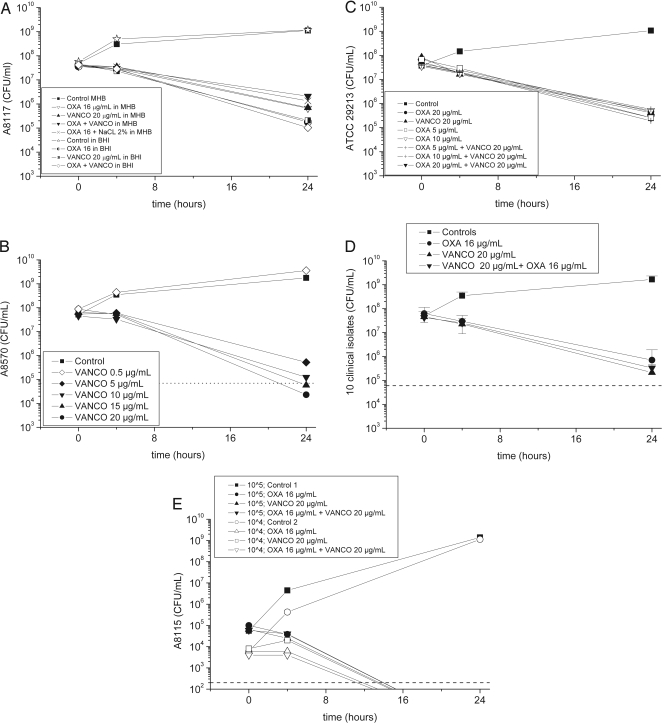
Prior to the start of the time-kill experiments, we determined the MICs of vancomycin and oxacillin for the pathogens. The respective MICs of the test strains ranged from 0.25 to 1.0 μg/ml for oxacillin and from 1.0 to 3.0 μg/ml for vancomycin. We then investigated the effects of different broth media on growth and antimicrobial activity (Fig. 1a). S. aureus A8117 grew similarly in both broth media in the absence of antibiotics. However, the level of killing by each antibiotic and by the combination was approximately 10-fold greater in BHI than in Mueller-Hinton broth (MHB), even after the supplementation of MHB with 2% sodium chloride. We elected to use BHI for all subsequent studies.
FIG. 1.
(a) Effect of growth medium (Mueller-Hinton broth versus brain heart infusion) supplemented with 2% sodium chloride on the killing of clinical isolate MSSA A8117. (b) Effects of various concentrations of vancomycin on killing activity (clinical isolate MSSA A8570). The dotted line indicates a 3-log10 decrease in the number of CFU/ml versus the number at the baseline (bactericidal effect). (c) Time-kill curves for S. aureus ATCC 29213 after incubation with oxacillin at concentrations ranging from 5 to 20 μg/ml alone or with vancomycin at a fixed concentration of 20 μg/ml. (d) Mean killing-versus-time profiles of 10 clinical MSSA isolates for vancomycin, oxacillin, and the combination. Error bars represent standard deviations. The dotted line indicates a 3-log10 decrease in the number of CFU/ml versus the number at the baseline (bactericidal effect). (e) Time-kill curves for isolate S. aureus A8115 for starting inocula of 104 and 105 CFU/ml. The horizontal dashed line highlights the limit of detection. OXA, oxacillin; VANCO, vancomycin.
Time-kill curves.
The initial inoculum in all experiments (except for the experiment whose results are presented in Fig. 1e) was approximately 8 log10 CFU/ml (Fig. 1a to d) and did not differ between groups. Incubation of the bacteria with vancomycin, oxacillin, or their combination decreased the number of viable bacteria by approximately 0.5 to 1.0 log10 CFU/ml at 4 h in all groups. At 24 h, the killing observed in the presence of vancomycin was comparable to that observed in the presence of oxacillin in all experiments performed (2.5 to 3.5 log10 CFU/ml) (Fig. 1a to d). The combination of vancomycin and oxacillin resulted in killing that was not different from that observed with either agent alone (Fig. 1d). Thus, no evidence of synergy or antagonism against any of the 11 MSSA isolates tested was detected.
Effect of drug concentration on killing.
As expected, vancomycin demonstrated concentration- or exposure-dependent killing when it was tested against S. aureus A8570 (Fig. 1b). In contrast, the lowest free concentration of oxacillin tested (5 μg/ml; Fig. 1c) was as effective as higher concentrations, which is consistent with the concept that the PK-pharmacodynamic index fT > MIC (where f indicates free) is predictive of the level of antimicrobial killing and efficacy for ß-lactams. For this isolate and for all others used in this study, which had oxacillin MICs that ranged from 0.25 to 1.0 μg/ml, each of these concentrations tested corresponded to an fT > MIC of 100%.
Effect of bacterial inoculum on killing.
The results of time-kill studies performed with inocula of 104 and 105 CFU/ml were compared with those performed with approximately 108 CFU/ml (Fig. 1e). With the two lower inocula, the colony counts at 24 h were below the lower limit of detection. However, with each inoculum, the use of each of the antimicrobials as well as their combination resulted in comparable colony counts.
Dynamic concentration-versus-time kill model.
In the dynamic concentration-versus-time kill model part of the study, S. aureus ATCC 29213 was exposed to antibiotics at levels that reflected a free drug concentration-versus-time profile in plasma after the intravenous administration of 1 g of vancomycin every 12 h after the administration of an initial loading dose of 2 g and after the administration of oxacillin at a dose of 1 g every 6 h (Fig. 2a). The time-kill profile observed in this dynamic experiment was very comparable to that observed in the static killing model (Fig. 2b).
DISCUSSION
Although it has not been proven by randomized, double-blind prospective studies, it is generally believed that treatment with a β-lactam is superior to treatment with vancomycin for serious MSSA infections. This concept is supported by a number of retrospective and observational studies (5, 12, 15, 18). One explanation for this might be that the bactericidal activity of β-lactams against MSSA may be superior to that of vancomycin. Small and Chambers (17) compared the bactericidal activities of nafcillin and vancomycin, each at four times the MIC, against 10 clinical isolates of MSSA using time-kill curves. Starting from an inoculum of approximately 107 CFU/ml in log phase, the bactericidal activities of these two antibiotics were comparable at 4 h of incubation, but the level of killing at 24 h was approximately 1.5 log10 CFU/ml greater with nafcillin than with vancomycin.
These results led us to hypothesize that the practice of adding a β-lactam to vancomycin for empirical therapy of suspected bacteremic S. aureus infection might be fruitless, as the bacteriostatic agent, vancomycin, might antagonize the killing activity of the more bactericidal β-lactam. The present study was undertaken to answer that question.
To our surprise, over a range of concentrations that would encompass achievable peak and trough free vancomycin concentrations in plasma (20 to 10 μg/ml), the level of killing of MSSA isolates at 24 h was comparable to that achieved by oxacillin. As a result, we observed no antagonism with the combination of the two antibiotics either in the fixed-concentration time-kill model or in the dynamic model. Thus, from these results, we would predict no microbiological disadvantage from the use of this therapeutic approach.
These results do not explain the apparent clinical advantages of β-lactam therapy suggested from the findings of the clinical studies cited above. For example, Lodise et al. (15) found not only that empirical vancomycin treatment of intravenous drug users with MSSA endocarditis was associated with greater mortality than empirical β-lactam therapy (individuals who received vancomycin plus a β-lactam were included among the subjects receiving empirical β-lactam therapy) but also that those who were subsequently switched to a β-lactam from vancomycin at a median of 3 days fared no better than those who remained on vancomycin. In contrast, in a study by Stryjewski et al. (18), while hemodialysis patients with MSSA bacteremia ultimately failed vancomycin treatment more often than cefazolin treatment, this difference was apparent only at the predetermined end point at 12 weeks after the first positive culture; the proportions of patients in each group who had responded to treatment at the time of discharge did not differ descriptively. In addition, in an experimental model of MSSA endocarditis, both vancomycin and cloxacillin were effective in reducing the numbers of viable bacteria within cardiac vegetations at doses simulating the levels achieved in humans (3).
Our in vitro results do not exclude alternative mechanisms by which oxacillin alone or together with vancomycin may achieve outcomes superior to those seen with vancomycin monotherapy in serious MSSA infections. The drugs may achieve different levels of penetration into sites of sequestered bacteria either intra- or extracellularly. In one study, although the bactericidal effects of both vancomycin and oxacillin over 24 h were markedly lower against intracellular S. aureus than against extracellular organisms, the magnitude of intracellular killing by oxacillin was approximately 10-fold greater than that by vancomycin (1). These drugs may also have different activities against microbial biofilms in vivo, although studies suggest that the in vitro activities of both agents against biofilm-associated S. aureus isolates are substantially compromised (6, 19).
There are several possible reasons why our results differ from those of Small and Chambers (17). In the present investigation, we used a different broth medium, which better supported the growth of the strains, and most importantly, we used a higher inoculum. Both vancomycin and nafcillin demonstrate inoculum effects over a period of 72 h (14). Nevertheless, our results with the low inocula did not suggest any important differences between vancomycin, oxacillin, or the combination.
Of note, it is worth mentioning that the therapeutic combination of vancomycin and ß-lactams has already been shown to exert synergistic killing activity against MRSA, glycopeptide-intermediate-resistant S. aureus (GISA), and vancomycin-resistant S. aureus (VRSA) strains (7, 9, 11) and therefore might be of relevance in these particular cases.
In conclusion, we observed no evidence of antagonism or synergism between oxacillin and vancomycin against MSSA strains tested at high inocula using both fixed-concentration and dynamic time-kill methods. These indifferent interactions suggest that decisions involving the use of these antibiotics for empirical therapy should be based on factors other than in vitro killing in broth media.
Acknowledgments
We have no conflicts relating to the antimicrobial agents studied in this paper.
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. VanBambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl A):125-130. [DOI] [PubMed] [Google Scholar]
- 3.Cantoni, L., M. P. Glauser, and J. Bille. 1990. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob. Agents Chemother. 34:2348-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cercenado, E., G. M. Eliopoulos, C. B. Wennersten, and R. C. Moellering, Jr. 1992. Absence of synergistic activity between ampicillin and vancomycin against highly vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 36:2201-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, F. Y., J. E. Peacock, Jr., D. M. Musher, P. Triplett, B. B. MacDonald, J. M. Mylotte, A. O'Donnell, M. M. Wagener, and V. L. Yu. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine 82:333-339. [DOI] [PubMed] [Google Scholar]
- 6.Chuard, C., P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1993. Susceptibility of Staphylococcus aureus growing on fibronectin-coated surfaces to bactericidal antibiotics. Antimicrob. Agents Chemother. 37:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Climo, W. M., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and β-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn, J. R., D. L. Jungkind, and J. S. Baker. 1980. In vitro antagonism by erythromycin of the bactericidal action of antimicrobial agents against common respiratory pathogens. Antimicrob. Agents Chemother. 18:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domaracki, B. E., A. M. Evans, and R. A. Venezia. 2000. Vancomycin and oxacillin synergy for methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 44:1394-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fätkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. S. aureus Endocarditis and Bacteremia Study Group. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 11.Fox, P. M., R. J. Lampen, K. S. Stumpf, G. L. Archer, and M. W. Climo. 2006. Successful therapy of experimental endocarditis caused by vancomycin-resistant Staphylococcus aureus with a combination of vancomycin and beta-lactam antibiotics. Antimicrob. Agents Chemother. 50:2951-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry, C. A., K. A. Rodvold, R. M. Novak, R. C. Hershow, and O. J. Naderer. 1997. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 17:990-997. [PubMed] [Google Scholar]
- 13.Krogstad, D. J., R. C. Moellering, Jr., and D. J. Greenblatt. 1980. Single-dose kinetics of intravenous vancomycin. J. Clin. Pharmacol. 20:197-201. [DOI] [PubMed] [Google Scholar]
- 14.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodise, T. P., Jr., P. S. McKinnon, D. P. Levine, and M. J. Rybak. 2007. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3731-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruedy, J. 1966. The effects of peritoneal dialysis on the physiological disposition of oxacillin, ampicillin and tetracycline in patients with renal disease. Can. Med. Assoc. J. 94:257-261. [PMC free article] [PubMed] [Google Scholar]
- 17.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stryjewski, M. E., D. R. Graham, S. E. Wilson, W. O'Riordan, D. Young, A. Lentnek, D. P. Ross, V. G. Fowler, A. Hopkins, H. D. Friedland, S. L. Barriere, M. M. Kitt, and G. R. Corey. 2008. Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683-1693. [DOI] [PubMed] [Google Scholar]
- 19.Wu, J. A., C. Kusuma, J. J. Mond, and J. F. Kokai-Kun. 2003. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 47:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]