Abstract
Resistance to antimicrobial agents is emerging in a wide variety of nosocomial and community-acquired pathogens. The development of alternative therapies against nosocomial infections caused by clinically relevant pathogens represents a major public health concern. RLP068/Cl is a novel Zn(II) phthalocyanine proposed as a photosensitizer suitable for antimicrobial photodynamic therapy (APDT) for localized infections. Its ability, following activation by light, to induce resistance in three major human pathogens after 20 daily passages was studied. Simultaneously for the same strains, the ability of daily sequential subcultures in subinhibitory concentrations of RLP068/Cl to develop resistant mutants without illumination was evaluated. We demonstrate that 20 consecutive APDT treatments with RLP068/Cl did not result in any resistant mutants and that, in dark conditions, only Staphylococcus aureus strains had increased MICs of RLP068/Cl. However, even in this case, the susceptibility of the mutated bacteria to APDT was not affected by their MIC increase.
The emergence of strains resistant to antimicrobial agents may be bringing to an end the so called “antibiotic era” (22). The widespread and increasing resistance of bacterial and fungal pathogens to commonly used antibiotics and chemotherapeutics has provided the necessary impetus to find alternative drugs and/or therapies to which microorganisms will not easily develop resistance. One example of these relatively novel strategies (therapies) is antimicrobial photodynamic therapy (APDT), which is expected to be useful in the treatment of localized infections (6, 15). APDT is a treatment that utilizes a combination of light, a chemical known as a photosensitizer (PS) that is capable of being activated by light, and oxygen to achieve a cytotoxic effect. The process involves delivering light of the appropriate wavelength to the PS molecule to bring to its excited singlet state, which subsequently crosses to a more stable, lower-energy triplet state. The interaction between the PS excited states and the endogenous oxygen in the proximity of the target cells provides the cytotoxic effects through the production of in situ reactive oxygen species (ROS) (3). The main advantages of APDT are high target specificity (provided that the PS is localized in the microorganisms without major involvement of the surrounding tissues or cells and by the selection of a suitable illumination protocol [17]), with few undesired side effects (the drug is inactive in the dark and becomes active only when exposed to light) (21). A standard APDT protocol, in fact, includes incubation of the target cells or diseased tissue in the dark with the PS (which is intrinsically nontoxic), followed by a short illumination to generate ROS, which are responsible for irreversible oxidative damage to essential cellular constituents of the microorganisms to which the PS is bound. The ability of bacteria and yeast to develop resistance to this peculiar kind of stress is still under debate (10). However, the short time of incubation of the PS with the microorganisms and the typically multitarget nature of photosensitized inactivation processes, usually involving a number of membrane proteins and lipid domains, prevent the possible expression of protective factors (e.g., the biosynthesis of stress proteins), thus minimizing the risk of the emergence of resistant strains. To confirm this hypothesis, we performed multipassage studies to investigate the ability of Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans strains to develop resistance upon exposure to RLP068/Cl, a new tetracationic Zn(II) phthalocyanine derivative synthesized in our laboratories and found to be suitable for APDT. A set of experiments was performed to assess any change in the sensitivity of the bacterial and fungal cells to repeated APDT treatments, as well as the onset of cytotoxic effects upon repeated and prolonged exposure of the cells to RLP068/Cl in the dark.
MATERIALS AND METHODS
Pathogens and culture conditions.
P. aeruginosa GiaS and S. aureus 28C65 are bacterial strains susceptible to all commonly used antibiotics. P. aeruginosa GW-1 is characterized by expanded-spectrum-cephalosporin resistance (16), and S. aureus 26A7 is a methicillin-resistant strain. C. albicans 3518 is an antifungal-agent-susceptible strain, while C. albicans 17 shows high azole resistance (20). All the bacterial and yeast strains were kindly supplied by G. M. Rossolini except for C. albicans 17, which was kindly supplied by T. C. White. All the bacterial strains were cultured in tryptic soy Broth (TSB) at 37°C, while the two C. albicans strains were cultured in Sabouraud dextrose broth (SDB) at 37°C unless otherwise specified.
Photosensitizer.
RLP068/Cl, a tetracationic Zn(II) phthalocyanine chloride supplied by Molteni Farmaceutici, was used as the photosensitizer (5). RLP068/Cl was dissolved in sterile MilliQ water to give a 1.78 mM stock solution and stored at −20°C in the dark until used.
Light source.
The illumination was carried out using a noncoherent halogen lamp (PDT 1200 L; Waldmann, Villingen-Schwenningen, Germany) with a band pass filter isolating the 600- to 700-nm-wavelength range. The delivered light energy was 30 J cm−2 (resulting from 10 min illumination at 50 mW cm−2).
Intrinsic-toxicity assay.
The toxicity of RLP068/Cl was evaluated by measuring its MIC for all three microorganisms in the absence of light. The in vitro susceptibility of each strain was determined by the microdilution broth method (12, 13). MICs for S. aureus were determined in TSB media, MICs for P. aeruginosa were estimated in TSB and protein-free minimal medium M9 (33.9 g/liter Na2HPO4, 15 g KH2PO4, 2.5 g NaCl, 1 ml MgSO4 [1 M], 1 ml biotin [1 mg/ml], 1 ml thiamine [1 mg/ml]. 1 g NH4Cl, 3 g glucose, 2 ml metal trace solution, and MilliQ water up to 1 liter), and MICs for C. albicans were estimated in RPMI 1640 medium containing 25 mM l-glutamine. The final concentrations of RLP068/Cl ranged from 1,163 μg/ml to 36 μg/ml. All experiments were performed in triplicate. Results were recorded after incubation at 37°C for 18 to 24 h.
Multistep resistance selection in the absence of light.
Tubes containing 1 ml of the appropriate medium were initially inoculated with approximately 107 CFU/ml of bacterial strains and 105 CFU/ml of yeast strains in the presence of RLP068/Cl at concentrations ranging from the MIC to three doubling dilutions below the MIC. The tubes were incubated in the dark at 37°C for 24 h. For subsequent daily passage, 10-μl inocula were taken from the first tube containing a subinhibitory drug concentration and subcultured into a new tube containing the same concentration of photosensitizer. Daily subculturing was carried out 20 times. An aliquot of cells grown in the presence of subinhibitory drug concentration was collected and stored at −80°C in glycerol stock solution for further analysis. Resistance selection was studied by repeating the MIC determination every 5 days. To ensure the stability of acquired resistance, resistant clones were subcultured for 10 days in drug-free media.
Phototoxicity assay.
Bacterial and yeast strains were grown in TSB and SDB, respectively, at 37°C with orbital shaking overnight. Cells were harvested by centrifugation (4,000 rpm for 10 min) and washed twice in phosphate-buffered saline (PBS), pH 7.4. The cells were then resuspended in the same buffer to a final concentration of 108 CFU/ml for bacteria and 106 CFU/ml for yeasts. Samples of bacterial cell suspensions were incubated in the dark with the appropriate RLP068/Cl concentrations at room temperature for 5 min, while samples of yeast cell suspensions were incubated under the same experimental conditions for 1 h, and then all samples were illuminated with a fluence rate of 50 mW cm−2 for 10 min (30 J cm−2). Controls were carried out without illumination with and without RLP068/Cl as well as with illumination without RLP068/Cl.
Illuminated and nonilluminated samples were assayed by serially 10-fold dilution in PBS and then plated on solid growth medium. After incubation of the plates at 37°C for 24 h, the number of CFU per ml was counted and the minimal bactericidal concentration (MBC) or minimal fungicide concentration (MFC), defined as the lowest concentration of RLP068/Cl that results in more than 99.9% (3 log10) killing, was calculated.
Photodynamic multistep resistance selection.
For each strain, a single colony surviving APDT in the presence of an RLP068/Cl concentration corresponding to the MBC or MCF was picked from the plate, inoculated in 5 ml of appropriate medium, and grown at 37°C. After 24 h cells, were harvested by centrifugation (4,000 rpm for 10 min) and washed twice in PBS, pH 7.4. The cells were then resuspended in the same buffer to a final concentration of 108 CFU/ml for bacteria and 106 CFU/ml for yeasts and mixed with RLP068/Cl at the MBC or MCF. The photodynamic treatment was repeated as described above. Ten microliters of each illuminated sample was then subcultured again to perform the subsequent cycles. Following this protocol, 20 daily passages were performed for each strain. The determination of MBC and MFC was repeated every 5 cycles.
RESULTS
We tested the APDT efficacy of RLP068/Cl, a new tetracationic Zn(II) phthalocyanine chloride, against three major human pathogens: the Gram-positive bacterium S. aureus, the Gram-negative bacterium P. aeruginosa, and the yeast C. albicans. The efficacy of RLP068/Cl was evaluated based on the determination of the number of visible CFU per milliliter. In agreement with the data reported in the literature (1, 2, 9, 10) for other cationic PSs, S. aureus showed higher susceptibility to APDT with RLP068/Cl than P. aeruginosa (Table 1), while C. albicans was successfully treated with concentrations of RLP068/Cl near those effective for S. aureus (Table 1). The susceptibility of drug-resistant pathogens to APDT treatment with RLP068/Cl was found to be very similar to that of the corresponding drug-sensitive strain. Control experiments (see Fig. 1, 2, and 3) showed that cell viability of all the tested pathogens was unaffected by illumination alone and by dark incubation with the tested concentrations of RLP068/Cl.
TABLE 1.
Multistep resistance selection by RLP068/Cl in the presence of lighta
| Strain | Initial MBC (μg/ml) for RLP068/Cl | Selected resistance |
Repeated MBC or MFC(μg/ml) after resistance selection in the absence of light at days 10 and 20 | |
|---|---|---|---|---|
| Passage | MBC or MFC (μg/ml) | |||
| Bacteria | ||||
| S. aureus 26A7 | 0.016 | 5 | 0.016 | ND |
| 10 | 0.016 | 0.016 | ||
| 15 | 0.016 | ND | ||
| 20 | 0.016 | 0.016 | ||
| S. aureus 28C65 | 0.008 | 5 | 0.027 | ND |
| 10 | 0.016 | 0.016 | ||
| 15 | 0.016 | ND | ||
| 20 | 0.016 | 0.016 | ||
| P. aeruginosa GiaS | 13.2 | 5 | 6.6 | ND |
| 10 | 3.3 | 6.6 | ||
| 15 | 6.6 | ND | ||
| 20 | 6.6 | 13.2 | ||
| P. aeruginosa GW-1 | 13.2 | 5 | 3.3 | ND |
| 10 | 6.6 | 6.6 | ||
| 15 | 13.2 | ND | ||
| 20 | 6.6 | 13.2 | ||
| Yeasts | ||||
| C. albicans 3518 | 1.3 | 5 | 1.3 | ND |
| 10 | 1.3 | 1.3 | ||
| 15 | 0.33 | ND | ||
| 20 | 0.66 | 1.3 | ||
| C. albicans 17 | 1.3 | 5 | 3.3 | ND |
| 10 | 3.3 | 1.3 | ||
| 15 | 1.3 | ND | ||
| 20 | 3.3 | 1.3 | ||
Data are results of multistep resistance selection studies with RLP068/Cl in the presence of light activation and repeated MBC/MFC determination after day 10 and 20 of resistance selection in the absence of light. ND, not determined.
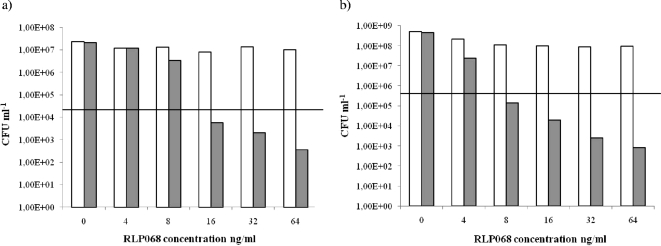
FIG. 1.
Efficacy of killing of Staphylococcus aureus 26A7 (a) and Staphylococcus aureus 28C65 (b) after 5 min of incubation in the dark with concentrations of RLP068/Cl ranging from 0 ng/ml to 64 ng/ml, with illumination (gray bars) and without illumination (white bars). Values below the horizontal line represent ≥99.9% (MBCs) efficacy of bacterial killing relative to the untreated controls (bacteria, but no light and no photosensitizer).
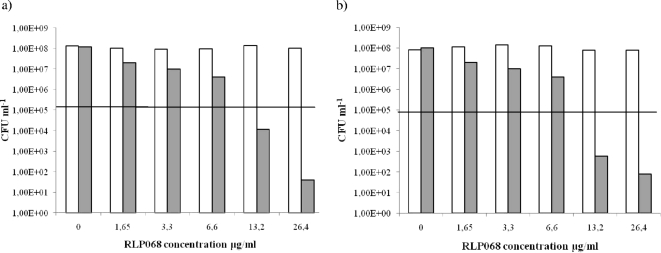
FIG. 2.
Efficacy of killing of Pseudomonas aeruginosa GiaS (a) and Pseudomonas aeruginosa GW-1 (b) after 5 min of incubation in the dark with concentrations of RLP068/Cl ranging from 0 μg/ml to 26.4 μg/ml, with illumination (gray bars) and without illumination (white bars). Values below the horizontal line represent ≥99.9% (MBCs) efficacy of bacterial killing relative to the untreated controls (bacteria, but no light and no photosensitizer).
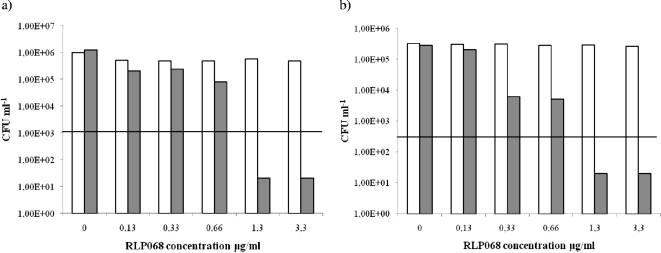
FIG. 3.
Efficacy of killing of Candida albicans 3518 (a) and Candida albicans 17 (b) after 1 h of incubation in the dark with concentrations of RLP068/Cl ranging from 0 μg/ml to 3.3 μg/ml, with illumination (gray bars) and without illumination (open bars). Values below the horizontal line represent ≥99.9% (MFCs) efficacy of yeast killing relative to the untreated controls (yeast, but no light and no photosensitizer).
Photoinactivation of S. aureus by RLP068/Cl.
Incubation of S. aureus strains with 4 to 64 ng/ml of RLP068/Cl caused a biologically relevant decrease of viability upon illumination (Fig. 1). RLP068/Cl at a concentration of 16 ng/ml for S. aureus strain 26A7 and 8 ng/ml for S. aureus strain 28C65 exhibited a killing efficacy of more than 99,9% (corresponding to ≥3 magnitudes of log10 reduction) (Fig. 1). Antibacterial activity was increased further at 64 ng/ml (Fig. 1). The growth of all bacterial samples without photosensitizer was not affected with or without illumination, demonstrating that the light dose of 50 mW cm−2 used during the experiments does not have an antibacterial effect itself (Fig. 1). At the same time, samples incubated with RLP068/Cl but not illuminated were not affected, showing that RLP068/Cl in the dark does not have antibacterial effects at the concentrations used (Fig. 1).
Photoinactivation of P. aeruginosa by RLP068/Cl.
Incubation of P. aeruginosa strains with 1.65 to 26.4 μg/ml of RLP068/Cl caused a relevant decrease in viability after illumination (Fig. 2), with a decrease of more than 99,9% (corresponding to ≥3 magnitudes of log10 reduction) at 13.2 μg/ml RLP068/Cl. An increase in photosensitizer concentration up to 26.4 μg/ml corresponded to an increase in killing efficacy (Fig. 2). Viability of P. aeruginosa strains was not affected by light itself or by RLP068/Cl in the absence of illumination (Fig. 2).
Photoinactivation of C. albicans by RLP068/Cl.
Illumination of C. albicans strains after incubation with RLP068/Cl in the range 0,13 to 3.3 μg/ml revealed a significant killing efficacy of more than 99.9% (corresponding to ≥3 magnitudes of log10 reduction) at a concentration of 1.3 μg/ml (Fig. 3). Antibacterial activity seemed to plateau at concentrations beyond 3.3 μg/ml (Fig. 3). As for S. aureus and P. aeruginosa, the viability of C. albicans strains was not affected by light itself or by RLP068/Cl without illumination (Fig. 3).
Multistep APDT resistance in vitro selection.
To determine development of resistance to RLP068/Cl with illumination in all the experimental strains used, we monitored the variation of MBCs or MFCs every 5 days during 20 daily repeated APDT treatments. A summary of the MBCs and MFCs obtained for all the strains after APDT at day 0, 5, 10, 15, and 20 is reported in Table 1. No significant variations in values were observed for all the strains tested.
Dark toxicity and resistance.
To verify the development of resistance in all the experimental strains to RLP068/Cl without illumination, we monitored their MICs every 5 days during 20 daily repeated cell cultures in the presence of RLP068/Cl PS-positive pressure in the dark. An overview of the MICs obtained is presented in Table 2. A first analysis, comparing the data obtained for bacteria in TSB, suggests that P. aeruginosa is less susceptible to the intrinsic activity of RLP068/Cl than S. aureus (Table 2). For each organism, the two selected strains showed similar MICs. Dark multistep resistance selection after 20 cycles (Table 2) showed no significant change in the MICs for P. aeruginosa and C. albicans strains, while for the two S. aureus strains a significant shift was observed. Both S. aureus strains retained the higher MICs even after 10 daily passages on drug-free media (Table 2). This suggests that the acquired resistance may remain stable in the absence of RLP068/Cl pressure, as was also been described for some fluoroquinolones, such as clinafloxacin (11). Moreover, after 20 cycles of dark multistep resistance selection, all the S. aureus resistant mutants selected showed the same susceptibility to the RLP068/Cl APDT treatment as the corresponding wild-type strain (Table 1).
TABLE 2.
Multistep resistance selection by RLP068/Cl in the absence of lighta
| Strain | Initial MIC (μg/ml) for RLP068/Cl | Selected resistance |
Repeated MIC (μg/ml) of RLP068/Cl retested after 10 antibiotic-free subcultures | |
|---|---|---|---|---|
| Passage | MIC (μg/ml) TSB | |||
| Bacteria | ||||
| S. aureus 26A7 | 73 | 5 | 581 | 1,163 |
| 10 | 581 | |||
| 15 | 1,163 | |||
| 20 | >1,163 | |||
| S. aureus 28C65 | 145 | 5 | 264 | 1,163 |
| 10 | 264 | |||
| 15 | 1,163 | |||
| 20 | 1,163 | |||
| P. aeruginosa GiaS | >1,163 (TSB), 264 (M9) | 5 | 264 | — |
| 10 | 264 | |||
| 15 | 264 | |||
| 20 | 264 | |||
| P. aeruginosa GW-1 | >1,163 (TSB), 264 (M9) | 5 | 264 | — |
| 10 | 264 | |||
| 15 | 264 | |||
| 20 | 264 | |||
| Yeasts | ||||
| C. albicans 3518 | 36 | 5 | <36 | — |
| 10 | <36 | |||
| 15 | 36 | |||
| 20 | 36 | |||
| C. albicans 17 | 73 | 5 | <36 | — |
| 10 | <36 | |||
| 15 | 36 | |||
| 20 | 36 | |||
Results of multistep resistance selection by RLP068/Cl in the absence of light. MIC determinations were performed in triplicate in TSB (S. aureus), M9 (P. aeruginosa, unless otherwise indicated), or SDB (C. albicans). —, no resistance selection.
DISCUSSION
The worldwide increase in antimicrobial resistance among different species of human pathogens had led to searches for alternative antimicrobial treatments to complement the currently available therapies. This in vitro study demonstrates that RLP068/Cl associated with light activation can be conveniently used in the APDT approach, as it exhibits a concentration-dependent killing efficacy against three prevalent human pathogens associated with skin and soft tissue infections. A >3-log10 killing efficacy was observed for all the microorganisms tested at RLP068/Cl concentrations lower than 13.2 μg/ml. Importantly, activity was retained against multidrug-resistant strains of all the species tested. Today, drug resistance in human pathogens includes target alteration, overexpressed efflux capability, and drug inactivation. As with all other treatments involving therapeutic compounds, the first step is to make the drug available with fast, efficient, and exclusive accumulation in the target region. Many traditional antimicrobial agents act by inhibiting metabolic processes and therefore need to enter and accumulate inside the microorganism to exert their action within the cytoplasm. This process often requires transport mechanisms, such as porins, which allow the influx of low-molecular-mass compounds (<600 to 700 Da) and exclude compounds above this molecular mass (14). This is the first point at which microbial species may develop resistance, by modifying characteristics or diminishing a number of transport systems required by the drug. In addition, the presence of multidrug efflux pumps further enhances the level of intrinsic resistance. Tegos and Hamblin (18) demonstrated that phenothiazinium photosensitizers are substrates of the microbial efflux pumps (MEP) and proposed that MEP inhibitors might enhance the antimicrobial effect of APDT. In further studies, Tegos et al. found that the uptake of phenothiazinium dye by the bacterial cells and the extent of light-mediated bacterial killing were inversely proportional to the level of MEP expression (19). Cell walls and membranes seem to be the main targets for APDT cationic drugs with high molecular masses, such as RLP068 Zn(II) phthalocyanine chloride (>1,300 Da), and so this kind of drug does not necessarily need to enter the microbial cell (4, 8, 9). Specific and proper adhesion to these structures is sufficient for light-activated destruction of the target cell, and this bypasses the issue of developing resistance by stopping uptake or increasing export of the drug. Furthermore, none of the capabilities of resistant organisms listed above covers the intermediacy of singlet oxygen. Antioxidant enzymes such as peroxide dismutase, catalase, and peroxidase give protection against some ROS but not against singlet oxygen. Indeed, singlet oxygen has been shown to inactivate these enzymes (7).
To our knowledge, this is one of the first studies in the field of selection of resistance in microorganisms due to repeated APDT treatments. For this reason, we decided to perform a sort of multistep resistance selection by RLP068/Cl. In the presence of light, none of the strains tested yielded resistance clones after 20 daily passages. This absence of resistance validates the multitarget nature of the photodynamic process. In this case, the targets involved are many, and to identify them individually would be difficult. We can only assume that microbial photoinactivation using RLP068/Cl resembles that described for other photosensitizers with high molecular weights. In the absence of illumination, only S. aureus strains showed an increase in MICs. Resistance was usually stable (MICs remained the same after 10 serial daily passages on drug-free medium). At the moment, we do not know which kind of resistance occurred. Further analysis is necessary to explain the mechanism by which this occurred resistance works on RLP068/Cl pressure in the absence of light. The fact that such resistance did not influence the susceptibility of the S. aureus strains to RLP068/Cl APDT may suggest that light activation of the dye easily circumvents resistance and breaks down S. aureus selected mutants. However, RLP068/Cl concentrations in multistep resistance selection in the absence of illumination experiments are higher than those effective in APDT on S. aureus, resulting in an increased probability of developing resistance compared with conditions used in light activation experiments.
Despite the limited, though representative, number of strains used in these experiments, we consistently found no tendency to induce mechanisms of resistance after light activation of RLP068/Cl, suggesting that APDT can be regarded as a convenient innovative strategy for the treatment of localized infections, such as oral and mucosal candidosis, periodontitis, or chronic wounds of various types and origins. In conclusion, we show that APDT, due to its peculiar mechanism of action, exhibits a low propensity for inducing resistance in the three important human pathogens tested. Further studies with a larger number of microorganisms and resistant strains may be needed in order to confirm these preliminary, albeit encouraging, results.
Acknowledgments
We are grateful to G. M. Rossolini and T. C. White, who kindly supplied us with the clinically isolated bacterial and yeast strains. We are grateful to Guido Carpini, Giacomo Chiti, and Alessandro Fiani for helpful scientific discussions. Thanks also go to Stuart Hindle for his contribution to the final revision of the manuscript.
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Banfi, S., E. Caruso, L. Buccafurni, V. Battini, S. Zazzaron, P. Barbieri, and V. Orlandi. 2006. Antibacterial activity of tetraaryl-porphyrin photosensitizers: an in-vitro study on Gram-negative and Gram-positive bacteria. J. Photochem. Photobiol. 85:28-38. [DOI] [PubMed] [Google Scholar]
- 2.Bertoloni, G., F. Rossi, G. Valduga, G. Jori, H. Ali, and J. E. Van Lier. 1992. Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios 71:33-46. [PubMed] [Google Scholar]
- 3.Demidova, T. N., and M. R. Hamblin. 2004. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 17:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demidova, T. N., and M. R. Hamblin. 2005. Effect of Cell-Photosensitizer Binding and Cell Density on Microbial Photoinactivation. Antimicrob. Agents Chemother. 49:2329-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabris, C., M. Soncin, E. Mazzon, P. Calzavara-Pinton, F. Lia, C. Giacomo, D. Dei, S. Tampucci, G. Roncucci, and G. Jori. 2005. A novel tetracationic phthalocyanine as a potential skin phototherapeutic agent. Exp. Dermatol. 14:675-683. [DOI] [PubMed] [Google Scholar]
- 6.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, S. Y., O. J. Kwon, and J. W. Park. 2001. Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie 83:437-444. [DOI] [PubMed] [Google Scholar]
- 8.Lambrechts, S. A. G., M. C. G. Aalders, and J. Van Marle. 2005. Mechanistic study of the photodynamic inactivation of Candida albicans by a cationic porphyrin. Antimicrob. Agents Chemother. 49:2026-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantareva, V., V. Kussovski, I. Angelov, E. Borisova, L. Avramov, G. Schnurpfeil, and D. Wöhrle. 2007. Photodynamic activity of water-soluble phthalocyanine zinc(II) complexes against pathogenic microorganisms. Bioorg. Med. Chem. 15:4829-4835. [DOI] [PubMed] [Google Scholar]
- 10.Minnock, A. D., I. Vernon, J. Schofield, J. Griffiths, J. H. Parish, and S. B. Brown. 1996. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B Biol. 32:159-164. [DOI] [PubMed] [Google Scholar]
- 11.Nagai, K., T. A. Davies, G. A. Punkuch, B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro selection of resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumonia. Antimicrob. Agents Chemother. 44:2740-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M07-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 13.National Committee for Clinical Laboratory Standards. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 3rd ed., M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 14.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Riordan, K., O. E. Akilov, and T. Hasan. 2005. The potential for photodynamic therapy in the treatment of localized infections. Photodiagnosis Photodyn. Ther. 2:247-262. [DOI] [PubMed] [Google Scholar]
- 16.Poirel, L., G. F. Weldhagen, T. Naas, C. De Champs, M. G. Dove, and P. Nordmann. 2001. GES-2, a class A beta-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 45:2598-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soncin, M., C. Fabbris, A. Busetti, D. Dei, D. Nistri, G. Roncucci, and G. Jori. 2002. Approaches to selectivity in the Zn(II)-phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus. Photochem. Photobiol. Sci. 1:815-819. [DOI] [PubMed] [Google Scholar]
- 18.Tegos, G. P., and M. R. Hamblin. 2006. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 50:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tegos, G. P., K. Masago, F. Aziz, A. Higginbotham, F. R. Stermitz, and M. R. Hamblin. 2008. Inhibitors of bacterial multidrug efflux pumps potentiate antibacterial photoinactivation. Antimicrob. Agents Chemother. 52:3202-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White, T. C., M. A. Pfaller, M. G. Rinaldi, J. Smith, and S. W. Redding. 1997. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 3(Suppl. 1):S102-S109. [DOI] [PubMed] [Google Scholar]
- 21.Winckler, K. D. 2007. Special section: Focus on anti-microbial photodynamic therapy (PDT). J. Photochem. Photobiol. B Biol. 86:43-44. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa, T. T. 2002. Antimicrobial resistance and aging: beginning of the end of the antibiotic era?. J. Am. Geriatr. Soc. 50:S226-S229. [DOI] [PubMed] [Google Scholar]