Abstract
Farnesol interacts with Candida albicans as both a quorum-sensing molecule and toxic agent, but confusion abounds regarding which conditions promote these distinct responses. Farnesol sensitivity was measured when inoculum cell history and size, temperature, and growth media were altered. Parameters for farnesol tolerance/sensitivity were defined, validating previous studies and identifying new variables, such as energy availability. This study clearly defines what farnesol concentrations are lethal to C. albicans, based on environmental conditions.
Candida albicans, a medically important dimorphic fungus, is a model system for quorum sensing in fungi (7). C. albicans excretes the quorum-sensing molecule (QSM) farnesol, which blocks the yeast-to-filament conversion when extracellular levels exceed 1 to 5 μM (11). Exogenous farnesol levels up to 300 μM do not alter the growth rate; instead, the cells grow as yeasts rather than as filaments (11). Farnesol blocks biofilm formation (15), and it is a virulence factor during systemic infection (13) and a protective factor during mucosal infection (6). Farnesol production is regulated because it is turned off in opaque cells (4) and anaerobiosis (3) but is elevated in some mutants (9) and upon treatment with sublethal levels of sterol biosynthesis inhibitors (12).
Twenty to fifty micromolar farnesol inhibits or kills other fungi, opaque C. albicans cells, several mammalian cell lines, and some bacteria (reviewed in reference 10). Thus, C. albicans can exhibit exceptional tolerance to farnesol (7). This view was challenged by Shirtliff et al. (16), who reported that farnesol, at concentrations as low as 40 μM, killed C. albicans. Thus, in the spirit of constructive dialogue, we draw attention to differences between the growth conditions used in our previous work on farnesol as a signaling molecule and those under which Shirtliff et al. (16) observed cell death.
Previous studies of C. albicans farnesol sensitivity (4, 8, 16, 17) used different assay conditions, adding to the confusion. Critically, Shirtliff et al. (16) used cells that were grown overnight, washed, and resuspended in phosphate-buffered saline (PBS) for farnesol sensitivity assays. Farnesol has detergent-like properties because it has both hydrophilic and hydrophobic portions, limited water solubility (7), and micelle-forming ability. Since bacterial detergent resistance is energy dependent (1, 14), farnesol resistance in C. albicans may also be similarly energy dependent. We examined farnesol sensitivity under different growth conditions. Cell growth was followed by observing optical density (OD) and cell death by methylene blue staining (5). C. albicans cells were grown to mid-log phase (OD at 600 nm [OD600] = 0.5) or stationary phase (unbudded cells; obtained from cultures inoculated at an OD600 of 0.1 and grown at 30°C for 16 to 18 h), washed three times in PBS, and inoculated at the indicated levels with variable concentrations of farnesol. We used 10 and 100 mM stocks of E, E-farnesol in methanol so that the final methanol concentration never exceeded 1%, a concentration that had no effect on cell growth or death (7).
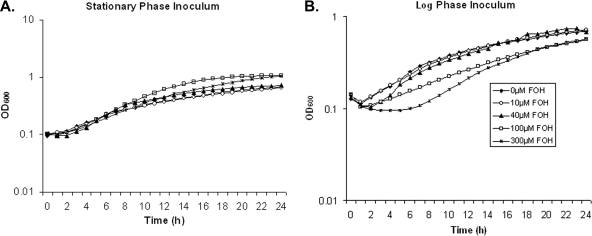
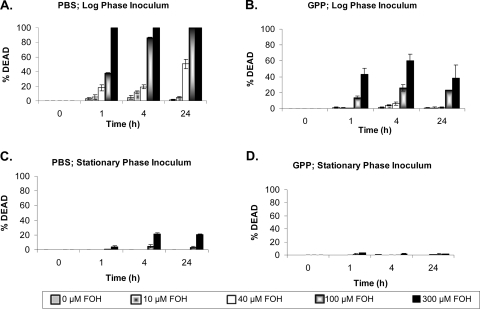
In rich growth medium (YPD), minimal cell death was observed at farnesol concentrations up to 300 μM, and growth inhibition was observed only with ≥300 μM farnesol (data not shown). Using a defined glucose-phosphate-proline (GPP; pH 6.8) (9) medium (Fig. 1A), similar growth curves were observed with concentrations of farnesol up to 300 μM when starting with stationary-phase inocula. The growth experiments were simultaneously examined for cell death by methylene blue staining (Fig. 2D). Minimal cell death occurred in GPP with stationary-phase inocula, our standard growth conditions (7), and up to 300 μM farnesol. However, with inocula of exponentially growing cells, 40 μM farnesol partially inhibited growth, and higher farnesol levels prolonged the lag phase (Fig. 1B). Log-phase cells were killed by 100 and 300 μM farnesol at 1 and 4 h in GPP (Fig. 2B), consistent with the prolonged lag phase. These results support the growth phase-dependent sensitivity described by Uppuluri et al. (17).
FIG. 1.
Effect of farnesol on C. albicans cell growth. (A) Stationary-phase inoculum. (B) Exponential-phase inoculum. Cells were grown in duplicate on at least two separate occasions in defined GPP medium with the indicated levels of farnesol at 30°C in 96-well plates, and OD600 values were recorded on an automated plate reader (Molecular Devices, Sunnyvale, CA). Note the different y-axis scales in graphs A and B.
FIG. 2.
Effect of farnesol on C. albicans cell death. Percentage of cell death was determined by methylene blue staining (5). Cells were incubated in either PBS (A and C) or GPP (B and D) with the indicated levels of farnesol in 96-well plates at 30°C. Incubations were initiated with either exponential (A and B)- or stationary (C and D)-phase inoculum.
To examine the effects of different media on farnesol sensitivity, cells were compared under both growth (GPP) and storage (PBS) conditions, using both exponential- and stationary-phase inocula (Fig. 2). For exponential-phase cells inoculated in PBS, even low levels of farnesol, i.e., 40 μM, caused cell death (Fig. 2A), consistent with the findings of Shirtliff et al. (16). The cells in PBS were far more sensitive to farnesol when they had come from an exponential-phase inoculum than when they had come from a stationary-phase inoculum (Fig. 2A and C). Interestingly, both exponential- and stationary-phase cells showed increased tolerance to farnesol when incubated in growth media (GPP or YPD) than when incubated in PBS. Similar results were obtained for both growth curves and cell death at 25°C, 30°C, and 37°C (data not shown). These observations suggest a role for energy source(s) in C. albicans farnesol tolerance.
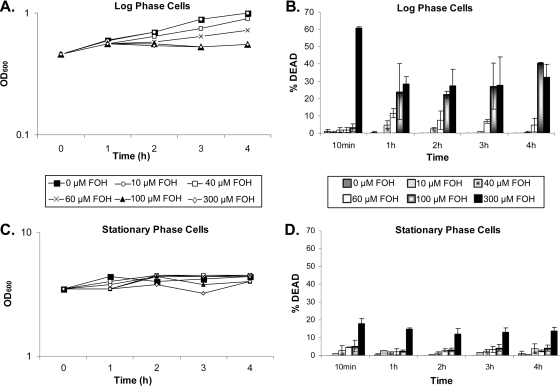
The previous experiments were conducted in 96-well plates with farnesol added at time zero to washed cells. Because plastic may absorb farnesol (2), we confirmed the farnesol sensitivity of exponentially growing cells by adding farnesol directly to two growing cultures (unwashed) in glass flasks (Fig. 3). We compared the farnesol sensitivities of C. albicans cells in exponential phase (OD600 = 0.5) and stationary phase (OD600 = 4.0). The results with the direct addition of farnesol to cultures in glass flasks were consistent with those obtained with plastic 96-well plates.
FIG. 3.
Toxicity of farnesol (FOH) to exponential cultures of C. albicans. One culture (A and B) was subdivided (into six) when it had reached exponential phase (OD600 = 0.5), and the other (C and D) was subdivided when it had reached stationary phase (OD600 = 4.0). Cultures were not washed prior to subdivision and farnesol addition. Cultures containing the indicated levels of farnesol were shaken at 30°C at 250 rpm for 4 h, and at the indicated times, cell growth (A and C) and percentage of cell death (B and D) were determined. All cultures were in glass flasks in GPP both before and after subdivision.
A benefit of this controversy is the identification of the conditions under which C. albicans tolerates farnesol when other cell types are killed by it. Throughout this work, cell death was not accompanied by cell lysis, because there was no drop in OD600 and the methylene blue-positive cells remained intact. This lack of cell lysis with C. albicans white cells is in marked contrast with opaque cells, for which ≥40 μM farnesol caused rapid cell lysis (4). These different farnesol susceptibilities in different environments suggest that farnesol tolerance is a physiological adaptation. Although a precise mechanisms(s) for farnesol tolerance remains unclear, conditions under which C. albicans is either sensitive or resistant to farnesol are now defined.
In summary, stationary-phase cells inoculated into growth media are suitable for studying farnesol signaling because they are not inhibited by high farnesol concentrations. This generalization was true for temperatures of 25°C, 30°C, and 37°C as well as starting cell densities of an OD600 of 0.05 or 0.10. Conversely, optimal conditions for studying farnesol-mediated cell death use log-phase cells under energy-starved conditions. Understanding these environmental distinctions may provide unambiguous conditions to compare farnesol's competing effects on C. albicans.
Acknowledgments
This work was supported by a faculty seed grant from the Constance Miriam and Ethel Corrine Syford Memorial Fund (to A.L.A.), the Hammond-Maude Fling Fellowship (to M.L.L.), and the Farnesol and Candida albicans Research Fund, University of Nebraska Foundation (to K.W.N.).
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Aspedon, A., and K. W. Nickerson. 1993. A two-part energy burden imposed by growth of Enterobacter cloacae and Escherichia coli in sodium dodecyl sulfate. Can. J. Microbiol. 39:555-561. [DOI] [PubMed] [Google Scholar]
- 2.Davis-Hanna, A., A. E. Piispanen, L. I. Stateva, and D. A. Hogan. 2008. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol. Microbiol. 67:47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumitru, R., J. M. Hornby, and K. W. Nickerson. 2004. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 48:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumitru, R., D. H. Navarathna, C. P. Semighini, C. G. Elowsky, R. V. Dumitru, D. Dignard, M. Whiteway, A. L. Atkin, and K. W. Nickerson. 2007. In vivo and in vitro anaerobic mating in Candida albicans. Eukaryot. Cell 6:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson, J., A. Sood, and D. A. Hogan. 2009. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 75:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hisajima, T., N. Maruyama, Y. Tanabe, H. Ishibashi, T. Yamada, K. Makimura, Y. Nishiyama, K. Funakoshi, H. Oshima, and S. Abe. 2008. Protective effects of farnesol against oral candidiasis in mice. Microbiol. Immunol. 52:327-333. [DOI] [PubMed] [Google Scholar]
- 7.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabra-Rizk, M. A., M. Shirtliff, C. James, and T. Meiller. 2006. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 6:1063-1073. [DOI] [PubMed] [Google Scholar]
- 9.Kebaara, B. W., M. L. Langford, D. H. Navarathna, R. Dumitru, K. W. Nickerson, and A. L. Atkin. 2008. Candida albicans Tup1 is involved in farnesol-mediated inhibition of filamentous-growth induction. Eukaryot. Cell 7:980-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford, M. L., A. L. Atkin, and K. W. Nickerson. 2009. Cellular interactions of farnesol, a quorum sensing molecule produced by Candida albicans. Fut. Microbiol. 4:1353-1362. [DOI] [PubMed] [Google Scholar]
- 11.Mosel, D. D., R. Dumitru, J. M. Hornby, A. L. Atkin, and K. W. Nickerson. 2005. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Biol. 71:4938-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarathna, D. H., J. M. Hornby, N. Hoerrmann, A. M. Parkhurst, G. E. Duhamel, and K. W. Nickerson. 2005. Enhanced pathogenicity of Candida albicans pre-treated with subinhibitory concentrations of fluconazole in a mouse model of disseminated candidiasis. J. Antimicrob. Chemother. 56:1156-1159. [DOI] [PubMed] [Google Scholar]
- 13.Navarathna, D. H., J. M. Hornby, N. Krishnan, A. Parkhurst, G. E. Duhamel, and K. W. Nickerson. 2007. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect. Immun. 75:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal, S., N. Eis, M. Bhattacharya, and K. W. Nickerson. 2003. Membrane-derived oligosaccharides (MDOs) are essential for sodium dodecyl sulfate resistance in Escherichia coli. FEMS Microbiol. Lett. 223:25-31. [DOI] [PubMed] [Google Scholar]
- 15.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirtliff, M. E., B. P. Krom, R. A. Meijering, B. M. Peters, J. Zhu, M. A. Scheper, M. L. Harris, and M. A. Jabra-Rizk. 2009. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 53:2392-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uppuluri, P., S. Mekala, and W. L. Chaffin. 2007. Farnesol-mediated inhibition of Candida albicans yeast growth and rescue by a diacylglycerol analogue. Yeast 24:681-693. [DOI] [PubMed] [Google Scholar]