Abstract
We have examined the potential bactericidal activities of several tetramic acids derived from Pseudomonas autoinducers against Clostridium difficile, a cause of antibiotic-associated pseudomembranous colitis. Clinical isolates of C. difficile (n = 4) were incubated in broth with a chemically synthesized Pseudomonas autoinducer and its tetramic acid derivatives. The structure-activity relationship and the mechanisms of action were examined by a time-killing assay and by determination of the morphological/staining characteristics. The use of some tetramic acids derived from N-3-oxododecanoyl l-homoserine lactone resulted in more than 3-log reductions in the viability of C. difficile within 30 min at 30 μM. The outer membrane was suggested to be one of the targets for the bactericidal activity of tetramic acid, because disturbance of the bacterial outer surface was demonstrated by alteration of the Gram-staining characteristic and electron microscopy. The data for the tetramic acid derivatives demonstrate that the keto-enol structure and the length of the acyl side chain of tetramic acid may be essential for the antibacterial activity of this molecule. These results suggest the potential for tetramic acid derivatives to be novel agents with activity against C. difficile.
Clostridium difficile is an anaerobic, Gram-positive, spore-forming rod and is well recognized as an etiologic agent for C. difficile-associated diarrhea (CDAD) (13, 15, 18). This organism is known to produce several toxins, such as toxins A and B, which are associated with the pathogenesis of this disease. CDAD is one of the most common nosocomial infections and is a frequent cause of morbidity and mortality among elderly hospitalized patients (15, 18). In recent years, dramatic increases in the incidence of CDAD have been documented in many hospitals internationally (16, 23, 27). In addition, approximately 3 to 8% of CDAD patients have been reported to develop fulminant diseases, defined as diseases whose courses are complicated by perforation, severe ileus with toxic megacolon, and hypotension (12, 17). The rates of mortality from fulminant CDAD are reported to be 30 to 80% (12). Although vancomycin and metronidazole are the first-line treatment options for CDAD, significant numbers of cases of treatment failure after treatment with these agents have been detected in the United States, Europe, and other countries (12). There is a need to develop more effective choices of treatments against CDAD.
Quorum-sensing systems that allow communication in bacterial societies through the production of autoinducer molecules have been the subject of much recent research activity (5, 19). Among human pathogens, the quorum-sensing system in Pseudomonas aeruginosa is one of the most investigated (2, 25). The production of virulence factors by this organism, such as exotoxins, pigments, and exopolysaccharide, is finely regulated by the coordinated production of autoinducer molecules, such as N-3-oxododecanoyl l-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL) (5, 22). Furthermore, recent progress in this field demonstrated that autoinducer molecules may play a role not only in intraspecies communication but also in interspecies and interkingdom communication, suggesting the multifunctional potential of autoinducers. Importantly, Kaufmann and colleagues have reported that the tetramic acid (TA) degradation products of 3-oxo-C12-HSL possess antibacterial activity against Gram-positive organisms, such as Staphylococcus aureus (14). These data suggest the antibacterial potential of bacterial autoinducer molecules and their TA degradation products, although understanding of their antibacterial spectra, in addition to their mechanisms of actions, is limited. Furthermore, the structure-activity relationship of TAs remains fully unknown.
In the present study, we examined the antibacterial activities of P. aeruginosa autoinducers and several synthetic TAs against C. difficile. The structure-activity relationship was explored to search for TA derivatives with stronger activity.
(The results of parts of this study were presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, October 2008, Washington, DC [abstr. B-3803].)
MATERIALS AND METHODS
Bacterial strains and culture method used.
C. difficile strains (n = 4) isolated from patients with pseudomembranous colitis in the hospital of the Toho University School of Medicine were used. The bacteria were grown under anaerobic conditions (Anaero Pack-Anaero; Mitsubishi Gas Chemical, Co., Inc., Japan) in brain heart infusion broth (BHI; Difco, Becton-Dickinson) supplemented with 1% (wt/vol) yeast extract (Difco, Becton-Dickinson) and 0.1% (wt/vol) l-cysteine (Sigma) at 37°C. For agar plate culture, Mueller-Hinton agar supplemented with 5% defibrinated sheep blood was used.
P. aeruginosa autoinducers and TAs used.
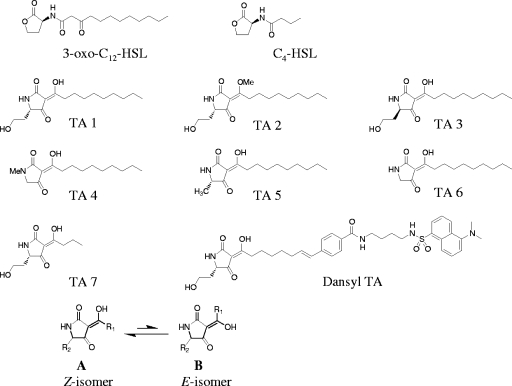
P. aeruginosa autoinducers (3-oxo-C12-HSL, C4-HSL) and other acyl-HSL analogs as precursors of TAs (TA1 to TA7) were chemically synthesized, as described previously (11, 20). Conversion of the acyl-HSL analogs into TAs (TA1 to TA7) was basically done by the procedure previously described for the preparation of TA1 (14) (Fig. 1). The structures of the Z-isofom and the E-isoform of the tetramic acid derivatives are shown in Fig. 1A and B, respectively (21).
FIG. 1.
Chemical structures of P. aeruginosa autoinducers (3-oxo-C12-HSL, C4-HSL) and synthetic TAs (TA1 to TA7, dansyl-TA).
General procedure for synthesis of TAs.
The condensation of 1 equivalent of 3-oxocarboxylic acid (1.0 mmol) and 1.2 equivalents of amino acid ester hydrochloride or hydrobromide (1.2 mmol) in the presence of 1-hydroxy-7-azabenzotriazole (1.2 mmol), N-methylmorpholine (1.5 mmol), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI·HCl) in acetonitrile at room temperature for 12 h furnished N-3-oxo-acyl-amino acid ester at a moderate yield. The acylamide obtained was converted into the corresponding TA by treatment with 1 equivalent of sodium tert-butoxide in methanol (0.2 mol/liter) at 55°C for 2 h. Purification of the TAs was performed by reverse-phase high-pressure liquid chromatography with a Daisopak SP-120-5-ODS-AP column (20 mm by 250 mm; Daiso Co., Ltd.) and a linear gradient of 36% to 72% (vol/vol) CH3CN containing 0.1% (vol/vol) trifluoroacetic acid for 30 min, followed by an isocratic of 72% (vol/vol) CH3CN containing 0.1% (vol/vol) trifluoroacetic acid for 15 min at a flow rate of 10 ml/min. Monitoring for the TAs was at 254 nm with an SPD-M10A photodiode array detector (Shimadzu, Kyoto, Japan), and the TAs were collected in fractions of 10 ml each. Each fraction was confirmed to be pure by Shimadzu liquid chromatography (LC)-ion-trap-time-of-flight mass spectrometry (MS) with a Cadenza CD-C18 column (2 mm by 100 mm; Imtakt Corp.) and a linear gradient of 30% to 80% (vol/vol) CH3CN containing 0.1% (vol/vol) formic acid over 20 min. The pure fractions were collected and lyophilized. 1H nuclear magnetic resonance (NMR) spectra were recorded at 400 and 500 MHz (Jeol EX-400 and Burker DMX-500 NMR spectrometers). The chemical shifts were measured relative to the signals for residual CHCl3 (7.26 ppm). The data from 1H-NMR and LC-MS characterizing each compound are described in the supplemental material.
Alterations of Gram-staining characteristics and morphology of bacteria.
After incubation at 37°C for 24 h, C. difficile was harvested by centrifugation at 5,000 rpm for 5 min. The bacteria were incubated with TAs or P. aeruginosa autoinducers for the indicated times. The bacteria were stained by Gram's method and were observed by light microscopy.
Effects of TAs on viability of bacteria.
C. difficile was cultured in the presence of TAs or Pseudomonas autoinducers at 37°C for the indicated times. After incubation, the numbers of viable bacteria were evaluated by cultivation of the samples after they were serially diluted 10-fold. In some experiments, bacteriolysis by TAs was monitored by observing the optical density of the bacterial suspensions at 600 nm.
Changes in cell surface structures and accumulation of TA1 by electron microscopy.
For electron microscopy, bacterial cells were immediately immersed in a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 1 h. After the cells were rinsed in phosphate-buffered saline (PBS), they were postfixed for 1 h in 1% OsO4 in PBS, followed by dehydration in ethanol, and they were then embedded in Epon 812. For the immunocytochemical observation, bacterial cells were immersed in a mixture of 0.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 h at 4°C. After the cells were washed in PBS for 1 h with no osmium treatment, all cells were dehydrated in graded ethanol and embedded in Unicryl resin. Ultrathin sections were incubated with 1.5% bovine serum albumin in 0.01 M PBS for 20 min, followed by incubation with rabbit anti-dansyl antibody (Molecular Probes) diluted 1:100 with 1% BSA and PBS for 2 h at room temperature. Negative control sections were incubated with nonimmune rabbit serum at the same concentration. After incubation, the sections were rinsed with PBS and incubated with a goat anti-rabbit IgG conjugate with 10-nm colloidal gold particles (BioCell Research Laboratories, Cardiff, United Kingdom) diluted 1:20 with PBS at room temperature for 30 min.
Preparation of multilamellar vesicles and solid-state NMR spectroscopy.
In the model membrane, 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC) and 1,2-dimyristoyl-sn-glycero-3-phosphatidylglycerol (DMPG) were utilized as lipids at a molar ratio of 4:1. The membrane system was made of lipids and cosolubilized with 3-oxo-C12-HSL or TA1 at an additive/lipid (A/L) molar ratio of 1:20 in chloroform-methanol (2:1). After evaporation of the solvent under vacuum for one night, the lipid film was hydrated with buffer (20 mM Tris-HCl, 100 mM NaCl [pH 7.6]) and vortex mixed. The suspension was freeze-thawed for 10 cycles and centrifuged. The supernatant was removed to adjust the water content to ∼80% (wt/wt), and the suspension was transferred to NMR tubes capped to prevent dehydration.
Solid-state NMR spectra were acquired on a CMX Infinity 300 spectrometer (Chemagnetics, Varian, Palo Alto, CA) operating at a proton resonance frequency of 300 MHz. 31P spectra were acquired by using a 5-μs single-excitation pulse with 30-kHz continuous-wave (CW) 1H decoupling during acquisition. The dwell time was 50 ms, and 256 to 1,024 transient readings were accumulated for each free induction decay (FID) with a 3-s relaxation delay. The 31P chemical shifts were referenced externally to that of 85% H3PO4 (0 ppm).
RESULTS
Effects of TA on viability of C. difficile.
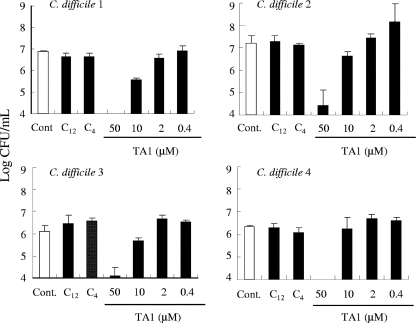
We evaluated the activities of TA1 and the Pseudomonas autoinducers against four strains of C. difficile after 24 h of incubation. As shown in Fig. 2, no reduction in bacterial numbers was observed for any strains examined in the presence of 3-oxo-C12-HSL (50 μM) and C4-HSL (50 μM). In contrast, TA1 reduced the numbers of all bacterial isolates examined in a concentration-dependent manner.
FIG. 2.
Effect of TA1 on growth of C. difficile. The bacteria (C. difficile 1 to 4) were grown in broth for 24 h with 50 μM autoinducers 3-oxo-C12-HSL (C12), C4-HSL (C4), or various concentrations of TA1. The bacterial numbers were then examined (n = 3). Cont., control.
Effects of TA on Gram-staining characteristics and cell surface structures of C. difficile.
We compared the Gram-staining characteristics of the C. difficile strains treated with TA1 (50 μM) or not treated. Drastic alterations in the Gram-staining characteristics and morphological changes to the cells were observed (see Fig. S1 in the supplemental material). Even a brief treatment with TA1 (30 min) clearly induced a change in the Gram-staining characteristics.
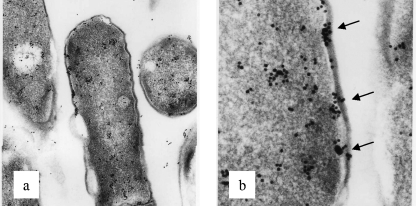
Therefore, we next examined the cell surface structures of C. difficile cells treated with TA1 or 3-oxo-C12-HSL by electron microscopy. There was no change in the surface structures of the cells treated with 3-oxo-C12-HSL compared with those of the control bacterial cells. In contrast, TA1 treatment induced alterations in the bacterial cell surface structures, such as irregular margins on the cell surface and a peptidoglycan layer with an increased width (see Fig. S2 in the supplemental material). The deposition and accumulation of dansyl-TA were demonstrated on some areas of the cell surfaces, and scattered dansyl-TA was detected in the cytoplasmic spaces of the bacteria (Fig. 3a and b). Collectively, these data suggest that TA1 accumulates in the cells, probably in the cell surface layer, which may be associated with changes to the Gram-staining characteristics and a loss of viability.
FIG. 3.
Localization of TA in C. difficile. Dansyl-TA-treated bacteria were reacted with anti-dansyl-TA antibody and gold-labeled anti-rabbit antibody and were then examined by electron microscopy. Arrows, the accumulation of TA1 on the bacterial surface. Magnifications, ×25,000 (a) and ×80,000 (b).
Structure and activity relationship of TAs.
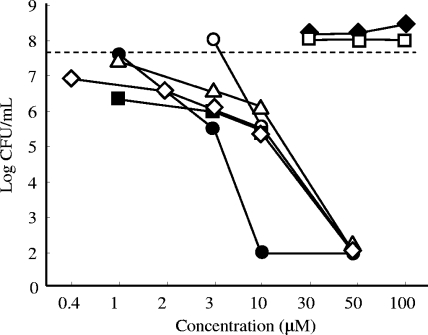
To obtain an understanding of the structure-activity relationship and to search for TAs with stronger activity, we have synthesized seven TAs with different structures and compared their activities on the viability of C. difficile (Fig. 1). As shown in Fig. 4, the present data suggest that there are structurally critical points for the antibacterial activities of the TAs. We clearly observed that TA2 and TA7 had less activity than TA1, suggesting that the keto-enol structure and the length of the acyl side chain play critical roles. The TAs were generally observed to have antibacterial effects at concentrations of 3 to 50 μM and in a concentration-dependent manner. Interestingly, TA6 was observed to have the strongest effects, in which a more than 105-fold reduction in bacterial numbers was demonstrated with TA6 at 10 μM.
FIG. 4.
Comparison of activities of TAs against C. difficile. The bacteria were grown for 24 h in BHI broth in the presence of various concentrations of TAs. The bacterial numbers were then examined by cultivation of the samples after serial 10-fold dilutions. The detection limit is 2 log CFU/ml. Dashed line, bacterial numbers without TAs; ⋄, TA1; ♦, TA2; ▵, TA3; ○, TA4; ▪, TA5; •, TA6; □, TA7.
Induction of bacteriolysis by TAs.
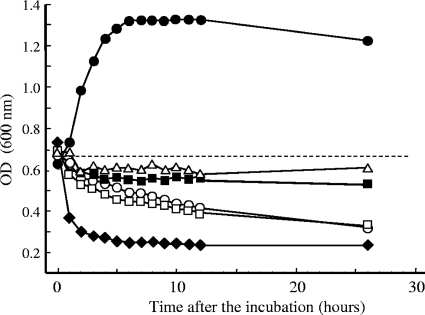
We further examined the effects of the TAs on the growth and bacteriolysis of C. difficile. As shown in Fig. 5, a rapid increase in the optical density at 600 nm was observed for the C. difficile control, and the optical density reached a maximum at 6 h after incubation. The TAs examined suppressed increases in the optical density by 24 h after incubation. In particular, TA6 demonstrated the strongest activity, in which an abrupt reduction in the optical density was observed within a couple of hours of incubation. These results suggest that the TAs may induce bacteriolysis in C. difficile, probably through disorganization of the cell surface structures.
FIG. 5.
Induction of bacteriolysis by TAs. The bacteria were incubated with several TAs at different concentrations for 26 h. At each time point, the optical density (OD) at 600 nm was determined. Dashed line, inoculum dose of C. difficile; •, control; ▪, TA1 (50 μM); ▵, TA3 (50 μM); □, TA4 (30 μM); ○, TA5 (50 μM); ♦, TA6 (30 μM).
Analysis of multilamellar vesicles by solid-state NMR spectroscopy.
To examine the effect of tetramic acid on the lipid membrane, the 31P NMR spectra of the lipid membranes in the absence and the presence of 3-oxo-C12-HSL or TA1 were measured under static conditions. For the control experiment in the absence of 3-oxo-C12-HSL or TA1, an axially symmetric, motionally averaged powder pattern was observed (see Fig. S3 in the supplemental material). This pattern indicates that the lipids from the multilamellar vesicles and lipid bilayers are mainly oriented in the magnet, with their normal pattern being perpendicular to the magnetic field (3). In the presence of 3-oxo-C12-HSL, a peak similar to that in the control sample was observed. The external appearance was also milky white for the control sample. In the presence of TA1, however, an isotropic peak was observed. Furthermore, the external appearance was a little see-through white rather than milky white. This isotropic component may be ascribable to the fast isotopic tumbling of the phospholipids, which is likely to be caused by the formation of micelles, small unilamellar vesicles, or small discoidal bilayers (4).
DISCUSSION
Kaufmann and collaborators have reported that TA1, a degradation product of the P. aeruginosa autoinducer 3-oxo-C12-HSL, has activity against Gram-positive organisms, such as Bacillus cereus and Staphylococcus aureus (14). The concentrations of TA1 effective against C. difficile (10 to 50 μM) are similar to those described in the previous report (10 to 30 μM for B. cereus and S. aureus [14]). These concentrations may be biologically relevant, because high concentrations of 3-oxo-C12-HSL (>600 μM) have previously been detected in a P. aeruginosa biofilm (1). It is plausible that P. aeruginosa may utilize TA as an interference strategy to preclude encroachment by competing bacteria in a microbial community, although it is not known whether this organism produces an autoinducer under anaerobic conditions. In relation to this point of view, C. difficile is an inhabitant of the human intestinal tract and may have a chance to colocalize and interact with a variety of intestinal organisms. Although it is likely that normal commensal bacteria suppress the overgrowth of C. difficile and protect the intestinal tract from pseudomembranous colitis, the activities of endogenous TAs and TA-producing bacteria in the gut and their ability to prevent CDAD are not known.
Even a brief TA1 treatment induced changes in the Gram-staining characteristics, suggesting an alteration of the cell surface peptidoglycan structures. Consistent with these findings, electron microscopy revealed that bacteria exposed to TA1 bacteria had an ill-defined cell surface margin and an increase in the width of the peptidoglycan layer. Furthermore, a fluorescent derivative of TA1 and gold-labeled anti-TA1 antibody were found to be accumulated and deposited in C. difficile. The 31P NMR spectrum of a model membrane in the presence of TAs indicated the existence of an isotropic component, which may be caused by the formation of micelles. These data indicate that TAs directly interact with lipids, as determined by use of a model membrane; i.e., the enolate anion of TAs may interact with the ammonium cation of phosphatidylcholine, DMPC. This finding is consistent with the fact that anti-C. difficile activity is eliminated in the presence of excessive Fe3+ (data not shown). On the other hand, an interaction between 3-oxo-C12-HSL and the ammonium cation of phospholipids would not occur because of the lower acidity of the enolate. The interaction of TAs and lipids may destabilize the cytoplasmic membrane and seriously affect the control of peptidoglycan metabolism. Collectively, these data suggest that certain types of TAs may be bactericidal for C. difficile, probably because of the damage to the cell surface structures of C. difficile, such as the peptidoglycan layer and cytoplasmic membrane, that they cause.
Several natural products containing a tetramic acid motif, such as reutericycline, streptolygidin, and tenuazonic acid, have been demonstrated to possess mycotoxic, antibacterial, antiviral, and antioxidant activities (9, 24, 26). Reutericycline is a well-known tetramic acid isolated from the sourdough isolate Lactobacillus reuteri LTH2584 (7, 10). This compound has been demonstrated to be bactericidal against Gram-positive bacteria by acting as a proton ionophore, thereby dissipating the transmembrane change in pH and leading to cell lysis (8). The efficacy of this mechanism is a function of the high degree of hydrophobicity of reutericycline, which therefore favors partitioning into the cytoplasmic membrane (6, 8). Consistent with these data, the structure-activity relationship found in the present study clearly indicates the importance of the hydrophobic acyl side chain in TAs. In addition, the presence of a keto-enol structure at the C-3 position was shown to be essential for the bactericidal activities of the TAs against C. difficile. These data further suggest that tetramic acid may destabilize the membrane, which results in antibacterial activity.
It may be important to consider the potential application and limitation of TAs in the clinical setting. Elimination of the anti-C. difficile activity by metal cations may prevent the clinical application of this compound. On the other hand, it is possible that TAs may act in synergy with other classes of antibiotics. To this end, we observed an exaggeration of the anti-C. difficile activity of aminoglycosides in the presence of TAs at sub-MICs (data not shown). Further investigations of the effects of TAs in an in vivo model of C. difficile infection may be necessary to correctly define the potential of TAs to be therapeutic agents against this disease.
Supplementary Material
Acknowledgments
We thank Barbara H. Iglewski for the kind gift of P. aeruginosa PAO1. We express our deep appreciation to Tse-Hsien Koh for his critical comments and careful review of the manuscript.
This study was supported by a grant for strategic research for nongovernmental schools of the Heisei 20th from the Ministry of Education, Culture, Sports, Science and Technology of Japan. No specific funding was received for this study.
Footnotes
Published ahead of print on 16 November 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Charlton, T. S., R. de Nys, A. Netting, N. Kumar, M. Hentzer, M. Givskov, and S. Kjelleberg. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2:530-541. [DOI] [PubMed] [Google Scholar]
- 2.de Kievit, T. R. 2009. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 11:279-288. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey, C. E., and A. Watts. 1987. A deuterium and phosphorus-31 nuclear magnetic resonance study of the interaction of melittin with dimyristoylphosphatidylcholine bilayers and the effects of contaminating phospholipase A2. Biochemistry 26:5803-5811. [DOI] [PubMed] [Google Scholar]
- 4.Dufourc, E. J., J. F. Faucon, G. Fourche, J. Dufourcq, T. Gulik-Krzywicki, and M. Le Marire. 1986. Reversible disc-to-vesicle transition of melittin-DPPC complexes triggered by the phospholipid acyl chain melting. FEBS Lett. 201:205-209. [Google Scholar]
- 5.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 6.Ganzle, M. G. 2004. Reutericyclin: biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 64:326-332. [DOI] [PubMed] [Google Scholar]
- 7.Ganzle, M. G., A. Holtzel, J. Walter, G. Jung, and W. P. Hammes. 2000. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 66:4325-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganzle, M. G., and R. F. Vogel. 2003. Studies on the mode of action of reutericyclin. Appl. Environ. Microbiol. 69:1305-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatenbeck, S., and J. Sierankiewicz. 1973. Microbial production of tenuazonic acid analogues. Antimicrob. Agents Chemother. 3:308-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtzel, A., M. G. Ganzle, G. J. Nicholson, W. P. Hammes, and G. Jung. 2000. The first low molecular weight antibiotic from lactic acid bacteria: reutericyclin, a new tetramic acid. Angew. Chem. Int. Ed. Engl. 139:2766-2768. [PubMed] [Google Scholar]
- 11.Horikawa, M., K. Tateda, E. Tuzuki, Y. Ishii, C. Ueda, T. Takabatake, S. Miyairi, K. Yamaguchi, and M. Ishiguro. 2006. Synthesis of Pseudomonas quorum-sensing autoinducer analogs and structural entities required for induction of apoptosis in macrophages. Bioorg. Med. Chem. Lett. 16:2130-2133. [DOI] [PubMed] [Google Scholar]
- 12.Jaber, M. R., S. Olafsson, W. L. Fung, and M. E. Reeves. 2008. Clinical review of the management of fulminant Clostridium difficile infection. Am. J. Gastroenterol. 103:3195-3203. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, S., and D. N. Gerding. 1998. Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 26:1027-1034. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann, G. F., R. Sartorio, S. H. Lee, C. J. Rogers, M. M. Meijler, J. A. Moss, B. Clapham, A. P. Brogan, T. J. Dickerson, and K. D. Janda. 2005. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. U. S. A. 102:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly, C. P., and J. T. LaMont. 2008. Clostridium difficile-more difficult than ever. N. Engl. J. Med. 359:1932-1940. [DOI] [PubMed] [Google Scholar]
- 16.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 17.McFarland, L. V., H. W. Beneda, J. E. Clarridge, and G. J. Raugi. 2007. Implications of the changing face of Clostridium difficile disease for health care practitioners. Am. J. Infect. Control 35:237-253. [DOI] [PubMed] [Google Scholar]
- 18.McFarland, L. V., C. M. Surawicz, and W. E. Stamm. 1990. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J. Infect. Dis. 162:678-684. [DOI] [PubMed] [Google Scholar]
- 19.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 20.Miyairi, S., K. Tateda, E. T. Fuse, C. Ueda, H. Saito, T. Takabatake, Y. Ishii, M. Horikawa, M. Ishiguro, T. J. Standiford, and K. Yamaguchi. 2006. Immunization with 3-oxododecanoyl-l-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J. Med. Microbiol. 55:1381-1387. [DOI] [PubMed] [Google Scholar]
- 21.Nolte, M. J., P. S. Steyn, and P. L. Wessels. 1980. Structural investigations of 3-acylpyrrolidine-2,4-diones by nuclear magnetic resonance spectroscopy and X-ray crystallography. J. Chem. Soc. Perkin Trans. 1:1057-1065. [Google Scholar]
- 22.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepin, J., L. Valiquette, M. E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pepin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can. Med. Assoc. J. 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen, T., P. B. Fernandes, M. A. Marovich, L. Shen, J. Mao, and A. G. Pernet. 1989. Aromatic dienoyl tetramic acids. Novel antibacterial agents with activity against anaerobes and staphylococci. J. Med. Chem. 32:1062-1069. [DOI] [PubMed] [Google Scholar]
- 25.Smith, R. S., and B. H. Iglewski. 2003. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J. Clin. Invest. 112:1460-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toda, S., S. Nakagawa, T. Naito, and H. Kawaguchi. 1980. Bu-2313, a new antibiotic complex active against anaerobes. III. Semi-synthesis of Bu-2313 A and B, and their analogs. J. Antibiot. (Tokyo) 33:173-181. [DOI] [PubMed] [Google Scholar]
- 27.Warny, M., J. Pepin, A. Fang, G. Killgore, A. Thompson, J. Brazier, E. Frost, and L. C. McDonald. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079-1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.