Abstract
This study represents the first phase III trial of the safety, tolerability, and effectiveness of tafenoquine for malaria prophylaxis. In a randomized (3:1), double-blinded study, Australian soldiers received weekly malaria prophylaxis with 200 mg tafenoquine (492 subjects) or 250 mg mefloquine (162 subjects) for 6 months on a peacekeeping deployment to East Timor. After returning to Australia, tafenoquine-receiving subjects received a placebo and mefloquine-receiving subjects received 30 mg primaquine daily for 14 days. There were no clinically significant differences between hematological and biochemical parameters of the treatment groups. Treatment-related adverse events for the two groups were similar (tafenoquine, 13.4%; mefloquine, 11.7%). Three subjects on tafenoquine (0.6%) and none on mefloquine discontinued prophylaxis because of possible drug-related adverse events. No diagnoses of malaria occurred for either group during deployment, but 4 cases (0.9%) and 1 case (0.7%) of Plasmodium vivax infection occurred among the tafenoquine and mefloquine groups, respectively, up to 20 weeks after discontinuation of medication. In a subset of subjects recruited for detailed safety assessments, treatment-related mild vortex keratopathy was detected in 93% (69 of 74) of tafenoquine subjects but none of the 21 mefloquine subjects. The vortex keratopathy was not associated with any effect on visual acuity and was fully resolved in all subjects by 1 year. Tafenoquine appears to be safe and well tolerated as malaria prophylaxis. Although the volunteers' precise exposure to malaria could not be proven in this study, tafenoquine appears to be a highly efficacious drug for malaria prophylaxis.
The continuing spread of multidrug-resistant Plasmodium species and concerns about adverse effects associated with antimalarial drugs has made the prevention of malaria problematic for nonimmune subjects, such as tourists and soldiers who travel to malaria endemic areas. No antimalarial drug is completely effective in preventing malaria (10); however, an ideal prophylactic drug would be highly effective against all malaria-inducing species, very well tolerated, and taken infrequently to enhance compliance (21). Currently, mefloquine, doxycycline, and atovaquone-proguanil are recommended for malaria prophylaxis (5, 23). These drugs are highly effective in preventing malaria but have shortcomings that limit their effectiveness, such as adverse effects, expense, and the difficulty of monitoring daily compliance within deployed military populations. Furthermore, none of these recommended drugs prevents the development and relapse of Plasmodium vivax and P. ovale dormant liver stages (hypnozoites).
Tafenoquine, a long-acting 8-aminoquinoline, is currently being codeveloped by GlaxoSmithKline (GSK) Research & Development Limited and the Walter Reed Army Institute of Research as a replacement for primaquine and for the prevention of malaria. Like primaquine, tafenoquine produces hemolysis in glucose-6-phosphate dehydrogenase (G6PD)-deficient recipients (21). Tafenoquine acts on all stages of the malaria parasite, with the potential to protect against all species of malaria parasites. Previous studies with a challenge model (4) and of indigenous populations in areas in which malaria is endemic have shown that tafenoquine was highly efficacious in preventing P. falciparum malaria and well tolerated (9, 13, 21). Tafenoquine was also shown to be efficacious in preventing both P. falciparum and P. vivax malaria for up to 6 months in Thai soldiers (22).
This first phase III study of tafenoquine for malaria prophylaxis was a randomized, double-blind, active controlled study carried out with healthy Australian soldiers deployed to East Timor as part of a United Nations (UN) peacekeeping mission. The primary study objective was to compare the safety and tolerability of tafenoquine with those of mefloquine in malaria prophylaxis for 6 months. A subset of 98 subjects underwent extra safety assessments to investigate the possible effects of phospholipidosis, methemoglobin, and cardiac safety. Since a placebo arm to document exposure was not possible, the key secondary objective was to assess the efficacy of tafenoquine in preventing P. falciparum and P. vivax malaria during and following deployment.
(This study was presented in part at the 51st Annual Meeting of the American Society of Tropical Medicine and Hygiene, Denver, CO, November 2002.)
MATERIALS AND METHODS
Study site and subjects.
The subjects were Australian soldiers deployed on UN peacekeeping duties to East Timor from October 2000 to April 2001. The soldiers were deployed to the Bobonaro District, on the western border of East Timor. The study included male and female subjects who were between 18 and 55 years of age, judged to be healthy by a medical history and physical examination with normal hematological and biochemical values, G6PD normal, and willing and able to give written informed consent and comply with the study protocol. Females were excluded if they were pregnant, lactating, or unwilling/unable to comply with recognized contraceptive methods. Subjects with a history of psychiatric disorders and/or seizures were also excluded. All subjects gave written informed consent, and the study protocol was approved by the Australian Defence Human Research Ethics Committee (ADHREC protocol no. 216/00) and the U.S. Army Human Subject Research Review Board.
Study design and drug administration.
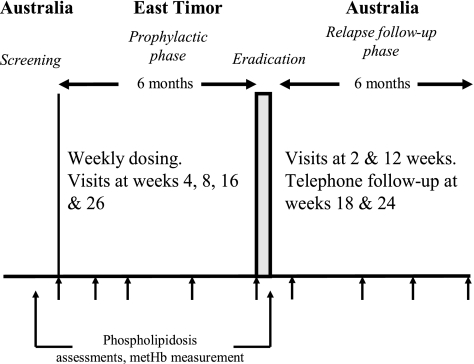
This comparative, randomized, double-blind, active controlled study had 4 phases: screening, loading, prophylactic phase, and relapse follow-up (Fig. 1). Following a loading-dose regimen of 200 mg tafenoquine or 250 mg mefloquine daily for 3 consecutive days, the subjects then received an oral weekly maintenance dose of 200 mg tafenoquine or 250 mg mefloquine for 26 ± 4 weeks, respectively. Subjects were directed to take their study medication at the same time each week with food (breakfast/dinner) to enhance drug bioavailability. Upon their return to Australia, subjects commenced a hypnozoite eradication regimen, receiving primaquine 15 mg twice a day (for the mefloquine group) or matched placebo twice a day (for the tafenoquine group) for 14 days. Drug compliance was observed and recorded for each subject by using medication logs.
FIG. 1.
Drug administration and safety analysis schedule for tafenoquine and mefloquine. metHb, methemoglobin.
Randomization.
A coding memo block randomization system (block size = 8) to provide a 3:1 ratio of tafenoquine-receiving subjects to mefloquine-receiving subjects was used to assign the subjects to a treatment group. Study drugs were prepackaged and prelabeled with a unique study number.
Drug sources.
Tafenoquine was supplied by GlaxoSmithKline in an opaque, hard gelatin capsule (Capsugel), each containing a 200-mg tafenoquine base. Placebo tafenoquine capsules were of identical appearance. Mefloquine (Lariam; 250-mg base tablet) was obtained from Hoffman-La Roche, and primaquine (15-mg base tablet) was supplied by GlaxoSmithKline. The matched placebos for mefloquine and primaquine were identical in external appearance to active capsules. All medication was provided in blinded individual foil blister packs and stored between 15°C to 30°C.
Safety and tolerability.
Assessment of adverse events and sample collection for hematological and blood chemistry parameters were carried out at the loading stage and then at weeks 4, 8, 16, and 26 during the prophylactic phase and at weeks 2 and 12 during the relapse follow-up phase. Adverse event monitoring was supplemented by review of subjects' medical records. For a subset of 98 subjects (77 on tafenoquine and 21 on mefloquine), more-detailed safety assessments were performed. These subjects were assessed for phospholipidosis and its effects (by ophthalmic assessments, lung function tests, and electron microscopy of peripheral blood lymphocytes) and methemoglobin assessment and an electrocardiogram were performed (to assess QT interval) at screening and at the end of the prophylactic phase. Following the identification of corneal deposits at the end of this study, a wider range of ophthalmic assessments was included at follow-up.
Disclosure of adverse events was elicited by the investigator asking the subject the nonleading question, “Do you feel differently in any way since starting the new treatment?” A study physician assessed the level of relationship of any adverse event on the basis of the subject's response and any temporal association and/or known adverse responses to the drug. The physician graded the severity of adverse events as mild (not affecting daily activities), moderate (with some interference in daily activities), and severe (when daily duties could not be completed). A causal relationship to the study drug was judged by the physician to be not related, unlikely, suspected, or probable.
Efficacy assessment.
Thick and thin blood smears were collected from all subjects at screening, at weeks 4, 8, 16, and 26 during the prophylactic phase, and at weeks 2 and 12 during the relapse follow-up phase or if symptoms suggestive of malaria developed. Telephone interviews with all subjects were carried out at weeks 18 and 24 during the relapse follow-up phase to determine their general health status. The Giemsa stain-treated blood smears were each read twice for malaria parasites by blinded microscopists at 2 separate institutions. A blood slide was considered negative if an examination of 200 oil immersion thick fields (magnification, ×1,000) showed no parasites. Any discrepant findings were to have been read by a third blinded expert microscopist and were to be used to define a prophylaxis failure if symptoms consistent with malaria were present.
Statistical analysis.
With at least 450 subjects on tafenoquine and 150 subjects on mefloquine, the study had 94% power to detect a 10% difference in failure rates, assuming an underlying failure rate of 10% in each treatment group (15). Safety and tolerability analyses were performed on data from all subjects who took at least one dose of prophylactic study medication (tafenoquine or mefloquine). Hematological/blood chemistry values for the two groups were compared by a paired Student's t test, and 95% confidence intervals (CIs) were calculated. The efficacy analysis was performed for the per-protocol population, which was defined as the subjects who met the inclusion criteria, were protocol compliant, and completed the prophylactic and relapse follow-up phases. Proportions were examined by using a χ2 test with Yates' correction or by Fisher's exact test. No adjustment was made for multiple testing.
RESULTS
Subject population.
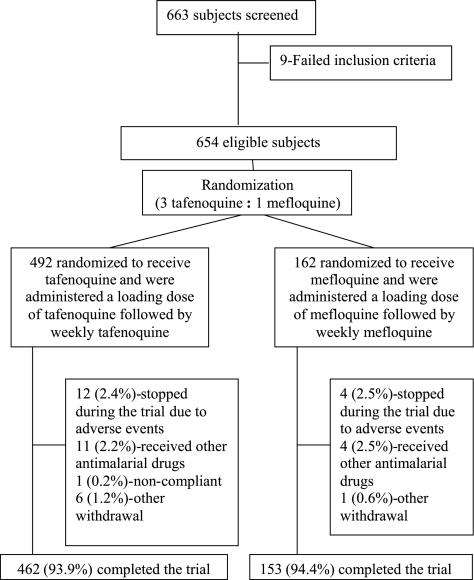
In total, 663 subjects were screened, and of these, 9 subjects failed the inclusion criteria. Of the remaining eligible subjects, 492 subjects were randomized to receive tafenoquine, and 162 subjects were randomized to receive mefloquine. Thirty-nine subjects (30 [6.1%] of the 492 tafenoquine subjects and 9 [5.6%] of the 162 mefloquine subjects) violated the protocol or did not complete the study, due to adverse events or other withdrawal reasons (Fig. 2). There were no marked differences between the groups in the proportions of subjects with protocol violations or withdrawals from the study (data not shown). The treatment groups were well balanced with respect to baseline demographic characteristics and history of malaria (Table 1), with the majority of subjects being white, male, and <35 years of age.
FIG. 2.
Flow diagram of subject accountability during the study.
TABLE 1.
Baseline demographic characteristics and previous malarial histories of subjects on tafenoquine and mefloquine for malaria prophylaxis
| Characteristic | Value for subjects who received: |
|
|---|---|---|
| Tafenoquine (n = 492) | Mefloquine (n = 162) | |
| No. (%) of subjects | ||
| Gender | ||
| Male | 478 (97.2) | 154 (95.1) |
| Female | 14 (2.8) | 8 (4.9) |
| Age (yr) | ||
| 18-25 | 286 (58.1) | 97 (59.9) |
| 26-35 | 178 (36.2) | 48 (29.6) |
| 36-45 | 27 (5.5) | 16 (9.9) |
| 46-55 | 1 (0.2) | 1 (0.6) |
| Race | ||
| White | 484 (98.4) | 160 (98.8) |
| Aboriginal/Torres Strait Islander | 4 (0.8) | 1 (0.6) |
| Other | 4 (0.8) | 1 (0.6) |
| Previous history of malaria | 15 (3.0) | 4 (2.5) |
| Having malaria attacks in 6 mo prior to deployment | 9 (1.8) | 1 (0.6) |
| Age | ||
| Mean (SD) | 25.4 (5.3) | 26.0 (6.5) |
| Range | 18-47 | 18-51 |
| Weight (kg) | ||
| Mean (SD) | 80.9 (11.9) | 81.3 (12.2) |
| Range | 50-135 | 53-135 |
| Height (cm) | ||
| Mean (SD) | 177.8 (7.0) | 177.1 (6.7) |
| Range | 155-198 | 157-192 |
Compliance.
As a result of observed therapy, compliance was high in both treatment groups (100% for the loading dose, 99% for the weekly regimens, and 96% for the follow-up antihypnozoite regimen).
Routine laboratory tests.
For most laboratory variables, the proportion of subjects with results that fell outside an extended normal range during the prophylactic phase was <5% (data not shown). In addition, the proportions of subjects with clinically significant changes from baseline values were similar across the treatment groups for most laboratory parameters. The parameters that were exceptions were hematocrit, bilirubin, and creatinine.
Decreases in hematocrits were seen in both subjects on tafenoquine and subjects on mefloquine, with up to 98 (20%) of the 492 tafenoquine subjects having a 15% decrease from the baseline at any one visit, compared to 23 (14.4%) of the 162 mefloquine subjects. However, only 2 subjects, both on tafenoquine, had a clinically significant hematocrit value (<85% of the lower limit of normal range) during the study. A higher proportion of tafenoquine subjects was reported to have an increase in bilirubin (>2 μmol/liter from the baseline) at any one visit during the study (10% of tafenoquine subjects versus 3.2% of mefloquine subjects). Of these, only 13 (2.6%) tafenoquine subjects and 1 (0.6%) mefloquine subject had a clinically significant bilirubin value (>150% of the upper limit of normal range) at some point during the study. Serum creatinine increases (>125% baseline value) were seen in both the tafenoquine and mefloquine groups, with an increase in serum creatinine in up to 19% of tafenoquine subjects at any one visit versus 10% of mefloquine subjects. At the follow-up, 6 to 8% of subjects in both groups had creatinine values that were still 25% above the baseline; however, few subjects had values outside the normal range, and none of these values was considered clinically significant.
Safety evaluation subgroup.
The ophthalmic assessments in the subgroup of subjects on tafenoquine and mefloquine are summarized in Table 2. At the end of prophylaxis, vortex keratopathy (corneal deposits) was found in 69 (93.2%) of 74 tafenoquine subjects but was absent in the 21 mefloquine subjects (Table 2). These changes were not associated with any visual disturbances and there were no differences between the groups in visual acuity, Amsler grid score, or Ishihara (color vision) score. All subjects with vortex keratopathy were followed up until resolution, with the incidence reducing to 39% at 3 months and 10% at 6 months; there was complete resolution by all subjects by 1 year. Based on the initial findings, fundoscopic examinations were carried out on 86 subjects at the 3-month postprophylaxis follow-up. Abnormalities (e.g., granularity/pigmentation of retinal pigment epithelium or hard drusen) were noted for 27 (39.1%) of 69 tafenoquine subjects and 4 (23.5%) of 17 mefloquine subjects. Retinal fluoroscein angiograms were performed on 14 tafenoquine subjects and 1 mefloquine subject for whom possible retinal findings had been observed. Of these, 4 (28.6%) tafenoquine subjects and 1 (100%) mefloquine subject were considered possibly abnormal. However, review by an expert ophthalmology review board concluded that the retinal findings may well have been normal variations and that there was no evidence to support drug-related visual disturbances. It should be noted that fundoscopic examination of the retina at follow-up was not blinded, because the examination was carried out with the knowledge that corneal deposits were present and no baseline data were available for comparison.
TABLE 2.
Ophthalmic assessments of a subgroup of subjects on tafenoquine or mefloquine
| Activity | Screening | Posttreatment assessment |
|---|---|---|
| Visual field tests | Amsler grid | Amsler grid |
| Humphrey perimetry | ||
| Visual acuity | Snellen chart | Snellen chart |
| Color vision | Ishihara test | Ishihara test |
| Standard pseudoisochromatic plates part 2 | ||
| Farnsworth-Munsell 100 hue test | ||
| Physical examination | Fundoscopy | Fundoscopy |
| Corneal examination | Corneal examination | |
| Digital retinal photography | ||
| Digital corneal photography | ||
| Fundus fluorescein angiograma |
Small number of subjects with possible retinal findings only.
In addition to undergoing phospholipidosis assessments, the safety subgroup also underwent methemoglobin assessment and electrocardiograms for assessment of QT interval. Mean methemoglobin levels increased by 1.8% in the tafenoquine group and by 0.1% in the mefloquine group at the end of prophylaxis, but by week 12 of follow-up, the increase in methemoglobin had resolved. In the tafenoquine group, there was a small reduction in the mean QT interval (difference of −4.5 ms; 95% CI, −9.7 to 0.7 ms), whereas a small increase in the interval was seen in the mefloquine group (difference of 1.6 ms; 95% CI, −12.1 to 15.4 ms) at the end of prophylaxis. There were no subjects for which there was a clinically dangerous prolongation of the QT interval. None of the safety findings impacted participants' well-being or was considered clinically significant.
Tolerability.
During the prophylactic phase, 454 (91.9%) of 492 tafenoquine subjects and 143 (88.3%) of 162 mefloquine subjects reported at least one adverse event. The most common adverse events (occurring in >5% of subjects) are summarized in Table 3. There was no significant difference between the 2 treatment groups in the number or type of adverse events, with the most common events being gastroenteritis and injury, which occurred in >30% of subjects in both treatment groups. The majority of adverse events were mild or moderate in severity. In total, there were 21 severe adverse events (18 [4%] tafenoquine subjects and 3 [2%] mefloquine subjects). The most common severe events were gastroenteritis (6 [1.2%] tafenoquine subjects and 0 mefloquine subjects) and injury (3 [0.6%] tafenoquine subjects and 2 [1.2%] mefloquine subjects). During the relapse follow-up phase, 203 (41.3%) tafenoquine/placebo subjects and 53 (33.9%) mefloquine/primaquine subjects reported adverse events; however, there was no notable difference between the treatment groups in the incidence or nature of events.
TABLE 3.
Adverse events occurring in >5% of subjects on tafenoquine or mefloquine (prophylactic phase)a
| Adverse event | No. (%) of subjects by AE severity and treatment group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mild |
Moderate |
Severe |
Total |
|||||
| Tafenoquine | Mefloquine | Tafenoquine | Mefloquine | Tafenoquine | Mefloquine | Tafenoquine | Mefloquine | |
| At least one AE | 431 (88) | 140 (86) | 194 (39) | 46 (28) | 18 (4) | 3 (2) | 454 (92) | 143 (88) |
| Gastrointestinal | ||||||||
| Gastroenteritis | 109 (22) | 36 (22) | 80 (16) | 17 (11) | 6 (1) | 0 | 182 (37) | 51 (32) |
| Diarrhea | 77 (16) | 28 (17) | 0 | 2 (1) | 1 (<1) | 0 | 77 (16) | 30 (19) |
| Nausea | 27 (6) | 13 (8) | 1 (<1) | 0 | 0 | 0 | 28 (6) | 13 (8) |
| Abdominal pain | 19 (4) | 11 (7) | 5 (1) | 3 (2) | 1 (<1) | 0 | 24 (5) | 13 (8) |
| Vomiting | 19 (4) | 8 (5) | 2 (<1) | 1 (<1) | 0 | 0 | 21 (4) | 8 (5) |
| Musculoskeletal | ||||||||
| Injury | 149 (30) | 46 (28) | 45 (9) | 4 (3) | 3 (<1) | 2 (1) | 178 (36) | 49 (30) |
| Back pain | 65 (13) | 24 (15) | 12 (2) | 2 (1) | 0 | 0 | 74 (15) | 26 (16) |
| Arthralgia | 52 (11) | 17 (11) | 9 (2) | 1 (<1) | 0 | 0 | 55 (11) | 18 (11) |
| Respiratory | ||||||||
| URTI | 97 (20) | 30 (19) | 6 (1) | 2 (1) | 0 | 0 | 101 (21) | 32 (20) |
| Pharyngitis | 24 (5) | 2 (1) | 2 (<1) | 1 (<1) | 0 | 0 | 25 (5) | 3 (2) |
| Dermatological | ||||||||
| Rash | 70 (14) | 20 (12) | 1 (<1) | 1 (<1) | 0 | 0 | 70 (14) | 21 (13) |
| Fungal dermatitis | 43 (9) | 8 (5) | 1 (<1) | 0 | 0 | 0 | 44 (9) | 8 (5) |
| Headache (constitutional AE) | 59 (12) | 18 (11) | 2 (<1) | 2 (1) | 0 | 0 | 61 (12) | 20 (12) |
| Viral infection | 23 (5) | 7 (4) | 16 (3) | 6 (4) | 1 (<1) | 0 | 39 (8) | 13 (8) |
In total, there were 492 tafenoquine subjects and 162 mefloquine subjects. AE, adverse event; URTI, upper respiratory tract infection.
In total, 64 (13.0%) tafenoquine subjects and 23 (14.2%) mefloquine subjects reported neuropsychiatric adverse events, the most common being vertigo, dizziness and various sleep disorders (Table 4). There was no significant difference between the treatment groups in the incidence and type of neuropsychiatric events, and all were reported as mild or moderate.
TABLE 4.
Neuropsychiatric events in subjects on tafeoquine or mefloquine (prophylactic phase)a
| Adverse event | No. (%) of subjects by AE severity and treatment group |
|||||
|---|---|---|---|---|---|---|
| Mild |
Moderate |
Total |
||||
| Tafenoquine | Mefloquine | Tafenoquine | Mefloquine | Tafenoquine | Mefloquine | |
| Vertigo | 22 (5) | 7 (4) | 0 | 1 (<1) | 22 (5) | 8 (5) |
| Somnolence | 12 (2) | 6 (4) | 0 | 0 | 12 (2) | 6 (4) |
| Abnormal dreams | 7 (1) | 2 (1) | 0 | 0 | 7 (1) | 2 (1) |
| Dizziness | 5 (1) | 2 (1) | 0 | 0 | 5 (1) | 2 (1) |
| Insomnia | 4 (<1) | 3 (2) | 1 (<1) | 0 | 5 (1) | 3 (2) |
| Abnormal coordination | 2 (<1) | 1 (<1) | 0 | 0 | 2 (<1) | 1 (<1) |
| Anxiety | 2 (<1) | 0 | 0 | 0 | 2 (<1) | 0 |
| Agitation | 2 (<1) | 0 | 0 | 0 | 2 (<1) | 0 |
| Euphoria | 2 (<1) | 0 | 0 | 0 | 2 (<1) | 0 |
| Tremor | 2 (<1) | 0 | 0 | 0 | 2 (<1) | 0 |
| Depression | 0 | 0 | 1 (<1) | 1 (<1) | 1 (<1) | 1 (<1) |
| Paroniria | 1 (<1) | 0 | 0 | 0 | 1 (<1) | 0 |
| Amnesia | 1 (<1) | 0 | 0 | 0 | 1 (<1) | 0 |
In total, there were 492 tafenoquine subjects and 162 mefloquine subjects. There were no severe adverse events (AEs) of this type.
Fifteen subjects withdrew from the study as a result of adverse events (12 [2.4%] tafenoquine subjects and 3 [1.9%] mefloquine subjects). Four tafenoquine subjects sustained injuries requiring evacuation from the study area, while 2 experienced arthralgia (1 subject on each drug). Three tafenoquine subjects withdrew for possible treatment-related adverse events, namely, abdominal pain (severe), depression (moderate), and hyperesthesia (moderate). The incidences of severe adverse events in the 2 groups were comparable (18 [3.7%] tafenoquine subjects and 5 [3.1%] mefloquine subjects).
In total, during the prophylactic phase, 66 (13.4%) tafenoquine subjects and 19 (11.7%) mefloquine subjects had adverse events with a suspected/probable relationship to treatment (Table 5). There were no significant differences between the treatment groups in the incidence or nature of treatment-related adverse events during the prophylactic phase. Only 1 subject on tafenoquine reported a severe adverse event (diarrhea and abdominal pain) suspected to be related to treatment.
TABLE 5.
Table of adverse events attributed as related to study drug during prophylactic phase in the safety populationa
| Adverse event | No. (%) of patients in treatment group |
|
|---|---|---|
| Tafenoquine (n = 492) | Mefloquine (n = 162) | |
| At least one AE | 66 (13.4) | 19 (11.7) |
| Nausea | 14 (2.8) | 4 (2.5) |
| Vertigo | 10 (2.0) | 2 (1.2) |
| Diarrhea | 9 (1.8) | 3 (1.9) |
| Abdominal pain | 7 (1.4) | 2 (1.2) |
| Abnormal dreaming | 6 (1.2) | 1 (0.6) |
| Somnolence | 6 (1.2) | 1 (0.6) |
| Headache | 3 (0.6) | 2 (1.2) |
| Insomnia | 3 (0.6) | 2 (1.2) |
Events occurring in >1% of subjects are shown. AE, adverse event.
Efficacy.
No symptomatic malarial infections occurred during the prophylactic phase in either treatment group. Smears collected from symptomatic subjects and during routine screening for malaria diagnosis were all negative. There were 4 cases (0.9%) of malarial infection in the tafenoquine group and a single case (0.7%) in the mefloquine group during the relapse follow-up phase (95% CI, −1.32 to 1.74; P = 1.0). All cases corresponded to P. vivax infection, which occurred between 16 and 20 weeks following the return from East Timor.
DISCUSSION
This phase III study describes the safety and tolerability of tafenoquine administered for malaria prevention in a nonimmune population of predominately young Caucasian males. Both tafenoquine and mefloquine were well tolerated. There were no clinically significant differences between hematological and blood chemistry results for the 2 treatment groups.
Assessment for phospholipidosis and its effects in a subgroup of 98 subjects showed at the end of the prophylactic phase a high incidence (93.2%) of mild vortex keratopathy (corneal deposits) in the tafenoquine group. Based on these findings, an independent expert ophthalmology board was asked to review the data. It concluded that the corneal changes were benign, fully reversible, and similar to those seen with several other drugs, including chloroquine, for which it is not considered to be a contraindication for continuous use (1). It also advised us that vision had not been impaired in any subject. A lack of baseline retinal photography data meant that the relevance of retinal findings could not be ascertained, but they reflected normal variability. Further assessment of the eye changes observed with tafenoquine will need to be undertaken to determine with certainty the overall significance of the observed changes and to clarify the retinal issues raised during the review.
As would be expected in a long-term study, the incidence of adverse events was high, with 92% of tafenoquine subjects and 88% of mefloquine subjects reporting one or more adverse events during the 6 months of prophylaxis. The majority of these events was mild or moderate in severity, and the events were typical of the type of events expected in a population of soldiers on active duty (e.g., injury or gastroenteritis). The number of withdrawals from the study was low for a long-term study, also reflecting the nature of the study population. There were no significant differences in the occurrence of treatment-related adverse events, including gastrointestinal and neuropsychiatric disturbances between the 2 treatment groups.
Limited comparative data on the tolerability of tafenoquine used for prophylaxis are available. In adult black Kenyans, the incidences of adverse events for subjects on placebo and on weekly 200 mg tafenoquine for 13 weeks were similar (21). Relative to our findings, the study of the Kenyans reported a higher incidence of headache (24% versus 12.4%) but lower incidences of diarrhea (7% versus 15.7%) and rashes (4% versus 14.2%) with the same maintenance dose. However, such comparisons are difficult to make when the subject populations differ so markedly in ethnicity, nutritional status, culture, employment, and tolerance to medication.
Mefloquine was well tolerated by the Australian soldiers, which is in accordance with the results of other randomized, double-blind studies of military populations (2, 6, 17). No soldiers on mefloquine withdrew from the study due to treatment-related adverse events, and no more than 2% of the soldiers on either tafenoquine or mefloquine experienced drug-associated neuropsychiatric disturbances. Severe neuropsychiatric adverse events in European travelers on mefloquine have been reported (18, 20), but such events were not observed in the present study. Neuropsychiatric adverse events related to mefloquine use are reported to be more common in females (20), and the somewhat atypical distribution of participants in this study should be considered when generalizing these findings.
Without a placebo control, the exposure to malaria experienced by the Australian soldiers could not be directly estimated. As an indication of the malaria exposure that the soldiers probably encountered, 2 malaria prevalence surveys were conducted (January 2001 and April 2001) in 7 East Timorese villages (about 200 residents in each village), all within 1 km of where the soldiers were located (3). The surveys showed that malaria was present in 6 of the 7 locations, with point prevalence rates ranging from 0 to 35.3% (P. falciparum, 0 to 14.4%; P. vivax, 0 to 16%). In addition to this evidence, several studies have confirmed a high incidence of malaria in East Timor (8, 11-12, 14, 19). While these studies are not conclusive proof that subjects in the present study were exposed to malaria, it is highly likely that the soldiers were exposed to both P. falciparum and P. vivax malaria. Because no prophylactic failures occurred during the treatment phase in East Timor, both treatments appeared to be effective in suppressing malaria infections. During the 6-month relapse follow-up period, 4 (0.9%) subjects on tafenoquine/placebo and 1 (0.7%) subject on mefloquine/primaquine developed P. vivax infections. These findings indicate that tafenoquine and primaquine are equally effective in preventing P. vivax relapse when primaquine compliance is monitored and confirm the results of previous studies in Papua New Guinea (16) and East Timor (7). Although the relapse rates for primaquine and tafenoquine appear to be similar, tafenoquine offers a major advantage in that there is no need to take additional medication after leaving the endemic area if tafenoquine is used for prophylaxis.
In summary, tafenoquine at 200 mg weekly is safe and well tolerated in nonimmune Caucasian subjects following 6 months of prophylaxis. Although mild vortex keratopathy was seen in the subjects on tafenoquine, this was benign and fully reversible. The most frequently recorded treatment-related adverse events for both tafenoquine and mefloquine were gastrointestinal disturbances, and these tended to be mild or moderate. Both treatments fully suppressed malarial infections during prophylaxis, and less than 1% of subjects developed postexposure malaria after either completion of tafenoquine prophylaxis or primaquine treatment. Tafenoquine is an effective alternative weekly antimalarial that can be used without the need for further medication after leaving an endemic area.
Acknowledgments
Tafenoquine Study Team members included Karl Rieckmann, Bob Cooper, Stephen Frances, Michael Reid, Alyson Auliff, Bruce Russell, Stephen McLeod-Robertson, John Staley, Kerryn Rowcliffe, John Ross, and Brian Potter from the Australian Army Malaria Institute, Keith Barker and Dominic Galvin from GlaxoSmithKline Research & Development Limited, and Ann Aultman from the U.S. Army Medical Materiel Development Activity.
We thank John Calagari, Stephen Ferndale, Damien Wood, and officers and soldiers of the 1st Battalion Group, Royal Australian Regiment, East Timor, who participated in the study for their support and cooperation. We are grateful to G. Dennis Shanks and Bob Cooper for commenting on the manuscript.
Financial support was from the U.S. Army Medical Materiel Development Activity, GlaxoSmithKline Research & Development Limited, and the Australian Defence Force.
P.P. and C.K. are employees of GlaxoSmithKline Research & Development Limited. For all other authors, there are no conflicts.
The opinions expressed are ours and do not necessarily reflect those of the Joint Health Command, Australian Defence Force, the U.S. Army, or any extant defense force policy.
Footnotes
Published ahead of print on 7 December 2009.
REFERENCES
- 1.Bernstein, H. N. 1983. Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am. J. Med. 75(Suppl. 1A):25-34. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau, E., B. Schuster, J. Sanchez, et al. 1993. Tolerability of prophylactic Lariam regimens. Trop. Med. Parasitol. 44:257-265. [PubMed] [Google Scholar]
- 3.Bragonier, R., H. Reyburn, P. Nasveld, et al. 2002. Rainy-season prevalence of malaria in Bobonaro district, East Timor. Ann. Trop. Med. Parasitol. 96:739-743. [DOI] [PubMed] [Google Scholar]
- 4.Brueckner, R. P., T. Coster, D. L. Wesche, et al. 1998. Prophylaxis of Plasmodium falciparum infection in a human challenge model with WR238605, a new 8-aminoquinoline antimalarial. Antimicrob. Agents Chemother. 42:1293-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Health information for international travel, 2003-2004. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA.
- 6.Croft, A. M., T. C. Clayton, and M. J. World. 1997. Side effects of mefloquine prophylaxis for malaria: an independent randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 91:199-203. [DOI] [PubMed] [Google Scholar]
- 7.Elmes, N. J., P. E. Nasveld, S. J. Kitchener, et al. 2008. The efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the Southwest Pacific. Trans. R. Soc. Trop. Med. Hyg. 102:1095-1101. [DOI] [PubMed] [Google Scholar]
- 8.Frances, S. P., A. M. Auliff, M. D. Edstein, et al. 2003. Survey of personal protection measures against mosquitoes among Australian Defence Force personnel deployed to East Timor. Mil. Med. 168:227-230. [PubMed] [Google Scholar]
- 9.Hale, B. R., S. Owusu-Agyei, D. J. Fryauff, et al. 2003. A randomized, double-blind, placebo-controlled, dose-ranging trial of tafenoquine for weekly prophylaxis against Plasmodium falciparum. Clin. Infect. Dis. 36:541-549. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman, S. L. 1992. Diagnosis, treatment and prevention of malaria. Med. Clin. North Am. 76:1327-1355. [DOI] [PubMed] [Google Scholar]
- 11.Kitchener, S., A. M. Auliff, and K. H. Rieckmann. 2000. Malaria in the Australian Defence Force during and after the participation in the International force in East Timor (INTERFET). Med. J. Aust. 173:583-585. [DOI] [PubMed] [Google Scholar]
- 12.Kolaczinski, J., and J. Webster. 2003. Malaria control in complex emergencies: the example of East Timor. Trop. Med. Int. Health 8:48-55. [DOI] [PubMed] [Google Scholar]
- 13.Lell, B., J. F. Faucher, M. A. Missinou, et al. 2000. Malaria chemoprophylaxis with tafenoquine: a randomised study. Lancet 355:2041-2045. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald, P. 2000. A clinical experience of malaria in East Timor. Aust Fam. Physician 29:1076-1078. [PubMed] [Google Scholar]
- 15.Makuch, R., and R. Simon. 1978. Sample size requirements for evaluating a conservative therapy. Cancer Treat. Res. 62:1037-1040. [PubMed] [Google Scholar]
- 16.Nasveld, P. E., S. Kitchener, M. D. Edstein, et al. 2002. Comparison of tafenoquine (WR238605) and primaquine in the post exposure prophylaxis of vivax malaria in Australian Defence Force personnel. Trans. R. Soc. Trop. Med. Hyg. 96:683-684. [DOI] [PubMed] [Google Scholar]
- 17.Ohrt, C., T. L. Richie, H. Widjaja, et al. 1997. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. Ann. Intern. Med. 126:963-972. [DOI] [PubMed] [Google Scholar]
- 18.Overbosch, D., H. Schilthuis, U. Bienzle, et al. 2001. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin. Infect. Dis. 33:1015-1021. [DOI] [PubMed] [Google Scholar]
- 19.Peragallo, M. S., A. M. Croft, and S. J. Kitchener. 2002. Malaria during a multinational military deployment: the comparative experience of the Italian, British and Australian armed forces in East Timor. Trans. R. Soc. Trop. Med. Hyg. 96:481-482. [DOI] [PubMed] [Google Scholar]
- 20.Schlagenhauf, P., A. Tschopp, R. Johnson, et al. 2003. Tolerability of malaria chemoprophylaxis in non-immune travelers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ 327:1078-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanks, G. D., A. J. Oloo, G. M. Aleman, et al. 2001. A new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malaria. Clin. Infect. Dis. 33:1968-1974. [DOI] [PubMed] [Google Scholar]
- 22.Walsh, D. S., C. Eamsila, T. Sasiprapha, et al. 2004. Efficacy of monthly tafenoquine for prophylaxis for Plasmodium vivax and multidrug-resistant P. falciparum malaria. J. Infect. Dis. 190:1456-1463. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2005. International travel and health. World Health Organization, Geneva, Switzerland. http:/www.who.int/malaria.