Abstract
S-013420 (EDP-420) is a novel bicyclolide (bridged bicyclic macrolide) antibacterial currently under development for the treatment of respiratory tract infections. The objective of the present study was to determine the plasma and intrapulmonary pharmacokinetic parameters of orally administered S-013420 in healthy volunteers. Twenty-eight healthy Japanese male subjects who never smoked were randomly allocated to seven groups of four subjects each who underwent bronchoalveolar lavage (BAL) at different times after dosing (2, 4, 6, 8, 10, 12, or 24 h). Blood samples were also taken at 0, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h after dosing. The S-013420 concentrations in plasma, epithelial lining fluid (ELF), and alveolar macrophages (AMs) were measured by using a combined high-performance liquid chromatography-mass spectrometric technique. A pharmacokinetic analysis of the plasma, ELF, and AM S-013420 concentration profiles was performed. S-013420 was rapidly absorbed in plasma, and the mean time to the maximum concentration in plasma was 2.27 h. S-013420 was rapidly distributed to the ELF and was slowly distributed to AMs. The areas under the concentration-time curves from time zero to 24 h (AUC0-24) for S-013420 were 20.3 times higher in ELF than in plasma and 244.6 times higher in AMs than in plasma. The mean maximum concentration in plasma was higher in ELF than in plasma and was much higher in AM than in plasma. Furthermore, pharmacodynamic calculations were done by using the AUC0-24/MIC90 ratio for common pneumonia pathogens (Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis). The AUC0-24 for plasma/MIC90s for these four organisms were 41.8, 83.6, 1.3, and 20.9, respectively. The AUC0-24 for ELF/MIC90s were 849.6, 1,699.2, 26.6, and 424.8, respectively. Considering the good efficacy shown in a subsequent phase 2 study (S. Kohno, K. Yamaguchi, Y. Tanigawara, A. Watanabe, A. Aoki, Y. Niki, and J. Fujita, Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-485), the good distribution of S-013420 in AMs and ELF observed in the present study is predictive of the good efficacy of S-013420 against respiratory pathogens.
During the development of antibacterial agents, knowledge of the drug concentrations in infected lesions is important for predicting the effectiveness of antimicrobial chemotherapy. In order to support the use of a drug for clinical indications such as respiratory tract infections, the bronchoalveolar lavage (BAL) technique can be utilized to investigate the distribution of the drug in the lungs of humans. The BAL technique allows measurement of the concentration of drug both in the epithelial lining fluid (ELF) and within alveolar macrophages (AMs). Measurement of the latter is especially important for macrolides, which can accumulate intracellularly at high concentrations.
Several investigations measuring the distributions of clarithromycin (CAM) (10, 11, 13, 15), azithromycin (AZM) (11, 13, 15, 20), and telithromycin (TEL) (5, 12) in the lungs of healthy subjects have been reported. Those studies have shown that macrolides have high degrees of distribution in ELF and AMs.
S-013420 (EDP-420) is a novel bicyclolide (bridged bicyclic macrolide) antibacterial and has been shown to have potent activity against various pathogens. It is notable that the drug has strong activity against multidrug-resistant Streptococcus pneumoniae, including penicillin G- and erythromycin-resistant strains, which have become a worldwide public health problem (4).
The spectrum of activity of S-013420 covers both common and atypical respiratory pathogens. Maki et al. (8) showed that S-013420 has in vitro activity against S. pneumoniae, Streptococcus pyogenes, and methicillin-susceptible Staphylococcus aureus (MIC90s ≤ 0.063 to 0.25 μg/ml). This activity was greater than the activities of CAM and AZM (MIC90s = 1 to >64 μg/ml) and comparable to the activity of TEL. The anti-Haemophilus influenzae activity of S-013420 (MIC90 = 8 μg/ml) was comparable to that of CAM and less than that of TEL (MIC90 = 4 μg/ml). Tsuji et al. investigated the in vivo activity of S-013420 against experimental animal infection models (18). In an animal model of infection with erythromycin-resistant S. pneumoniae, S-013420 was more effective than CAM and AZM and was as effective as TEL. S-013420 (30 mg/kg of body weight) reduced the number of viable cells of a strain of H. influenzae to the same extent that CAM did.
In general, ketolide analogues such as TEL are well known to have a low potential to induce resistance. S-013420 has also been demonstrated to have a low potential to induce resistance (19). These excellent antibacterial characteristics of S-013420 are the result of the inhibition of protein synthesis by binding to two sites in domain V and one site in domain II, a total of three sites, of the 23S rRNA for the 50S subunit of the bacterial ribosome.
For macrolide antibiotics, the ratio of area under the concentration-time curve (AUC) to the MIC, calculated by pharmacokinetic (PK) analysis, is well-known to correlate with efficacy against infections (3). The AUC for ELF/MIC is the most favorable parameter for predicting the ability of macrolides to eradicate respiratory pathogens.
In the present study, we investigated the pharmacokinetics of S-013420, including its lung distribution, using BAL multiple times for up to 24 h after the administration of a single oral dose of a S-013420 suspension to healthy volunteers. By means of in vitro PK and pharmacodynamic (PD) modeling with an H. influenzae strain (MIC = 8 μg/ml), the AUC from time zero to 24 h (AUC0-24)/MIC required to achieve 90% maximum bactericidal activity was calculated in order to predict the appropriate dosages for use in a subsequent phase 2 clinical trial (7).
MATERIALS AND METHODS
Subjects.
Healthy Japanese male subjects between the ages of 20 and 45 years who had normal body mass indexes (range, 18.5 to 24.9) and who had never smoked were eligible for inclusion in the study. All subjects provided written informed consent before they were screened. The subjects were excluded from this study if any of the following conditions existed: hypersensitivity to macrolides, lidocaine, or atropine; a history of bronchial asthma; a history of alcohol abuse or drug abuse; or any condition known to interfere with the absorption, distribution, metabolism, or excretion of drugs.
Study design and procedures.
This was an open-label study of the plasma and intrapulmonary pharmacokinetics of S-013420. Subjects were allocated to seven groups of four subjects each according to the time of BAL after oral dosing (2, 4, 6, 8, 10, 12, or 24 h). They were given a single oral dose of 400 mg of an S-013420 suspension. Each subject underwent fiber-optic bronchoscopy by BAL once at the respective time after administration of the dose of S-013420. Blood samples were also taken from each subject at 0, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h after dosing. The study was approved by the local ethics committee and was carried out in accordance with Japanese Good Clinical Practice guidelines.
BAL.
As pretreatments, inhalation anesthesia with 2% lidocaine through a nebulizer and the injection of atropine sulfate were started 15 min before fiber-optic bronchoscopy. After that, regional anesthesia of the pharynx with lidocaine through a Jackson-type spray was performed. Systemic sedation was not used. The bronchoscope was inserted up to the right middle lobe and was then wedged in place. BAL was carried out by the infusion of four 50-ml volumes of sterile saline into the subsegmental bronchus of the right middle lobe, and each specimen was immediately aspirated. The BAL procedure was performed within 1 min. The first aliquot recovered was discarded and the last three were pooled.
Blood samples.
Blood samples were collected from all subjects just before dosing on day 1 and at further selected time points (just before oral dosing and at 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h after dosing).
Sample analysis and handling.
The blood samples, which were used for determination of the S-013420 and urea concentrations, were centrifuged at 1,600 × g for 10 min at 4°C, and the plasma was stored at −20°C until it was assayed. BAL fluid was kept on ice and was centrifuged at 400 × g for 5 min at 4°C to separate the cells from the supernatant. The cells sedimented from the BAL fluid were retained for determination of the cell counts with a hemocytometer. Differential counting of the cells in the BAL fluid was performed with a stained cytocentrifuged sample of BAL fluid. The BAL cells and supernatant were then both stored at −20°C until they were assayed for their S-013420 and urea concentrations.
S-013420 and urea assay.
Urea diffuses freely throughout the body fluid, and therefore, the concentrations of urea in plasma and ELF are identical. The volume of ELF can be calculated from the dilution of urea in the BAL fluid.
Determination of the S-013420 concentration in plasma was performed by a validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) method by Analytical Chemistry, Developmental Research Laboratories, Shionogi & Co., Ltd. (Osaka, Japan). Determination of the S-013420 concentration in BAL fluid was performed by a validated LC/MS/MS method at Sumika Chemical Analysis Service, Ltd. (Osaka, Japan). S-013420 was extracted from ELF and AMs by deproteinization and was separated by liquid chromatography with electrospray ionization tandem mass spectrometry. The assay for S-013420 was linear (r = 0.9997) over the concentration range of 0.005 to 5 μg/ml. The concentrations in the interday quality control samples (surfactant-containing normal saline solution) for ELF and AMs (n = 5) of 0.004997, 0.009994, 0.4997, and 3.998 μg/ml had coefficients of variation (CVs) of 3.0, 3.9, 6.9, and 2.8%, respectively.
Pharmacokinetic calculations of plasma concentrations.
The maximum observed concentration in plasma (Cmax), the time to Cmax (Tmax), and the apparent plasma terminal-phase half-life (t1/2,z) were calculated on the basis of the plasma S-013420 concentrations by a model-independent approach with the WinNonlin program (version 4.01; Pharsight Corporation). The AUC0-24 was calculated by the trapezoidal method.
Calculation of S-013420 CELF and CAM.
Calculation of the S-013420 concentration in ELF (CELF) and the S-013420 concentration in AMs (CAM) was performed with the BAL fluid supernatant and the pulmonary cell pellet, respectively, from aspirates recovered from the 2nd, 3rd, and 4th saline infusions.
CAM was determined as follows: CHPLC/VAM, where CHPLC is the concentration of S-013420 in a 1-ml cell suspension measured by high-performance liquid chromatography (HPLC), and VAM is the volume of AM in a 1-ml cell suspension. Cells were counted by type to determine the number of AMs. A mean AM volume of 2.42 μl/106 cells was used to calculate the volume of AMs in the suspension (1).
The urea concentration in the supernatant of the BAL fluid was corrected for possible contamination with urea from blood (2). To determine the amount of urea present in the plasma from contamination with urea from blood, the ratio of the number of erythrocytes (RBCs) in BAL fluid to the number of RBCs in blood was used. The corrected amount of urea in the supernatant of the BAL fluid (ureaCORR) was derived from the following relationship: ureaBAL − (RBCBAL/RBCblood × VBAL × ureaplasma), where ureaBAL is the urea concentration in the supernatant of the BAL fluid, RBCBAL is the RBC count in BAL fluid, RBCblood is the RBC count in blood, VBAL is the volume of BAL fluid, and ureaplasma is the concentration of urea in plasma.
CELF was determined as follows: CBAL × (ureaplasma/ureaBAL), where CBAL is the concentration of S-013420 measured in the supernatant of the BAL fluid.
The ratios of the concentration in ELF and AM to the simultaneous concentration in plasma were calculated for each subject and were summarized for each group at each sampling time.
Safety.
A clinical examination was performed, and standard laboratory parameters (hematology parameters, blood chemistry analysis, and urinalysis) and vital signs, including evaluation by electrocardiography, were monitored as part of the screening before the administration of S-013420 on day −1 and on days 1, 2, 3, 4, 6, and 10. Chest X rays were also performed during the screening and on day 6. Adverse events were assessed through questioning and spontaneous reporting.
Statistical considerations.
On the basis of the findings of previous studies with other antimicrobials, it was estimated that 4 subjects per group and seven groups (a total of 28 subjects) would be sufficient for assessment of the concentration of S-013420 in the target tissue. Descriptive statistics and statistical analysis were performed with the SAS program (version 8.02; SAS Institute). The demographic parameters of age, height, weight, and serum creatinine concentration were compared for the seven groups. For all tests performed, a P value of <0.05 was considered significant.
The calculated ELF, AM, and plasma concentration data for S-013420 and the ratios of the drug concentration in ELF and AM to the concentration in plasma at each sampling time were summarized. The time courses for ELF, AM, and plasma concentrations were graphically represented.
The PK parameters of Cmax, Tmax, AUC0-24, and t1/2,z for the ELF and AM concentrations were calculated by a model-independent approach in the same manner used for the plasma concentration data.
For calculation of the PD parameters, the MIC90s of S-013420 for common respiratory pathogens were obtained from the recent literature. The PD parameters consisting of the AUC0-24/MIC90 ratios in ELF and plasma for common pneumonia pathogens (S. aureus, S. pneumoniae, H. influenzae, and Moraxella catarrhalis) were calculated.
RESULTS
Twenty-eight healthy Japanese males participated in this study. There were no significant differences in the average ages among the groups for whom BAL was performed at 2, 4, 6, 8, 10, 12, and 24 h after dosing (P > 0.05). There were some significant differences in height and weight between certain groups. The serum creatinine concentrations in the group for whom BAL was performed at 10 h (the 10-h group) were significantly higher than those in the 4-h group (P = 0.0231) (Table 1). The total cell count and the cellular fraction data for cells obtained from BAL fluid are given in Table 2.
TABLE 1.
Characteristics of the 28 subjects in the studya
| Time (h) | Age (yr) | Ht (cm) | Wt (kg) | Serum creatinine concn (mg/dl) |
|---|---|---|---|---|
| 2 | 21.3 ± 0.5 | 171.3 ± 5.3 | 55.3 ± 3.0d | 0.80 ± 0.11 |
| 4 | 22.0 ± 2.2 | 176.7 ± 4.5b | 64.1 ± 5.7 | 0.75 ± 0.07e |
| 6 | 21.5 ± 0.6 | 172.7 ± 4.7 | 65.3 ± 5.0d | 0.86 ± 0.03 |
| 8 | 22.5 ± 1.9 | 176.0 ± 3.3c | 63.7 ± 4.3 | 0.78 ± 0.09 |
| 10 | 23.3 ± 4.0 | 169.1 ± 2.7b | 57.4 ± 7.5 | 0.88 ± 0.05e |
| 12 | 22.8 ± 1.7 | 167.6 ± 4.8b,c | 62.0 ± 7.0 | 0.84 ± 0.07 |
| 24 | 22.0 ± 1.4 | 171.0 ± 7.1 | 60.0 ± 9.7 | 0.81 ± 0.09 |
The data are given as the means ± SDs for four subjects in each group. There were no significant differences among the groups by age (P > 0.05).
The heights of the 4-h group were significantly greater than those of the 10-h and 12-h groups (P <0.05).
The heights of the 8-h group were significantly greater than those of the 12-h group (P = 0.022).
The weights of the 6-h group were significantly greater than those of the 2-h group (P = 0.039).
The serum creatinine concentrations of the 10-h group were significantly higher than those of the 4-h group (P = 0.0231).
TABLE 2.
Total cell count and cellular fraction from BAL fluida
| Time (h) | Total no. of cells in BAL fluid | % |
||||
|---|---|---|---|---|---|---|
| Neutrophils | Lymphocytes | Eosinophils | AMs | Other | ||
| 2 | 5.18 × 106 ± 2.0 × 106 | 0.0 ± 0.0 | 15.3 ± 1.0 | 0.0 ± 0.0 | 84.8 ± 1.0 | 0.0 ± 0.0 |
| 4 | 6.30 × 106 ± 2.05 × 106 | 0.4 ± 0.5 | 12.4 ± 4.5 | 0.1 ± 0.2 | 87.1 ± 4.3 | 0.0 ± 0.0 |
| 6 | 3.35 × 106 ± 0.54 × 106 | 0.0 ± 0.0 | 13.0 ± 4.7 | 0.0 ± 0.0 | 86.9 ± 4.8 | 0.1 ± 0.3 |
| 8 | 4.28 × 106 ± 2.26 × 106 | 0.1 ± 0.3 | 9.3 ± 4.7 | 0.3 ± 0.5 | 90.4 ± 4.9 | 0.0 ± 0.0 |
| 10 | 5.13 × 106 ± 2.01 × 106 | 0.0 ± 0.0 | 13.6 ± 5.7 | 0.1 ± 0.3 | 85.8 ± 6.0 | 0.5 ± 0.6 |
| 12 | 4.70 × 106 ± 1.25 × 106 | 0.0 ± 0.0 | 13.3 ± 7.6 | 0.9 ± 1.2 | 85.9 ± 8.7 | 0.0 ± 0.0 |
| 24 | 5.83 × 106 ± 2.03 × 106 | 0.0 ± 0.0 | 10.8 ± 5.7 | 0.0 ± 0.0 | 89.3 ± 5.7 | 0.0 ± 0.0 |
Data are given as the means ± SDs (n = 4).
Issues from the subjects' medical histories or hypersensitivity to drugs that could have caused a problem with the conduct and evaluation of the findings of the present study were not found in any subjects. None of the 28 subjects experienced significant adverse events. Mild leukocytosis in four subjects, mild neutrophilia in eight subjects, and mild lymphopenia in nine subjects were observed on the 2nd day after the BAL, but no abnormalities were seen over the next 24 h. No subject required treatment for any adverse events during the study. A mild elevation in the alanine aminotransferase (ALT) concentration in one subject was observed 3 days after dosing, but this resolved by the 6th day after dosing. Only the elevation in the ALT concentration was judged to be a drug-related event.
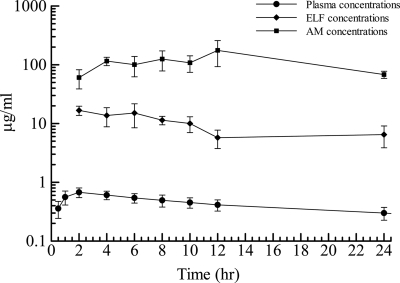
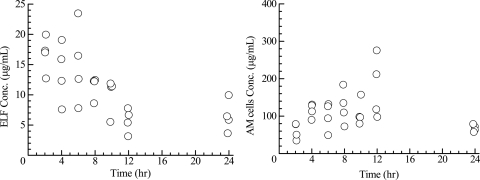
Prior to dosing, plasma S-013420 concentrations were below the quantitative limit of detection in all study subjects. The mean ± standard deviation (SD) drug concentrations in plasma, AM, and ELF are displayed in Table 3. Cmax (0.646 μg/ml) was reached at 2 h postdosing and gradually decreased to 0.315 μg/ml over 24 h. The ratios of the mean concentration in ELF to the mean concentration in plasma for S-013420 during the 24-h period after drug administration were in the range of 13.8 to 27.0. The ratios of the mean concentration in AM to the mean concentration in plasma for S-013420 ranged from 93 to 406. The ELF, AM, and plasma S-013420 concentrations over time are represented graphically in Fig. 1. The individual ELF and AM concentrations are shown in Fig. 2.
TABLE 3.
Concentration of S-013420 in plasma, ELF, and AMa
| Time (h) | Cplasma (μg/ml) | CELF (μg/ml) | CAM (μg/ml) | CELF/Cplasma | CAM/Cplasma |
|---|---|---|---|---|---|
| 2 | 0.646 ± 0.088 | 16.7 ± 3.0 | 61 ± 22 | 26.3 | 93 |
| 4 | 0.575 ± 0.075 | 13.7 ± 4.9 | 116 ± 19 | 24.8 | 201 |
| 6 | 0.540 ± 0.131 | 15.1 ± 6.6 | 100 ± 38 | 27.0 | 183 |
| 8 | 0.441 ± 0.070 | 11.4 ± 1.9 | 125 ± 47 | 25.9 | 283 |
| 10 | 0.429 ± 0.091 | 10.0 ± 3.0 | 108 ± 34 | 23.2 | 260 |
| 12 | 0.424 ± 0.088 | 5.8 ± 2.0 | 176 ± 83 | 13.8 | 406 |
| 24 | 0.315 ± 0.116 | 6.5 ± 2.6 | 68 ± 10 | 21.3 | 232 |
Data are given as the means ± SDs.
FIG. 1.
Mean concentrations ± standard deviations of S-013420 in plasma, ELF, and AM at time of BAL.
FIG. 2.
Time courses of ELF and AM concentrations (n = 28).
The results of the PK analysis for the plasma, ELF, and AM concentration profiles are summarized in Table 4. S-013420 was rapidly absorbed in plasma, and the mean Tmax in plasma was 2.27 h. S-013420 was rapidly distributed into the ELF, while it was slowly distributed into AMs. The mean Tmaxs in ELF and AMs were 2 h and 12 h, respectively. The mean Cmax in ELF was higher than the mean Cmax in plasma, and the mean Cmax in AMs was much higher than that in plasma. The ratios of the AUC0-24 for ELF and AMs to the AUC0-24 for plasma were 20.3 and 244.6, respectively.
TABLE 4.
Pharmacokinetics of S-013420 after a single 400-mg dosea
| Compartment | Cmax (μg/ml) | Tmax (h) | AUC0-24 (μg·h/ml) | t1/2,z (h) |
|---|---|---|---|---|
| Plasma | 0.687 | 2.27 | 10.46 | 17.5 |
| ELF | 16.8 | 2.00 | 212.4 | 14.8 |
| AM | 175.9 | 12.00 | 2559 |
For plasma, n = 28. For ELF and AMs, the calculations were based on the mean concentration at each sampling time. AUC0-24 was calculated by the linear trapezoidal method. It was not possible to calculate the t1/2,z of AM.
Estimates of the PDs from the AUC0-24/MIC90 ratio for the common respiratory pathogens S. aureus, S. pneumoniae, H. influenzae, and M. catarrhalis are summarized in Table 5. The AUC0-24 for plasma/MIC90 ratios for these four pathogens were 41.8, 83.6, 1.3, and 20.9, respectively; and the AUC0-24 for ELF/MIC90 ratios were 849.6, 1,699.2, 26.6, and 424.8, respectively. For macrolide antibiotics, the drug concentration in the lungs is well correlated with efficacy. In the phase 2 study with the two dosages determined by PK/PD analysis as described above, the clinical cure rates were 92.5% and 85.0% (7). The high level of distribution of S-013420 into ELF and AMs showed that it has good PD characteristics.
TABLE 5.
MIC90s for the organisms tested and AUC0-24/MIC90 ratios for S-013420 after a single 400-mg oral dose
| Organism (no. of isolates) | MIC90 (μg/ml) | AUC0-24/MIC90 ratio |
|
|---|---|---|---|
| Plasma | ELF | ||
| S. aureus, erythromycin susceptible (65) | 0.25 | 41.8 | 849.6 |
| S. pneumoniae (115) | 0.125 | 83.6 | 1699.2 |
| H. influenzae (97) | 8 | 1.3 | 26.6 |
| M. catarrhalis (55) | 0.5 | 20.9 | 424.8 |
DISCUSSION
This study, in which the BAL method was used, clearly defined the distribution of S-013420 into the lung ELF and AM compartments. Understanding of the distribution of antimicrobial concentrations in ELF and AMs has been shown to be an important parameter in predicting the clinical outcome and the eradication of bacteria from infected respiratory tracts. ELF especially represents a major site of infection (pneumonia) caused by common bacterial pathogens. In this study, the elevated ELF concentrations of S-013420 were sustained and the mean concentrations of S-013420 in ELF were more than 20 times higher than those in plasma for 10 h after dosing. The increase in the drug concentration in ELF to peak levels showed no delay compared to the time to the increase in plasma (16).
In general, free drug levels are important for prediction of the clinical and bacteriological efficacies of a drug. The protein binding ratio of S-013420 in ELF is not known. However, Sutinen et al. (17) reported on the identification of several proteins in ELF. We performed an experiment and observed 23% protein binding of S-013420 in a mixture of 10 μg/ml S-013420 and 40 mg/ml human serum albumin. Considering the protein content in ELF, we estimate that the protein binding ratio of S-013420 in ELF is ca. 20%. Therefore, the free AUC0-24 for ELF/MIC90 of S-013420 against H. influenzae is speculated to be 21.2, which exceeds the 90% maximum bactericidal activity (10.8) determined from in vitro PK/PD modeling with an H. influenzae strain (MIC, 8 μg/ml). The high intrapulmonary AUC0-24 for ELF/MIC90 ratios support the possibility of the use of a once-daily dosing regimen for the treatment of respiratory infections, even infections caused by H. influenzae.
The daily AUC for ELF required to kill organisms causing infections can be calculated to be 86.4 μg·h/ml. For clinical use, two dosages corresponding to doses that would achieve about 1.5 times and 2 times the AUC of 86.4 μg·h/ml, respectively, were predicted from the following assumption. There is a proportional relationship between the AUC predicted for ELF and the AUC actually obtained for plasma after the administration of a single dose of S-013420. In fact, two dosages were adopted in the phase 2 clinical trial on the basis of these data, and as expected, good efficacy against H. influenzae was obtained (7).
It is well known that the delivery of macrolide analogues to infected regions by phagocytes contributes to the effectiveness of the macrolide analogues (9). Similar behavior by S-013420 should enable it to be effective as well. In this study, S-013420 was seen to be distributed into AMs, resulting in intracellular concentrations of up to 176 ± 83 μg/ml at 12 h after dosing, followed by a slow decline over 24 h to 68 ± 10 μg/ml, indicating that S-013420 has a good potential to be able to eradicate cytosolic Legionella spp. and chlamydiae in the phase 2 study (7). Therefore, it is necessary to know the lung distributions of S-013420 to support clinical trials.
Kiems et al. suggested that the lysis of AMs in ELF is the major technical problem in estimating the concentration in ELF (6). The possibility of technical errors must also be considered. The ranges of SDs for ELF are wider than those for plasma, as shown in Table 3, and this may possibly be caused by the lysis of AMs. However, the Tmax in AMs is 12 h and the Tmax in ELF is 2 h, which is similar to the Tmax in plasma; therefore, these findings do not support the possibility that the high concentration of drug in ELF mainly depends on the lysis of AMs.
Another technical problem is the problem of using urea for correction of the data, which results in overestimation of the ELF volume and a lower drug concentration. This is caused by the diffusion of urea into the BAL fluid recovered because of a prolonged dwell time during the lavage procedure (6, 14). With the insertion of a single dose of sterile saline and immediate aspiration, it is likely that at least 80% of the urea recovered is derived from the urea present in the ELF prior to the lavage procedure (14). In the phase 2 study, the two dosages (1.5 times and 2 times the required daily dosage that results in an AUC for ELF of 86.4 μg·h/ml) based on intrapulmonary pharmacokinetics showed good efficacy. It is therefore possible that the actual and the measured concentrations of S-013420 in ELF may show some errors, but the difference is small.
The sustained penetration of S-013420 in ELF and particularly into AMs, together with its spectrum of activity against typical and atypical pathogens, suggests that S-013420 is a promising new agent for the treatment of respiratory tract infections.
Acknowledgments
We conducted this clinical trial at the Medical Co. LTA Kyushu Clinical Pharmacology Research Clinic in 2006. We really appreciate all the support received from the dedicated staff.
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Baldwin, D. R., R. Wise, J. M. Andrew, J. P. Ashby, and D. Honeybourne. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3:886-890. [PubMed] [Google Scholar]
- 2.Conte, J. E., Jr., J. Golden, S. Duncan, E. Mckenna, E. Lin, and E. Zurlinden. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig, W. A. 2002. The role of pharmacodynamics in effective treatment of community-acquired pathogens. Adv. Studies Med. 2:126-134. [Google Scholar]
- 4.Felmingham, D., R. R. Reinert, Y. Hirakata, and A. Rodloff. 2002. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and comparative in vitro activity of the ketolide, telithromycin. J. Antimicrob. Chemother. 50(Suppl. 1):25-37. [DOI] [PubMed] [Google Scholar]
- 5.Kadota, J., Y. Ishimatsu, T. Iwashita, Y. Matsubara, K. Tomono, M. Tateno, R. Ishihara, C. Muller-Serieyes, and S. Kohno. 2002. Intrapulmonary concentrations of telithromycin, a new ketolide, in healthy Japanese volunteers. Antimicrob. Agents Chemother. 46:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiem, S., and J. J. Schentag. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohno, S., K. Yamaguchi, Y. Tanigawara, A. Watanabe, A. Aoki, Y. Niki, and J. Fujita. 2007. The efficacy, the safety and pharmacokinetics (PK) of S-013420, a bicyclolide in patients with community-acquired pneumonia (CAP), abstr. L-485. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 8.Maki, H., T. Fujimura, Y. Yamano, J. Shimada, and S. Kuwahara. 2005. S-013420, a new bridged bicyclicketolide. I. In vitro antibacterial activity, abstr. F-2029. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Nightingale, C. H. 1997. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr. Infect. Dis. J. 16:438-443. [DOI] [PubMed] [Google Scholar]
- 10.Noreddin, A. M., D. Roberts, K. Nichol, A. Wierzbowski, D. J. Hoban, and G. G. Zhanel. 2002. Pharmacodynamic modeling of clarithromycin against macrolide-resistant [PCR-positive mef(A) or erm(B)] Streptococcus pneumoniae simulating clinically achievable serum and epithelial lining fluid free-drug concentrations. Antimicrob. Agents Chemother. 46:4029-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen, K. M., G. S. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell, Jr. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong, C. T., P. K. Dandekar, C. Sutherland, C. H. Nightingale, and D. P. Nicolau. 2005. Intrapulmonary concentrations of telithromycin: clinical implications for respiratory tract infections due to Streptococcus pneumoniae. Chemotherapy 51:339-346. [DOI] [PubMed] [Google Scholar]
- 13.Patel, K. B., D. Xuan, P. R. Tessier, J. H. Russomanno, R. Quintiliani, and C. H. Nightingale. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnel, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 15.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentration of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada, J., Y. Saisho, and H. Fukase. 2007. The pharmacokinetics (PK), safety and tolerability of S-013420, a novel bicyclolide in healthy Japanese male subjects, abstr. A-799. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 17.Sutinen, S., H. Riska, R. Backman, S. H. Sutinen, and B. Fröseth. 1995. Alveolar lavage fluid (ALF) of normal volunteer subjects: cytologic, immunocytochemical, and biochemical reference values. Respir. Med. 89:85-92. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji, M., H. Miwa, M. Takema, E. Kanaoka, T. Yoshikawa, J. Shimada, and S. Kuwahara. 2005. S-013420, a new bridged bicyclicketolide. II. In vivo activity against experimental animal infection models, abstr. F-2035. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 19.Van Bambeke, F., J. M. Harms, Y. V. Laethem, and P. M. Tulkens. 2008. Ketolides: pharmacological profile and rational positioning in the treatment of respiratory tract infections. Expert Opin. Pharmacother. 9:267-283. [DOI] [PubMed] [Google Scholar]
- 20.Zhanel, G. G., M. DeCorby, A. Noreddin, C. Mendoza, A. Cumming, K. Nichol, A. Wierzbowski, and D. J. Hoban. 2003. Pharmacodynamic activity of azithromycin against macrolide-susceptible and -resistant Streptococcus pneumoniae simulating clinically achievable free serum, epithelial lining fluid and middle ear fluid concentrations. J. Antimicrob. Chemother. 52:83-88. [DOI] [PubMed] [Google Scholar]