Abstract
Steady-state pharmacokinetics of ertapenem were compared in patients after 1-g intravenous and subcutaneous (s.c.) infusions. Bioavailability was 99% ± 18% after s.c. administration, but peaks were reduced by about (43 ± 29 versus 115 ± 28 μg/ml) and times to peak were delayed. Simulations based on unbound concentrations show that time over the MIC should always be longer than 30% to 40% of the dosing interval, suggesting that s.c. infusion could be an alternative in patients with reduced vascular access.
Ertapenem is a recent long-acting, parenteral carbapenem antibiotic mainly indicated in the treatment of community-acquired infections or hospital-acquired infections without suspicion of Pseudomonas or Acinetobacter (5), as an alternative to penicillin-β-lactamase inhibitor combination (10, 19, 23). The pharmacokinetics of ertapenem has been extensively described (2, 3, 6-8, 15, 16, 18, 21). Ertapenem may be administered intravenously (i.v.) or intramuscularly (i.m.) for several days (13), but for many hospitalized patients the i.m. route might be contraindicated due to anticoagulant therapy. Subcutaneous (s.c.) administration is daily safely used with drugs and fluids mostly for dehydrated elderly patients or patients in palliative care when oral or i.v. administration is impossible (20) and could then appear as an interesting alternative. Advantages for the s.c. route over the i.v. route include a similar number of or even fewer complications, cost savings, greater patient comfort, and less nursing time to start and maintain the infusion (1, 22). The aim of this study was to compare the pharmacokinetics of ertapenem at steady state following 30-min i.v. and s.c. infusions in order to determine if s.c. administration of ertapenem, which is not yet approved, could be a viable alternative for i.v. infusion in patients with limited vascular sites.
The study was conducted at the University Hospital of Poitiers (France) after its approval by the local ethics committee (Region Poitou-Charentes CCPPRB, protocol no. 05.12.26). Written informed consent was obtained from each subject or their closest relative if the patient was unconscious. The study enrolled 6 adult male patients suspected of having an infection due to ertapenem-susceptible bacteria (Table 1). Ertapenem was the only antibiotic used, sedation was obtained with propofol and sufentanil, and no other drugs that could have been suspected of interacting with ertapenem pharmacokinetics such as vasopressors or midazolam were used. At study enrollment, patients were mechanically ventilated and exhibited a systemic inflammatory response syndrome. Infection sites justifying ertapenem administration were early-onset ventilator-associated pneumonia (n = 5) and surgical wound infection (patient no. 2). The microorganisms isolated at those infection sites were methicillin-susceptible Staphylococcus aureus (n = 2), Haemophilus influenzae (n = 1), Escherichia coli (n = 1), and Klebsiella pneumoniae (n = 2). Local tolerance was assessed during the 24 h following subcutaneous infusion by checking for erythema, pruritus, hematoma, or necrosis at the insertion site. Ertapenem (Invanz) was purchased from the pharmaceutical company Merck Sharp & Dohme-Chibret (Paris, France) as a dry powder and reconstituted in 50 ml normal saline just before being infused with a pump (Orchestra DPS; Fresenius Vial, Brezins, France). The administration sites were a central vein (i.v.) or the anterior side of a thigh (s.c.). Initially 1 g of ertapenem was administered i.v. over 30 min once daily, and blood samples for the i.v. pharmacokinetic study were collected between the 4th day and the 7th day. The next day, treatment was shifted to the s.c. route and a second series of blood samples was collected during the following 24 h for the s.c. pharmacokinetic study. Ertapenem administration was shifted back to the i.v. route until the end of therapy. Blood samples were drawn via an arterial catheter in heparinized tubes and immediately centrifuged for 10 min at 2,500 × g and 4°C to separate plasma, which was then transferred to storage vials and diluted (1:1) with a stabilizing solution consisting of 1:1 ethylene glycol and 2-(4-morpholino) ethylsulfonic acid at 0.1 mol/liter (pH 6.5). Plasma ultrafiltrates were obtained from plasma samples collected at times 0.5 h and 24 h postdosing by centrifugation with a Centrifree system (CF50A model; Amicon, Molsheim, France). Samples were stored at −80°C until analysis. Ertapenem concentrations were measured using a liquid chromatography method with tandem mass spectrometry detection (12). Within- and between-day variability of the method at various concentrations led to coefficients of variation no greater than 16.4% (n = 11) and accuracies ranging between 98.5% and 110.9%. A compartmental pharmacokinetic analysis for total plasma concentrations was conducted with WinNonLin version 4.0.1. (Pharsight Corporation, Mountain View, CA), using a two-open-compartment model with multiple zero order infusions after i.v. administrations followed by a one-open-compartment model with one zero order infusion after s.c. administration. Duration of infusion was set at a fixed value equal to 0.5 h after i.v. administrations, but estimated by the modeling after s.c. administration. A 1/y weight was used for all the analysis. Unbound concentrations were derived from measured total concentrations using a saturable two-class binding site model with one specific binding site and one nonspecific binding site, previously validated for ertapenem (7). The rate constants for specific and nonspecific binding sites were estimated from the 12 pairs of total and unbound concentrations measured at 0.5 and 24 h and using the mean concentration values of albumin (14.1 g/liter) and the remaining proteins (38.7 g/liter) characteristic of these patients. The same compartmental pharmacokinetic analysis as for total concentrations was conducted with unbound concentrations, and simulations were derived for each subject and each route of administration in order to estimate the percentage of dosing interval during which unbound concentrations would be higher than various breakpoint values, chosen as 1, 2, 4, and 8 mg/liter, in agreement with the Clinical Laboratory Standards Institute. Results are presented as means ± standard deviation (SD), and nonparametric Wilcoxon's rank test was used for statistical comparisons, with P < 0.05 considered as significant.
TABLE 1.
Patient characteristics and individual and mean ± SD pharmacokinetic parameters based on total ertapenem concentrations measured after i.v. and s.c. 30-min infusions of ertapenem (1 g/24 h) to 6 adult patients
| Parametera | Result for patient: |
Mean ± SDb | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Patient characteristics | |||||||
| Age (yr) | 67 | 52 | 19 | 63 | 55 | 81 | 56 ± 19 |
| Body wt (kg) | 72 | 60 | 76 | 90 | 100 | 66 | 77 ± 14 |
| Height (m) | 1.67 | 1.72 | 1.80 | 1.80 | 1.85 | 1.70 | 1.76 ± 0.07 |
| BMI (kg/m2) | 25.8 | 20.3 | 23.5 | 27.8 | 29.2 | 22.8 | 24.9 ± 3.0 |
| SAPS II on admission | 20 | 18 | 21 | 59 | 21 | 30 | 28 ± 14 |
| Albumin concn (g/liter) | 10.0 | 14.4 | 16.8 | 14.3 | 9.5 | 19.6 | 14.1 ± 3.6 |
| Creatinine concn (μmol/liter) | 27 | 37 | 43 | 66 | 86 | 72 | 55 ± 21 |
| Total concn of proteins (g/liter) | 48 | 57 | 65 | 49 | 40 | 58 | 53 ± 9 |
| Fluid balance (ml) | |||||||
| i.v. | + 350 | + 950 | + 200 | + 350 | + 800 | + 100 | +458 ± 340 |
| s.c. | + 500 | + 700 | + 200 | + 150 | + 650 | + 150 | +392 ± 255 |
| ICU outcome | Survived | Survived | Survived | Survived | Survived | Survived | |
| Pharmacokinetics | |||||||
| Cmax (μg/ml) | |||||||
| i.v. | 94 | 124 | 104 | 75 | 148 | 142 | 115 ± 28 |
| s.c. | 25 | 25 | 77 | 19 | 28 | 84 | 43 ± 29* |
| tmax (h) | |||||||
| i.v. | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| s.c. | 2.7 | 1.3 | 2.8 | 4.6 | 2.3 | 2.3 | 2.7 ± 1.1* |
| t1/2 (h) | |||||||
| i.v. | 3.4 | 2.3 | 4.7 | 3.0 | 4.9 | 5.3 | 3.9 ± 1.2 |
| s.c. | 5.1 | 6.3 | 2.8 | 6.6 | 6.4 | 5.3 | 5.4 ± 1.4* |
| AUC0-24 s.c./AUC0-24 i.v. | 0.87 | 1.02 | 1.03 | 1.18 | 0.70 | 1.14 | 0.99 ± 0.18 |
| CL (liters/h) i.v. | 4.7 | 4.4 | 3.1 | 6.2 | 2.6 | 1.7 | 3.8 ± 1.6 |
| Vss (liters) i.v. | 19.4 | 12.6 | 15.0 | 23.1 | 15.3 | 10.9 | 16.1 ± 4.5 |
| fu (%) | |||||||
| i.v. | 45.8 ± 4.4 | 43.4 ± 4.3 | 40.9 ± 3.9 | 45.8 ± 3.3 | 51.4 ± 5.5 | 46.8 ± 4.6 | |
| s.c. | 43.4 ± 1.7 | 41.4 ± 1.5 | 40.4 ± 2.8 | 44.1 ± 0.9 | 48.3 ± 2.2 | 45.3 ± 2.9 | |
BMI, body mass index; SAPS, simplified acute physiology score; ICU, intensive care unit; Cmax, maximal concentration of ertapenem; tmax, time to obtain maximal concentration; t1/2, half-life of elimination; AUC0-24, area under the curve from 0 to 24 h; CL, clearance; Vss, volume of distribution at steady state; fu, unbound fraction of ertapenem.
*, P < 0.05.
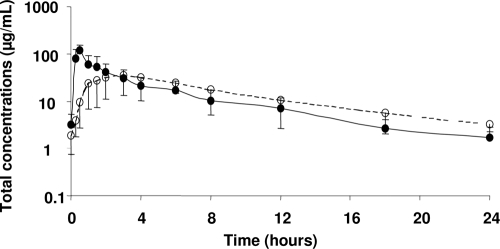
All patients completed the study without any local or systemic adverse effect attributable to ertapenem administration, and signs of infection had disappeared by the end of treatment. Ertapenem plasma concentration-time profiles were shifted to the right after s.c. infusion, with an approximately 3-fold reduction of peak concentrations (Cmax) and 5-fold increase of time to peak concentration (tmax) (Table 1). However, after 3 h postdosing on average, plasma concentrations became higher following s.c. infusion (Fig. 1), and AUCs were virtually identical after both routes of administration, attesting for complete bioavailability following s.c. infusion. However, because only unbound drug has the ability to distribute and to exert antimicrobial activity at the target site of infection, unbound concentrations should be considered to predict efficacy (4, 14). In this study, ertapenem protein binding demonstrated no sign of nonlinearity and was relatively limited, with unbound fractions (fu) ranging from 40.4% ± 2.8% to 51.4% ± 5.5% (Table 1), consistent with the value (fu = 54.8% ± 19.1%) recently reported by Burkhardt et al. in critical care patients (7), but much higher than the average value (16% unbound corresponding to 84% bound) currently reported in healthy volunteers (15, 20). Because ertapenem antimicrobial activity is considered to be time dependent (9), a peak reduction after s.c. administration may not have major consequences on its clinical efficacy. Instead, the dosing interval during which unbound drug concentration exceeds the MIC (t > MIC), represents the most relevant pharmacokinetics/pharmacodynamics parameter (17), and a t > MIC of 30 to 40% of the dosing interval should be effective (11). Conducted simulations suggested that for susceptible and intermediately susceptible microorganisms (MIC ≤ 4 mg/liter), t > MIC based upon unbound ertapenem concentrations should always be longer than 30% to 40% of the dosing interval, independently of the route of administration. In conclusion, this study suggests that s.c. infusion of ertapenem should be equivalent to i.v. infusions in terms of efficacy and could therefore represent an interesting alternative for patients with reduced vascular access, such as dehydrated elderly patients or patients in palliative care. However, this should be confirmed in a larger population of such patients.
FIG. 1.
Mean ± SD total ertapenem concentrations in plasma after multiple daily intravenous infusions (1 g over 30 min) followed by a subcutaneous infusion (1 g over 30 min) in 6 patients. Closed symbols and the solid line correspond to intravenous infusion, and open symbols and the dashed line correspond to subcutaneous infusion.
Acknowledgments
Vipul Kumar from the Medical School of Hannover (Germany) is gratefully acknowledged for providing information required to perform the binding modeling of ertapenem. We thank Pharsight Corporation for supplying WinNonLin through the PAL program.
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Barbot, A., N. Venisse, F. Rayeh, S. Bouquet, B. Debaene, and O. Mimoz. 2003. Pharmacokinetics and pharmacodynamics of sequential intravenous and subcutaneous teicoplanin in critically ill patients without vasopressors. Intensive Care Med. 29:1528-1534. [DOI] [PubMed] [Google Scholar]
- 2.Boselli, E., D. Breilh, M. C. Saux, J. B. Gordien, and B. Allaouchiche. 2006. Pharmacokinetics and lung concentrations of ertapenem in patients with ventilator-associated pneumonia. Intensive Care Med. 32:2059-2062. [DOI] [PubMed] [Google Scholar]
- 3.Brink, A. J., G. A. Richards, V. Schillack, S. Kiem, and J. Schentag. 2008. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents 33:432-436. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt, O., M. Brunner, S. Schmidt, M. Grant, Y. Tang, and H. Derendorf. 2006. Penetration of ertapenem into skeletal muscle and subcutaneous adipose tissue in healthy volunteers measured by in vivo microdialysis. J. Antimicrob. Chemother. 58:632-636. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt, O., H. Derendorf, and T. Welte. 2007. Ertapenem: the new carbapenem 5 years after first FDA licensing for clinical practice. Expert Opin. Pharmacother. 8:237-256. [DOI] [PubMed] [Google Scholar]
- 6.Burkhardt, O., C. Hafer, A. Langhoff, V. Kaever, V. Kumar, T. Welte, H. Haller, D. Fliser, and J. T. Kielstein. 2009. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrol. Dial. Transplant. 24:267-271. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt, O., V. Kumar, D. Katterwe, J. Majcher-Peszynska, B. Drewelow, H. Derendorf, and T. Welte. 2007. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J. Antimicrob. Chemother. 59:277-284. [DOI] [PubMed] [Google Scholar]
- 8.Chen, M., A. N. Nafziger, G. L. Drusano, L. Ma, and J. S. Bertino, Jr. 2006. Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob. Agents Chemother. 50:1222-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, W. 2002. Pharmacodynamics of antimicrobials: general concepts and application, p. 1-22. In C. H. Nightingale, M. T. Murakawa, and P. G. Ambrose (ed.), Antimicrobial pharmacodynamics in theory and clinical practice. Marcel-Dekker, New York, NY.
- 10.Dinubile, M. J., I. R. Friedland, C. Y. Chan, M. R. Motyl, H. Giezek, K. McCarroll, M. Shivaprakash, J. P. Quinn, R. A. Weinstein, and J. W. Chow. 2007. Bowel colonization with vancomycin-resistant enterococci after antimicrobial therapy for intra-abdominal infections: observations from 2 randomized comparative clinical trials of ertapenem therapy. Diagn. Microbiol. Infect. Dis. 58:491-494. [DOI] [PubMed] [Google Scholar]
- 11.Fonzo-Christe, C., C. Vukasovic, A. F. Wasilewski-Rasca, and P. Bonnabry. 2005. Subcutaneous administration of drugs in the elderly: survey of practice and systematic literature review. Palliat. Med. 19:208-219. [DOI] [PubMed] [Google Scholar]
- 12.Lefeuvre, S., N. Venisse, S. Marchand, M. Bachelet, and W. Couet. 2008. A simple and sensitive liquid chromatography-tandem mass spectrometry assay for the quantification of ertapenem in microdialysate. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 862:242-245. [DOI] [PubMed] [Google Scholar]
- 13.Legua, P., J. Lema, J. Moll, Q. Jiang, G. Woods, and I. Friedland. 2002. Safety and local tolerability of intramuscularly administered ertapenem diluted in lidocaine: a prospective, randomized, double-blind study versus intramuscular ceftriaxone. Clin. Ther. 24:434-444. [DOI] [PubMed] [Google Scholar]
- 14.Liu, P., and H. Derendorf. 2003. Antimicrobial tissue concentrations. Infect. Dis. Clin. North Am. 17:599-613. [DOI] [PubMed] [Google Scholar]
- 15.Majumdar, A. K., D. G. Musson, K. L. Birk, C. J. Kitchen, S. Holland, and J. McCrea. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob. Agents Chemother. 46:3506-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mistry, G. C., A. K. Majumdar, S. Swan, D. Sica, A. Fisher, Y. Xu, M. Hesney, L. Xi, J. A. Wagner, and P. J. Deutsch. 2006. Pharmacokinetics of ertapenem in patients with varying degrees of renal insufficiency and in patients on hemodialysis. J. Clin. Pharmacol. 46:1128-1138. [DOI] [PubMed] [Google Scholar]
- 17.Mouton, J. W., D. J. Touzw, A. M. Horrevorts, and A. A. Vinks. 2000. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin. Pharmacokinet. 39:185-201. [DOI] [PubMed] [Google Scholar]
- 18.Musson, D., A. Majumdar, S. Holland, K. Birk, P. Winckersham, and S. Li. 2004. Pharmacokinetics of total and unbound ertapenem in healthy elderly subjects. Antimicrob. Agents Chemother. 48:521-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namias, N., J. S. Solomkin, E. H. Jensen, J. E. Tomassini, and M. A. Abramson. 2007. Randomized, multicenter, double-blind study of efficacy, safety, and tolerability of intravenous ertapenem versus piperacillin/tazobactam in treatment of complicated intra-abdominal infections in hospitalized adults. Surg. Infect. 8:15-28. [DOI] [PubMed] [Google Scholar]
- 20.Nix, D. E., A. K. Majumdar, and M. J. DiNubile. 2004. Pharmacokinetics and pharmacodynamics of ertapenem: an overview for clinicians. J. Antimicrob. Chemother. 53:23-28. [DOI] [PubMed] [Google Scholar]
- 21.Pletz, M. W., M. Rau, J. Bulitta, A. De Roux, O. Burkhardt, and G. Kruse. 2004. Ertapenem pharmacokinetics and impact on intestinal microflora, in comparison to those of ceftriaxone, after multiple dosing in male and female volunteers. Antimicrob. Agents Chemother. 48:3765-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remington, R., and T. Hultman. 2007. Hypodermoclysis to treat dehydration: a review of the evidence. J. Am. Geriatr. Soc. 55:2051-2055. [DOI] [PubMed] [Google Scholar]
- 23.Solomkin, J. S., A. E. Yellin, O. D. Rotstein, N. V. Christou, E. P. Dellinger, and J. M. Tellado. 2003. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intra-abdominal infections: results of a double-blind, randomized comparative phase III trial. Ann. Surg. 237:235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]