Abstract
Building on previous findings that amiloride analogues inhibit HIV-1 replication in monocyte-derived macrophages (MDM), Biotron Limited has generated a library of over 300 small-molecule compounds with significant improvements in anti-HIV-1 activity. Our lead compound, BIT225, blocks Vpu ion channel activity and also shows anti-HIV-1 activity, with a 50% effective concentration of 2.25 ± 0.23 μM (mean ± the standard error) and minimal in vitro toxicity (50% toxic concentration, 284 μM) in infected MDM, resulting in a selectivity index of 126. In this study, we define the antiretroviral efficacy of BIT225 activity in macrophages, which are important drug targets because cells of the monocyte lineage are key reservoirs of HIV-1, disseminating virus to the peripheral tissues as they differentiate into macrophages. In assays with acutely and chronically HIV-1Ba-L-infected MDM, BIT225 resulted in significant reductions in viral integration and virus release as measured by real-time PCR and a reverse transcriptase (RT) activity assay at various stages of monocyte-to-macrophage differentiation. Further, the TZM-bl assay showed that the de novo virus produced at low levels in the presence of BIT225 was less infectious than virus produced in the absence of the compound. No antiviral activity was observed in MDM chronically infected with HIV-2, which lacks Vpu, confirming our initial targeting of and screening against this viral protein. The activity of BIT225 is post-virus integration, with no direct effects on the HIV-1 enzymes RT and protease. The findings of this study suggest that BIT225 is a late-phase inhibitor of the viral life cycle, targeting Vpu, and is a drug capable of significantly inhibiting HIV-1 release from both acute and chronically infected macrophages.
Vpu, encoded by human immunodeficiency virus type 1 (HIV-1) but not HIV-2, is an integral membrane protein with multiple activities that facilitate the budding and release of newly formed virions from infected human cells (for reviews, see references 5, 16, and 24). Vpu affects both the efficiency of release and the infectivity of new virus particles. It is therefore a potential target for antiviral drugs. Vpu is located primarily in the membranes of the endoplasmic reticulum and Golgi compartments in infected cells. The steady-state level of Vpu expressed at the plasma membrane varies from low to undetectable, depending on the virus isolate and Vpu variant. The mechanisms by which Vpu enhances virus release are complex and not well understood. Recent research has shown that Vpu counteracts the effects of the cell surface protein tetherin (CD137), which inhibits the release of a range of virus particles, including HIV-1 (26).
Macrophages are widely acknowledged to be a major target for infection by HIV-1, with vaginal macrophages reported to be highly permissive (35), and it has been argued previously that infection of individuals by macrophage-tropic viral strains is necessary and sufficient for the development of AIDS (33). In rhesus macaques that have been infected with highly pathogenic simian-human immunodeficiency virus (SHIV) strains, plasma viremia can be almost completely sustained by tissue macrophages following complete depletion of the CD4+ T cells (17). Macrophages are relatively long-lived cells that are less prone to cell death as a result of HIV-1 infection than other cells and are therefore likely to be a major site of viral latency. With the half-lives of macrophages reported to be of the order of months to years, depending on the specific type of macrophage (14), and with reports of higher concentrations of antiviral drugs being required to inhibit HIV-1 replication in macrophages than in T cells (3), infected macrophages pose a serious problem for viral eradication with current antiretroviral therapy (1, 2, 3, 15, 28, 36, 37, 44). In addition to the challenge of latently infected resting memory T cells, the problem of long-lived infected macrophages in the successful treatment of HIV-1 infection is highlighted by recently determined viral decay kinetics with current treatment regimens (25). Further, by using a single-copy viral load assay, more than 80% of patients receiving recommended antiretroviral therapies were found to have quantifiable viremia for at least 2 years after the initiation of therapy (21). The authors of the study suggest that persistent viremia during antiretroviral therapy is likely to be derived from reservoirs of long-lived virus-producing cells that are not affected by currently available drugs that target new cycles of viral replication. Lastly, infiltration by infected macrophages is the major route of infection of the central nervous system, causing HIV-1-associated AIDS dementia in 15 to 20% of late-stage AIDS patients (41). The poor penetration of some antiretroviral drugs into the cerebrospinal fluid may result in the development of a sanctuary site, where macrophages, in addition to microglial cells, can result in persistent viremia. If HIV-1 infection is to be cured, new strategies that will eradicate these reservoirs will be required.
The HIV-1 Vpu protein is a nonstructural virus protein with multiple roles in the viral life cycle. One of Vpu's activities is as a virus-encoded ion channel (also called a viroporin), and Biotron's antiviral compound library was initially developed and screened with the aim of finding inhibitors of the Vpu ion channel (11, 9). Subsequently, compounds that block the Vpu ion channel were found to have strong anti-HIV-1 activity in monocyte-derived macrophages (MDM) (10). Here, we describe the results for our lead compound, BIT225, which has been successful in preclinical safety and pharmacology studies and a recently completed phase I clinical investigation.
MATERIALS AND METHODS
Virus strains.
The laboratory-adapted R5-tropic virus HIV-1B-aL was grown in HIV-1-seronegative peripheral blood mononuclear cells (PBMC) to a level of 8 × 104 50% tissue culture infective doses (TCID50)/ml, and titers in PBMC were determined. Additional R5-tropic primary HIV-1 isolates G-92NG003-G3 (subtype G), 92TH001 (subtype A/E), and 93BR029 (subtype F) were obtained courtesy of the AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH) and were also grown in HIV-1-seronegative PBMC, after which titers in PBMC were determined.
Experiments using X4-tropic strains (92UG029 [subtype A] and 98IN017 [subtype C]) and drug-resistant strains (strain 5705.72, resistant to nucleoside reverse transcriptase [RT] inhibitors [zidovudine and lamivudine]; strain 1064.52, resistant to protease inhibitors [amprenavir, atazanavir, lopinavir {LPV}, indinavir, nelfinavir, ritonavir, and saquinavir {SQV}]; and strain MDR3761, resistant to multiple drugs [zidovudine, dideoxyinosine, lamivudine, stavudine, nevirapine, indinavir, SQV, and nelfinavir]) were performed by Southern Research Institute (SRI; Frederick, MD).
Two HIV-2 clinical isolates isolated from Gambian patients in 1988 were obtained courtesy of the AIDS Research and Reference Reagent Program (from Robin Weiss). An isolate from a patient with AIDS, HIV-2CBL-20 (2 × 105 TCID50/ml), a very cytopathic strain which grows rapidly and produces high levels of RT, was compared to the less cytopathic strain HIV-2CBL-23 (105 TCID50/ml), isolated from a patient without HIV-related symptoms. Unless otherwise stated, a multiplicity of infection (MOI) of 0.05 was used to infect MDM with HIV-1 and HIV-2.
Compounds.
BIT225 was manufactured under contract by Dr. Reddy's Laboratories Ltd. (Hyderabad, India), prepared by dissolving stock in dimethyl sulfoxide (DMSO), and further diluted in culture medium to working concentrations. The nonnucleoside RT inhibitor (NNRTI) efavirenz (EFV) and the protease inhibitors LPV and SQV were obtained from the NIH AIDS Research and Reference Reagents Program.
Analysis of drug interactions.
The assay format for the combination studies examined all possible combinations of five concentrations of drug A and eight concentrations of drug B in a checkerboard format. p24 was used for the assay end point, and a combination cytotoxicity analysis using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] end point was performed in parallel. The data obtained in these assays were imported into the Prichard and Shipman MacSynergy II program (29) for data analysis. Briefly, the MacSynergy II program calculates the theoretical additive interactions of the drugs based on the Bliss Independence mathematical definition of expected effects for drug-drug interactions and assumes that the drugs act independently of each other. Each data point was derived from five samples, and the data were used to create three-dimensional plots. The statistical relevance of the data was analyzed and plotted at one of three confidence levels (68, 95, and 99%).
Vpu phospholipid bilayer inhibition assay.
We have reported previously that the Vpu protein of HIV-1 forms cation-selective ion channels in planar phospholipid bilayers (11) and that certain amiloride analogues are able to inhibit the flow of ions through the Vpu channel (9). Essentially, the same methods were used here to test inhibition of Vpu channels by BIT225. Simply, a synthetic peptide corresponding to the transmembrane α-helical (TMH) domain (residues 1 to 32) of Vpu was reconstituted into a lipid bilayer, resulting in ion channel formation due to homo-oligomerization of the peptide. First, the cis and trans chambers of the bilayer apparatus were filled with buffer containing 50 mM KCl in 10 mM MES (morpholineethanesulfonic acid) buffer (pH 7.4). Then a lipid mixture of palmitoyl-oleoyl-phosphatidylethanolamine and palmitoyl-oleoyl-phosphatidylcholine (50:50) dissolved in n-decane was used to paint the bilayer across a small aperture (∼100 μm in diameter) separating the two chambers. Finally, the capacitance of the bilayer was characterized electrically to confirm nonconduction of ions prior to the addition of the Vpu peptide. The Vpu peptide was synthesized and purified as described previously (9), and aliquots were dissolved in 2,2,2-trifluoroethanol initially at 0.15 mg/ml. Samples (1 μl containing 150 ng of peptide) were added to both the cis and trans chambers, and solutions in both chambers were stirred to facilitate spontaneous incorporation of the peptide into the bilayer. Channel activity, measured as a K+ ion current, was detected after approximately 25 min of stirring at a holding potential of −100 mV (trans relative to cis). For recording, the holding potential was reduced to −40 mV and the data were filtered at 1 kHz and digitized at 2 kHz. For subsequent analysis and presentation, data traces were filtered at 200 Hz and mean currents were determined over a period of 10 to 30 s.
Preparation of cells. (i) MDM.
PBMC were isolated from seronegative blood samples (Australian Red Cross Blood Service, Sydney) via standard Ficoll-Paque gradient centrifugation. Monocytes were further isolated via adherence enrichment and plated into a 96-well flat-bottom plate at a density of 2 × 105 cells per well in a mixture containing Dulbecco's modified Eagle medium (DMEM; GIBCO [Invitrogen, Carlsbad, CA]), 10% heat-inactivated male AB serum (Sigma-Aldrich, St. Louis, MO), and 2 mM l-glutamine and 50 μg/ml gentamicin (Sigma-Aldrich) (hereinafter referred to as DMEM10+). Cells were further cultured for up to 14 days to allow the monocytes to differentiate into macrophages (18).
PBMC were activated using phytohemagglutinin (PHA; Sigma) at 2.5 μg/ml for 3 days and cultured in RPMI medium supplemented with 10% fetal calf serum (CSL Biosciences, Parkville, VIC, Australia), 20 mM l-glutamine (Sigma-Aldrich), and 200 U/ml penicillin-200 μg/ml streptomycin (GIBCO; Invitrogen) (R10+ medium) and in RPMI medium supplemented with 20 U of interleukin-2 (Roche Molecular Biochemicals, Indianapolis, IN).
(ii) Cell lines.
The indicator cell line TZM-bl and T-cell lines were obtained from the AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH; the TZM-bl cell line was courtesy of John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.). TZM-bl cells were cultured in DMEM supplemented with 10% fetal calf serum, and the T-cell lines were cultured in R10+ medium.
Evaluation of HIV-1 infection. (i) RT activity assay.
An RT activity assay was used to quantify the amounts of virus within the culture supernatants via a chemiluminescence enzyme-linked immunosorbent assay (ELISA) that detects the activity of the HIV RT enzyme (38). Briefly, CovaLink 96-well plates (NUNC, Rochester, NY) were coated with poly(A) (Roche Diagnostics), and the lysed virus was added to the plates. During the conversion of RNA into DNA and during DNA strand formation, the viral RT randomly incorporates a labeled nucleoside, dUTP-digoxigenin (Roche Diagnostics), which was detected with the addition of antidigoxigenin tagged with horseradish peroxidase (Roche Diagnostics). The amount of incorporated labeled nucleoside was determined via the color change when the enzyme conjugate was exposed to the substrate, 3,3′,5,5′-tetramethylbenzidine (Sigma), and the reaction was stopped with 1 M sulfuric acid. The intensity of the color was quantified by measuring the absorbance at 450 nm. By using a recombinant RT enzyme (Roche Diagnostics) to generate a standard curve, unknown samples were identified.
(ii) Real-time PCR assay.
A real-time PCR assay was used to determine the total amounts of HIV-1 DNA (gag) within cell lysates. Briefly, MDM were washed twice with phosphate-buffered saline (PBS), lysed, and stored at −80°C for real-time PCR. Real-time PCR for HIV-1 long terminal repeat (LTR)-gag DNA was performed using primers and molecular beacons as described previously (19). HIV-1 primers SL19 (5′-TCTCTAGCAGTGGCGCCCGAACA-3′) and SL20 (5′-TCTCCTTCTAGCCTCCGCTAGTC-3′) and the matching molecular beacon {5′-6-carboxyfluorescein-CGGGAGTACTCACCAGTCGCCGCCCCTCGCCCTCCCG-DABCYL [4-(4′-dimethylaminophenylazo)benzoic acid]-3′} cross the primer binding site and LTR and detect full-length provirus. Estimation of the cell number by using the albumin DNA copy number was performed with the primers 5′-TGCATGAGAAAACGCCAGTAA-3′ and 5′-ATGGTCGCCTGTTCACCAA-3′ and the molecular beacon 5′-6-carboxyfluorescein-CGCCATGACAGAGTCACCAAATGCTGCACAGAATGGCC-DABCYL-3′ under the conditions used for HIV-1 LTR-gag real-time PCR. Amplifications were performed using a model 7700 system (PerkinElmer, Branchburg, NJ). Albumin and HIV-1 DNA copy numbers were subsequently used to calculate the percentage of HIV-1-infected cells among the total cells in culture.
(iii) TZM-bl assay.
The TZM-bl indicator cell line contains a luciferase gene, which is activated upon HIV-1 integration into the host cell genome. HIV-1 integration was measured using lysed cells treated with luciferin, a substrate that is sensitive to luciferase enzymatic activity, from a luciferase reporter assay kit (BD Biosciences, San Jose, CA). Light emitted by the luciferin reaction was detected by an illuminometer (FLUOstar Optima; BMG Labtech, VIC, Australia). The percent inhibition of HIV-1 infection by BIT225 was determined relative to the level of infection in the DMSO-treated control.
In vitro antiviral activity assays with BIT225. (i) Dose-response assay.
MDM were allowed to differentiate over a 14-day period, with medium changes as required. Day 14 MDM were infected with HIV-1Ba-L for 3 h and washed three times with PBS (GIBCO; Invitrogen) at room temperature. Cells were cultured in DMEM10+ in the presence of BIT225 at concentrations decreasing from 10 to 0 μM, with the 0 μM preparation containing a volume of DMSO equivalent to the BIT225 volume in the other preparations (Sigma). Samples of culture supernatants were taken on day 7 postinfection and post-BIT225 addition and analyzed for RT activity. Raw data were converted into percentages of viral control growth, and nonlinear regression analysis was conducted using Prism GraphPad software to establish a dose-response curve and a 50% effective concentration (EC50).
(ii) In vitro acute infection assay.
As in the dose-response analysis, the macrophage monolayer was incubated with virus for 3 h and washed three times with PBS. Cells were cultured in DMEM10+ with 10 μM BIT225, with an equal amount of DMSO used as a control. Samples were routinely taken to measure replication kinetics.
(iii) In vitro chronic infection assay.
To encourage greater infection of the monolayer, the day 14 MDM were incubated with virus for 3 h and left in culture for a further 7 days without being washed to remove virus. Seven days postinfection, the monolayer was washed three times with PBS and 10 μM BIT225 was added. Samples of culture supernatant were routinely taken to measure viral replication kinetics by the RT activity assay.
(iv) Cytotoxicity assay with BIT225.
The cytotoxicity of BIT225 for MDM was measured using the metabolism of thiazolyl blue tetrazolium bromide {MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide]; Sigma} in a 96-well plate format. On day 7 post-compound addition, half of the medium was removed from the culture and 100 μg of MTT was added to each culture well in a volume of 20 μl of 5-mg/ml stock. The culture was incubated for 5 h at 37°C in 5% CO2 to ensure the development of formazan crystals within the viable cells. Upon completion of incubation, cells were permeabilized and formazan crystals were dissolved with acidified isopropanol containing 10% Triton X-100. The absorbance was read at a wavelength of 560 nm, with higher values associated with greater cell viability and therefore less toxicity. The percent cell viability was calculated relative to the quantity of viable cells in the DMSO-treated controls.
(v) Late-phase inhibitor assay.
TZM-bl cells were infected with HIV-1Ba-L in the presence of either BIT225 (0 to 10 μM) or the antiretroviral compound EFV (10 nM), LPV (100 nM), or SQV (10 nM). If compounds are capable of inhibiting the virus at the stages of preintegration and/or integration, the luciferase gene will not be expressed and luciferase activity will not be detected as described above.
(vi) Analysis of BIT225 interaction with RT enzyme.
In an analysis using the RT assay described above, BIT225 or antiretroviral compounds were added to the initial reverse transcription reaction mixture containing a known amount of recombinant RT enzyme. Inhibition of RT activity provides evidence of direct drug effects upon the RT enzyme or upon the process of reverse transcription.
(vii) Protease activity assay.
A cell-free fluorometric assay using synthetic HIV-1 protease substrate 1 (Sigma) was used to determine if BIT225 exerted any effects upon the HIV-1 protease enzyme (Sigma). The substrate contains a fluorophore emitter and a quencher on either side of a peptide cleavage site. The addition of the HIV-1 protease results in the cleavage of the peptide, and the light emitted over time is proportional to the activity of the protease enzyme when measured using a spectrophotometer. BIT225 was premixed with a 2 μM concentration of the protease substrate to obtain various BIT225 concentrations ranging from 1 to 100 μM. Fluorescence was read at 490 nm with an illuminometer (FLUOstar Optima) every 5 s for 5 min to determine background fluorescence and autofluorescence levels with an excitation wavelength set at 340 nm. HIV-1 protease was added at 1.5%, and the enzymatic degradation of the substrate was monitored for a further 5 min, with readings taken every 5 s in the presence or absence of the drug.
Transmission electron micrography (TEM).
Chronically infected MDM were plated onto six-well Primaria plates (BD Falcon, Franklin Lakes, NJ) at 9 × 106 cells/well. Following 7 days of BIT225 or DMSO treatment, cells were fixed for 3 h with Karnovsky's fixative and washed with MOPS (Sigma-Aldrich). Fixed MDM were dehydrated in increasing concentrations of anhydrous ethanol, 10, 30, 50, 70, 90, and 100%, for 30 min at each step, with two steps at 100% ethanol. Following dehydration, the cells were infiltrated with increasing concentrations of LR White resin, 25, 50, 75, and 100%, in ethanol for 8 h at each step. After a second change of 100% resin, the cells were embedded in fresh resin in gelatin capsules and allowed to gently sink to the bottom to form a loose pellet. The gelatin capsules were capped to exclude air, and the resin was polymerized in an oven at 60°C for 24 h. MDM samples embedded in resin blocks were sectioned with a Diatome diamond knife on a Leica Ultracut R microtome, and ultrathin sections (90 nm thick) were collected onto pioloform-coated 100-mesh hexagonal copper grids. The sections on grids were sequentially stained with 2% aqueous uranyl acetate for 10 min followed by a triple lead stain for 5 min and viewed with a Phillips CM120 Biotwin transmission electron microscope at 120 kV. Micrographs were captured with a Gatan MultiScan 600CW charge-coupled device camera.
Western blot analysis.
Briefly, chronically infected MDM lysates were obtained from cell cultures that had been grown with and without BIT225 and other antiretroviral compounds. Total protein was quantified using the Lowry method, and results for samples were normalized to ensure that equal amounts were loaded onto the gel. β-Actin expression levels confirmed that equal amounts of total protein were loaded into the lanes. Samples were electrophoresed on a precast 10% polyacrylamide gel (iGel; Gradipore, Hawthorne, NY) and transferred onto a nitrocellulose membrane (Amersham Bioscience, Sweden). Blots were probed with serum from an HIV-1-seropositive donor and washed before incubation with a secondary antibody, anti-human IgG conjugated with horseradish peroxidase. Proteins were detected using the ECL detection system (Amersham Biosciences).
RESULTS
BIT225 is a novel anti-HIV-1 compound targeting the Vpu ion channel.
We have reported previously that the Vpu protein of HIV-1 forms cation-selective ion channels in planar phospholipid bilayers (11) and that certain amiloride analogues, namely, hexamethylene amiloride and dimethyl amiloride, are able to inhibit the flow of ions through the Vpu channel (9). Based on these initial observations, an iterative program of chemical synthesis and activity screening was initiated to explore the chemical space around the amiloride analogues. This has led to structural diversification and the identification of new classes of compounds with greater potency and lower toxicity than previously existing compounds and the potential for further development. In all, a library of over 300 compounds was generated and screened, after which BIT225 was selected as the leading compound for further testing as a potential new anti-HIV compound (Fig. 1a).
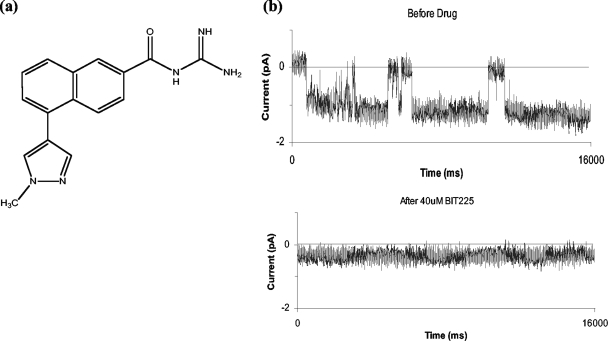
FIG. 1.
BIT225, a novel inhibitor of HIV-1 Vpu ion channels. Here we show the chemical structure of BIT225 (a), a novel compound that inhibits the Vpu ion channel when used in a phospholipid bilayer assay (b). The traces in panel b are continuous 16-s data sets representative of ion currents detected at a holding potential of −40 mV before and after addition of BIT225 (40 mM final concentration) to the cis and trans chambers.
BIT225 {N-[5-(1-methyl-1H-pyrazol-4-yl)-naphthalene-2-carbonyl]-guanidine; CAS no. 917909-71-8} is a novel compound, which planar lipid bilayer experiments confirm is able to inhibit the ion channel activity associated with the transmembrane domain of HIV-1 Vpu. After the addition of BIT225 (40 μM), typical channel opening and closing transitions seen prior to compound addition are absent from the data traces and the average ion current across the bilayer is reduced to near the baseline (Fig. 1b).
BIT225 inhibits the release of HIV-1 in a dose-dependent manner that is not a result of cellular toxicity.
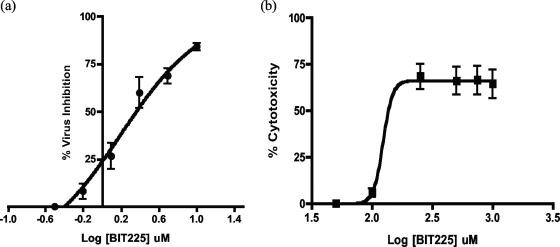
BIT225 was titrated in a descending concentration range from 10 to 0 μM in HIV-1Ba-L-infected, day 14 MDM. HIV-1 levels in the supernatant, quantitated using the RT activity assay, demonstrated a concentration-dependent curve, with the inhibition of virus release by BIT225 greatest at 10 μM and lowest at 0.625 μM (n = 6). Nonlinear regression analysis using Prism GraphPad software established a dose-response curve, and the mean EC50 of BIT225 ± the standard error (SE) was calculated to be 2.25 ± 0.23 μM (Fig. 2a).
FIG. 2.
Mean EC50 and TC50 dose-response curves for BIT225 treatment of HIV-1Ba-L-infected MDM. Shown are the mean dose-response curves for MDM, with bars representing standard errors of the means, indicating the effects of BIT225 on HIV-1Ba-L replication in human MDM (a) and the effects of BIT225 on day 14 MDM viability (b). The mean EC50 of BIT225 ± SE was 2.25 ± 0.23 μM (n, 6 separate experiments with each drug concentration assayed in triplicate), and the mean TC50 was 284 ± 22 μM (n = 3), resulting in a selectivity index of 126.
To ensure that the inhibition of HIV-1 release was not due to cytotoxic effects of BIT225, cytotoxicity assays of the treated MDM were always performed in parallel with the antiviral activity assays. Results consistently demonstrated minimal cytotoxicity associated with the treatment of MDM with BIT225 within the concentration range from 0 to 10 μM. By using the MTT assay, >96% cell viability in BIT225-treated samples compared DMSO-treated controls (n = 8) was detected. In addition, no cytotoxicity was observed when the TZM-bl indicator cell line was cultured in the presence of BIT225 at concentrations of up to 20 μM (n = 6) (data not shown). To establish a 50% toxic concentration (TC50) of BIT225 for HIV-1-infected MDM, the concentration range was extended to 1,000 μM (Fig. 2b). The percent cell viability was calculated, and the mean TC50 ± SE was determined to be 284 ± 22 μM (n = 3) by nonlinear regression analysis, resulting in a selectivity index of 126.
Profile of BIT225 activities against a panel of R5-, X4-, and dual-tropic and drug-resistant HIV-1 strains.
The extent of BIT225 antiviral activity was further assessed with a panel of HIV-1 primary isolates of various subtypes and tropisms. The greatest activity was observed with the R5-tropic and dual-tropic viruses, with similar EC50s determined for the different subtypes. Moderate activity against the primary X4 viruses treated with BIT225 was also observed. In addition, BIT225 greatly inhibited both single-drug- and multidrug-resistant viral isolates (Table 1). MDM were infected with various HIV-1 subtypes with different tropisms, and the effects of BIT225 were determined by comparing BIT225-treated samples to DMSO-treated controls. We also examined the effects of BIT225 on patterns of resistance to individual and multiple currently used antiretroviral drugs.
TABLE 1.
BIT225 demonstrates strong activities against a wide panel of HIV-1 isolatesa
| HIV strain | Descriptionb | Mean EC50 (mM) of BIT225 | Mean TC50 (mM) of BIT225 | Selectivity index |
|---|---|---|---|---|
| Ba-L* | R5, subtype B | **2.25 | 284 | 126 |
| G-92NG003-G3* | R5, subtype G | 1.6 | 210 | 131 |
| 92TH001* | R5, subtype A/E | 1.5 | 210 | 140 |
| 93BR029* | R5, subtype F | 1.9 | 210 | 111 |
| 92UG029 | X4, subtype A | 14.4 | 202 | 14 |
| 98IN017 | X4, subtype C | 42.2 | 202 | 4.8 |
| 5705.72 | RTI resistant | 5.1 | 202 | 39.3 |
| 1064.52 | PI resistant | 2.7 | 167 | 61.9 |
| MDR3761 | Multidrug resistant | 5.1 | 202 | 39.3 |
Shown are mean EC50s and TC50s from data generated by SRI, Frederick, MD (n = 3), and in-house experiments. Strains tested in in-house experiments are marked by asterisks. For in-house data, double asterisks indicate an n value of 6; otherwise, n is 3.
RTI, RT inhibitor; PI, protease inhibitor.
The anti-HIV-1 activity of BIT225 was evaluated in two-drug combination studies with EFV, LPV, and tenofovir (PMPA). Each combination was tested in human macrophages acutely infected with the laboratory-adapted strain HIV-1Ba-L. Viral inhibition was assessed, and the cytotoxicity of each combination was also evaluated in parallel with the antiviral activity. Analyses of drug interactions for each of the combinations were performed using the Prichard and Shipman MacSynergy II three-dimensional model for statistical evaluation of combination assays. For these studies, synergy is defined by the drug concentration yielding synergy volumes greater than 50 μM2%. A numerical value for the synergy volume (expressed in square micromolar percent) defines the level of synergism, as follows: >100, highly synergistic; 51 to 100, slightly synergistic; 50 to −50, additive; −50 to −100, slightly antagonistic; and <−100, highly antagonistic. BIT225 and PMPA were found to be additive with regard to antiviral activity, BIT225 and EFV were determined to be additive to slightly synergistic, and BIT225 and LPV were slightly to highly synergistic (SRI; data not shown).
Development of a robust HIV-1 model to determine the full extent of BIT225 anti-HIV-1 activity in MDM.
BIT225 anti-HIV-1 activity was evident at multiple time points during the differentiation of monocytes into macrophages. Monocytes isolated from peripheral blood and cultured for various numbers of days (from 3 to 15) using adherence to allow differentiation into macrophages are employed routinely in experiments to study HIV-1, as described in the literature (1, 4, 7, 10, 18, 30, 31, 34). Here, we aimed to determine the permissiveness of MDM to HIV-1 infection after various days in culture and to evaluate the effects of BIT225 treatment on viral replication. Blood monocytes were differentiated for 7, 10, and 14 days and acutely infected with HIV-1Ba-L for 3 h at MOIs of 0.001, 0.01, 0.1, 1, and 3 to determine the extent of BIT225 activity with the viral burden and with the degree of macrophage differentiation by using time as a possible surrogate marker. Following infection, the cells were washed three times with PBS and cultured for a further 7 days with BIT225 at 10 μM or the equivalent volume of DMSO. On day 7 postinfection, the supernatant was sampled for RT activity and the cells were lysed to evaluate HIV-1 infection by real-time PCR.
Levels of infection of the day 7, 10, and 14 MDM were comparable as measured by real-time PCR. HIV-1 gag copy numbers in the day 7 MDM ranged from 94 to 1,157, with a peak infection rate at an MOI of 0.01. Similarly, ranges for the day 10 and day 14 MDM were 171 to 1,378 and 98 to 613 copies, respectively, with peak infection rates at MOIs of 0.01 and 0.1. As a result, an MOI of 0.05 was used in all subsequent experiments. Despite increasing MOIs, the day 10 and 14 MDM exhibited very even levels of infection as measured by the HIV-1 gag copy number, suggesting that the number of MDM permissive to infection may be fixed within the cell population. After 7 days of treatment, BIT225 resulted in high rates of inhibition of the virus compared to the level of infection in the DMSO-treated controls, with the compound likely preventing secondary infection or viral spread in the day 7, 10, and 14 MDM at all MOIs.
De novo virus production in the day 7 MDM cultures as measured by RT activity was low (<2 RT U/ml), despite the presence of viral copy numbers comparable to those in the day 10 and 14 MDM, suggesting the establishment of latent infection. Virus production in the day 10 and 14 MDM generally correlated with virus input, with the RT activity levels increasing as the MOI increased in the DMSO-treated controls (data not shown). A reduction in HIV-1 output was observed in the day 10 MDM treated with BIT225; this effect was more pronounced at the lower MOIs, as ∼82% viral inhibition was observed at MOIs of 0.01 and 0.001. When the culture supernatants from the BIT225- and DMSO-treated day 10 MDM were assessed using the TZM-bl cell infectivity assay, BIT225 resulted in similar levels of viral inhibition, with 90.4 and 98.1% inhibition at the same MOIs of 0.01 and 0.001, respectively. BIT225 effects on HIV-1 production in the day 14 MDM were less pronounced than those in the day 10 MDM; however, as in the day 10 MDM, BIT225 demonstrated high levels of viral inhibition in the day 14 MDM across the range of MOIs. Maximum levels of viral inhibition of 77 and 90.3% were observed with an MOI of 0.001, as measured by the RT assay and the TZM-bl indicator cell line assay, respectively (data not shown).
In vitro activities of BIT225 against HIV-1Ba-L-infected MDM in both acute and chronic infection assays.
Day 14 MDM were acutely infected with HIV-1Ba-L at an MOI of 0.05 for 3 h, unbound virus was washed off, and the macrophages were cultured for a further 7 days with or without BIT225. BIT225 treatment resulted in a maximum of 38% inhibition of virus release compared to that in DMSO-treated controls as measured by RT activity in the supernatants on day 21 of culture. However, virus production was invariably low, <6 RT U/ml on day 21 (data not shown). To create a more stringent assay to examine the anti-HIV-1 effects of BIT225, day 7, 10, and 14 MDM were chronically infected with HIV-1Ba-L for 7 days before being washed and cultured with the drug for an additional 7 days. As observed previously, day 7 MDM exhibited low infection rates, <2 RT U/ml on day 21 in both DMSO- and BIT225-treated cultures. The culture supernatants of chronically infected day 10 and day 14 MDM had greater HIV-1 titers than those of infected day 7 cells, with peak RT production levels of ∼13 and ∼19 RT U/ml at 7 and 5 days postinfection, respectively. Despite the higher levels of infection in day 14 MDM, the greatest effects of BIT225 were observed with this infection model. Following a single day of BIT225 treatment, 60.7% inhibition of virus release was observed, with the inhibition of virus release peaking at 91.5% 5 days posttreatment (on day 26 in culture), when virus production was also the highest in the DMSO-treated controls (data not shown). As a result, day 14 MDM and a 7-day period of infection were used in all subsequent chronic infection assays. Combined, the data suggest that day 7 MDM are permissive but nonproductive with regard to HIV-1 replication and have low levels of virus release, day 10 MDM are more permissive to infection as measured by real-time PCR, day 14 MDM are more productive as determined by RT activity, and it is de novo HIV-1 production upon which BIT225 exerts its effects.
Interestingly, a single dose of BIT225 at 10 μM in culture demonstrated long-term inhibition of virus production, for at least 7 days post-BIT225 addition in the chronic infection assay. To establish if inhibition by BIT225 treatment resulted in a long-term effect, BIT225 was removed from chronically infected MDM cultures and the cells were maintained without the drug for an additional 20 days. After 7 days, viral recovery was observed, with a fivefold increase in the amount of virus released as measured by RT activity. The increased level of release was comparable to the level in DMSO-treated controls (data not shown), and this result suggests that BIT225 targets de novo virus production. Conversely, if acutely infected day 14 MDM were treated with BIT225 at later time points in culture, the de novo production of virus could be inhibited to levels approaching those in MDM cultures receiving earlier treatment. The addition of BIT225 to infected day 14 MDM at 11 and 15 days postinfection (days 25 and 29 of culture) resulted in 7.3- and 2.2-fold reductions in virus 2 and 3 days later, inhibiting virus production by 86.3 and 54.3% as measured by RT activity (data not shown).
In vitro activity of BIT225 against HIV-1NL4-3 in PBMC and T-cell lines.
Using T-cell lines and PHA-stimulated PBMC from an HIV-1-seronegative donor, we aimed to determine the cellular range of BIT225. PM1 cells, MT-2 cells, and PHA-activated day 3 PBMC were infected with HIV-1NL4-3 for 3 h at an MOI of 0.05. The cells were washed three times and cultured in a 96-well plate at the predetermined optimum seeding densities (5 × 103 cells/well for PM1 and MT-2 cells and 2 × 105 cells/well for the activated PBMC) for 7 days. Serial dilutions of BIT225 (25 and 10 μM BIT225 and concentrations diluted 1:5 from 5 to 0.0016 μM) were added, and HIV-1 replication in the supernatants was measured by evaluating RT activity on day 7 of cell culture, with the cell viability also assessed using the MTT assay on day 7 of culture. Percentages of inhibition were calculated by comparing the BIT225-treated cells to the DMSO-treated controls.
BIT225 antiviral responses in the T-cell cultures were again dose dependent. Treatment with BIT225 at 25 μM resulted in mean levels of inhibition of HIV-1 of 38.8, 93.1, and 95.8% in the MT-2 and PM1 cells and PBMC, respectively. At 10 μM BIT225, less viral inhibition was observed, with mean levels of inhibition of 24, 21, and 56.8%, respectively, for the three cell types. HIV-1 inhibition was further reduced with 5 μM BIT225; activity was apparent in the PBMC cultures only, with 25.7% inhibition of virus release (data not shown). While more favorable responses are observed with MDM, BIT225 activity in infected T cells is apparent, albeit at higher concentrations of the drug.
BIT225 anti-HIV-1 activity via the viral protein Vpu is supported by the drug's lack of activity toward HIV-2-infected MDM.
Unlike HIV-1, HIV-2 has evolved lacking the vpu gene. This makes for an ideal virological tool for helping unravel the mechanism of action of BIT225 with respect to this viral target. Based upon the results of the lipid bilayer experiments, with BIT225 antagonizing the Vpu viroporin (Fig. 1b), our hypothesis is that if BIT225 is acting upon Vpu, we will observe no antiviral activity in cells infected with HIV-2 compared to HIV-1-infected cells that have a fully functional vpu gene.
Day 14 MDM from HIV-seronegative donors (n = 3) were infected separately with both HIV-1 and HIV-2 in a chronic infection assay. HIV-1Ba-L was used in this instance as a positive control for BIT225's well-established anti-HIV-1 activity. Briefly, the macrophages were infected with HIV-1 or HIV-2 at an MOI of 0.05 for 7 days, subsequently washed to be free of extracellular virus, and cultured in the presence or absence of BIT225 at 10 μM for a further 7 days. Supernatants from day 28 cultures were assayed for HIV levels by the RT activity assay.
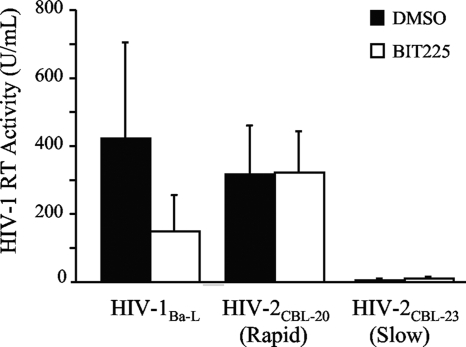
BIT225 activity was observed in chronically HIV-1-infected macrophages from all three of the MDM donors, with a mean level (±SE) of inhibition of 66.6% (±1.6%). In support of BIT225's exerting its effect via Vpu, no activity was observed in the macrophages infected with HIV-2, either the cytopathic strain (mean inhibition of 6.1% ± 3.3%) or the noncytopathic strain (mean inhibition of 13.4% ± 9.8%). These data suggest that BIT225 antiviral activity is indeed targeted at the HIV-1 protein Vpu in infected macrophages (Fig. 3).
FIG. 3.
BIT225 has no effect upon the replication of HIV-2, which lacks vpu. Day 14 MDM were infected with HIV-1Ba-L or with the rapid or slow-growing clinical isolate of HIV-2 (CBL-20 or CBL-23, respectively) at an MOI of 0.05 in the chronic infection assay. Starting at day 21, the infected macrophages were cultured in the presence or absence of BIT225 at 10 μM for an additional 7 days. Shown are the mean results with SE bars for samples from three separate MDM donors, with HIV replication measured by RT activity in the culture supernatant.
BIT225 has a unique mode of action. (i) BIT225 blocks a late step in virus replication.
TZM-bl cells were infected with HIV-1Ba-L in the presence of either BIT225 (at 0.3125 to 10 μM) or the antiretroviral compound EFV (10 nM) as a positive control or LPV (100 nM) or SQV (10 nM) as a negative control for the assay. If compounds are capable of inhibiting the virus at the stages of preintegration and/or integration, the luciferase gene will not be expressed.
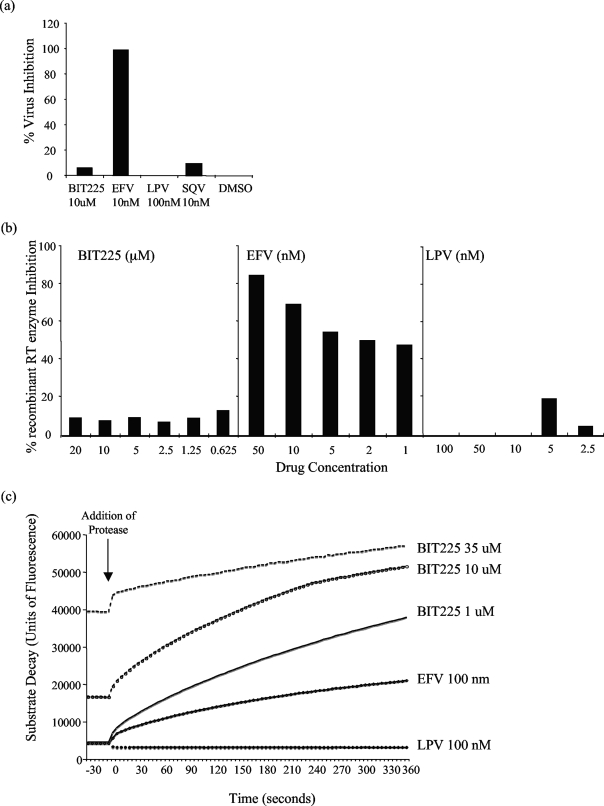
The results of infecting TZM-bl cells in the presence of BIT225 confirmed that the compound's activity occurs postintegration. BIT225 did not prevent the integration of HIV-1 into the host cell genome, and the levels of luciferase expression in the BIT225-treated samples were equal to those in both the DMSO-treated controls and the samples treated with protease inhibitors SQV and LPV, which both act in the late stages of HIV-1 replication. Compared to treatment with DMSO, treatment with BIT225 at 10 μM resulted in a nonsignificant mean level of inhibition of HIV-1 integration of 6%. The negative controls LPV and SQV resulted in comparably low levels of inhibition of viral integration of 0 and 9%, respectively. The positive control for early-phase inhibition, the NNRTI EFV, was able to inhibit reverse transcription and therefore resulted in a reduction in the levels of HIV-1 integration by 99% compared to those in the DMSO-treated controls (Fig. 4a).
FIG. 4.
The anti-HIV-1 effects of BIT225 are exerted late in the viral life cycle. Compared to the control drugs in each of the assays, BIT225 at 10 μM does not interfere with viral integration, as demonstrated by a lack of activity with the indicator cell line TZM-bl (a), does not interfere with the process of reverse transcription or with the viral RT enzyme (b), and does not affect the viral protease enzyme (c). Shown are data representative of results from three separate experiments. Concentrations of BIT225 are expressed as micromolar concentrations and those of EFV, LPV, and SQV are expressed as nanomolar concentrations.
(ii) Is BIT225 acting upon the HIV-1 enzymes RT and protease?
To further elucidate BIT225 antiviral activity, we used the RT activity assay to determine whether BIT225 acted directly on the RT enzyme of HIV-1 or on the process of reverse transcription. The effect of BIT225 on RT was determined by adding the compound directly into the RT ELISA mixture and comparing the observed activity to the activities of the drugs EFV and LPV, positive and negative controls, respectively, in the ELISA.
As expected, inhibition of the RT enzyme by EFV (50, 10, 5, 2, and 1 nM) in a dose-dependent manner was observed, while BIT225 in addition to LPV did not interrupt the enzyme activity. BIT225 was assessed in descending concentrations from 20 to 0.625 μM, with the greatest mean level ± standard deviation of virus inhibition, 17.6% ± 17.9% (n = 3), observed at 2.5 μM. These data add to the previous data suggesting that BIT225 antiviral activity is occurring post-reverse transcription (Fig. 4b).
To determine if BIT225 was affecting the activity of the HIV-1 enzyme protease, BIT225 inhibition was tested in a cell-free assay and compared to results for the positive control LPV and the negative control EFV. As expected, LPV resulted in inhibition of the protease activity, as cleavage of the artificial substrate was inhibited and no fluorescence was observed. Similar to the NNRTI EFV, BIT225 did not inhibit the activity of protease within the substrate-enzyme assay, emphasizing a novel and unique activity compared to those of the current late-phase inhibitors (Fig. 4c). HIV protease was able to continuously cleave the protease substrate in the presence of various BIT225 concentrations as high as 100 μM. Increasing autofluorescence of BIT225 was observed with increasing drug concentrations, a pattern observed previously in the TZM-bl cell assay and likely to be the result of the aromatic rings that are present in the molecular structure.
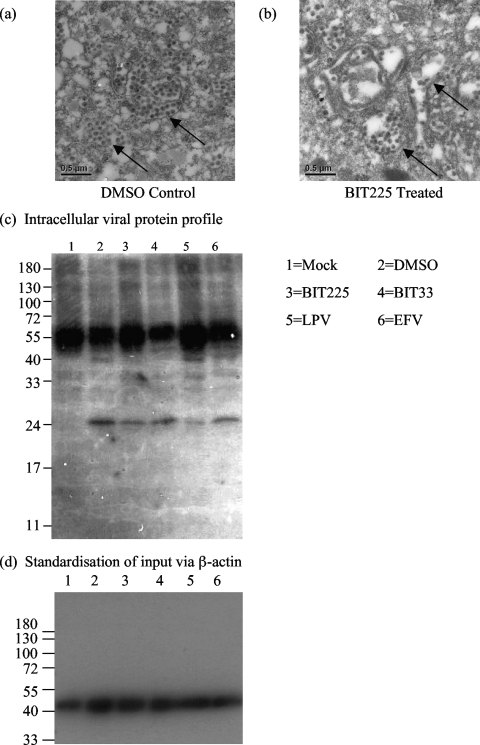
BIT225 affects virion packaging within the intracellular compartments of the infected macrophage.
Day 14 macrophages chronically infected with HIV-1Ba-L and subsequently treated with BIT225 at 10 μM for an additional 7 days showed abnormal virus-like particles within the intracellular compartments compared to the DMSO-treated controls when examined by TEM (Fig. 5a and b). The findings are consistent with BIT225's exerting its effect late in the HIV-1 life cycle and with the reduction of virus release into the culture supernatants. Western blot analysis of the intracellular viral proteins failed to reveal any gross changes in viral protein expression between treated and untreated HIV-1-infected MDM. With protein input standardized with respect to β-actin, a decrease in the level of p24 capsid with BIT225 treatment compared to the level in the DMSO-treated control was observed, suggesting either a reduction in intracellular virus or a decreased level of protein as a result of the irregular viral assembly (Fig. 5c). LPV treatment, as expected, results in no p24 band, as there is no cleavage of the Gag polyprotein. Nonspecific heavy-chain IgG bands at 50 kDa can be seen as a result of using human serum.
FIG. 5.
BIT225 results in defects in virion assembly. Day 14 MDM were infected chronically with HIV-1Ba-L and treated with BIT225 at 10 μM or with DMSO prior to being fixed for TEM on day 28. (a and b) Shown are DMSO-treated MDM with HIV-1-like particles (indicated by the arrows) (a) and BIT225-treated MDM with aberrant virus-like particles sequestered within intracellular compartments (b). (c and d) Additionally, the cells were lysed for Western blot analysis to determine gross protein differences between BIT225-treated and untreated MDM in equivalent amounts of total protein (c); equivalency of protein amounts was confirmed by analysis of β-actin expression (d).
DISCUSSION
In this study, we present our lead compound BIT225 as a novel drug targeting the Vpu protein of HIV-1 with preferential antiviral activity toward human macrophages. Dose-response analyses of both the anti-HIV-1 activity and cellular toxicity have enabled us to establish in vitro EC50s and TC50s of BIT225 and further calculate a selectivity index of 126. We have developed two infection systems to measure the ability of BIT225 to inhibit the release of HIV from MDM and have shown strong antiviral activity across a range of infection rates and monocyte-to-macrophage differentiation stages. BIT225 is nontoxic at high levels, with good antiviral activity in macrophages that have been infected at high rates or for long durations. We have shown that the activity of BIT225 is broad, resulting in strong viral inhibition in cells infected with R5-, X4-, and dual-tropic viral strains. Most important and clinically significant are the activities of BIT225 against single-drug- and multidrug-resistant strains of virus and the synergistic and additive effects with some of the current antiretroviral drugs in use during HIV-1 therapy. Activity of BIT225 against HIV-2 is absent, supporting the lipid bilayer data suggesting Vpu as the likely target. In addition, our data show that BIT225 targets a late-stage process in the virus life cycle where Vpu is of biological importance. BIT225 does not affect the HIV-1 enzyme RT or protease and appears to disrupt the formation of intracellular de novo virus particles.
Our findings with BIT225 demonstrate differences in anti-HIV-1 activity in macrophages and T cells, suggesting that the activity is cell type dependent. The role of Vpu in virus assembly within particular host cells is widely supported in the literature. Numerous studies have reported the importance of Vpu for virus replication in macrophages and T cells, presenting contrasting results. Balliet et al. reported a reduction in virus release of 3 orders of magnitude in primary macrophage cultures but virtually no effect with the same strain in activated T cells (4). In contrast, Schubert et al., using isogenic vpu+ and vpu-deficient primary and chimeric HIV-1 isolates, observed the effects on the kinetics of virus release in macrophages to be similar to, although more moderate than, those in T cells (32). It has also been shown previously that the magnitude of Vpu enhancement of replication is critically dependent on the viral inoculum and the rate of cell proliferation and may be a key factor behind such experimental variation. MDM are terminally differentiated, have limited potential for proliferation (39), and can display an extended viral replication cycle as a result of limitations in nucleotide precursors (27). In growth-arrested cells infected at low MOIs, the Vpu effect was of the order of 1,000-fold, whereas at high MOIs, only a 2- to 3-fold enhancement of virus release was observed (8). The antiviral efficacy of novel Vpu inhibitors could then, theoretically, be of particular value for individuals in which highly active antiretroviral therapy is maintaining low viral loads. Additionally, despite a higher number of infected cells in macrophage populations than in T-cell populations, there is a 10-fold difference between the virus output from macrophages and that from an equal number of T cells, with the majority of the virus remaining sequestered intracellularly within the macrophages and hidden from the host (23).
HIV-2 isolates have evolved a distinct mechanism to overcome the missing Vpu, with Env Rod10 demonstrating Vpu-like activity (6). Identical levels of enhancement of viral particle release were observed when Vpu and Rod10 were provided separately in trans to an NL4-3 Vpu mutant, and the two proteins were functionally interchangeable, each augmenting the release of simian immunodeficiency virus (SIV), HIV-1, and HIV-2 particles. This finding also supports the requirement for a membrane pore for the successful release of viral particles. Similar to Vpu, HIV-2 Env has an innate tendency to form homo-oligomeric complexes and requires the presence of a functional transmembrane domain for activity (6, 20, 31). The absence of BIT225 activity we observed in the HIV-2 assay strongly supports the selective targeting of the Vpu ion channel that is evident only in the HIV-1 MDM model.
Elucidating the mechanism of action of BIT225 in HIV-1 infection is proving challenging. Using simple assays, we have shown that BIT225 has a novel mode of action, does not interfere with the process of reverse transcription, and has no effect on the HIV-1 protease enzyme. The role of the viroporins in viral life cycles is poorly understood. Preliminary studies examining the effects of BIT compounds on viral assembly by TEM and Western blot analyses have revealed morphological differences between infected macrophages that have been treated with the compounds and untreated infected macrophages. It is interesting to speculate that we are interfering with intracellular microenvironments, making them less favorable for viral assembly, with this effect being more pronounced in macrophages than T cells, where virus replication occurs within late endosomes or multivesicular bodies that may or may not connect to the plasma membrane (7, 22, 30). Supporting this idea, the assembly of infectious HIV-1 requires, in addition to Gag, proper incorporation of other viral proteins. In particular, Vpu is known to affect the intracellular trafficking of the viral membrane glycoprotein Env. Infection with viruses expressing mutant forms of Vpu that do not have ion channel activity alters the cellular distributions of both Env and Gag, in addition to reducing particle release (40). The authors of the previous study speculate that “dissipation of ionic gradients within the endosomal system by Vpu may disrupt ESCRT-mediated sorting,” which is critical for proper virus budding. In support, they note that in yeast, mutation of the vps44 gene that encodes a sodium/proton exchanger leads to alteration of the endosomal compartment, similar to those changes caused by mutant ESCRT proteins.
Overall, despite some controversy, the vpu inhibition experiments clearly demonstrate that the increased numbers of viral particles produced by cells infected with vpu+ strains may facilitate the spread of virus throughout the body, possibly leading to more rapid progression of disease. Enhanced virus release is particularly pronounced in quiescent, terminally differentiated cells such as macrophages, and as already discussed, persistent infection of macrophages is widely acknowledged to have important roles in the establishment, spread, and pathogenesis of HIV-1 infection (12, 13, 32, 42, 43). Macrophages are long-lived cells, disseminating virus in trans (i.e., nonintegrated virus) for up to 6 weeks in culture, with intracellular virus decaying rapidly in the first 2 weeks postinfection but minimally thereafter (34). In addition to resting memory T cells, macrophages present a key hurdle in the successful treatment of HIV-1. BIT225, with a novel mechanism of action that targets Vpu and with activity preferentially against HIV-1 in infected macrophages, may assist in the eradication of HIV-1 reservoirs from the human host.
Acknowledgments
We thank Deb Hill and Roger Ptak at SRI (Frederick, MD) for the BIT225 studies with the drug-resistant HIV-1 isolates and the tropism and synergy studies; the HIV-1 Pathogenesis Laboratory, Westmead Millennium Institute, Sydney, Australia, for processing the real-time PCR assays, in particular Valerie Marsden and Sarah Mercier for their assistance in generating these data; and Simon Crawford, School of Botany, University of Melbourne, Victoria, Australia, for conducting the TEM studies. We also thank the contributors and the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, in particular John C. Kappes, Xiaoyun Wu, and Tranzyme Inc., for the TZM-bl cells and Robin Weiss for the HIV-2 clinical isolates (CBL-20 and CBL-23).
Footnotes
Published ahead of print on 7 December 2009.
REFERENCES
- 1.Aquaro, S., P. Bagnarelli, T. Guenci, A. De Luca, M. Clementi, E. Balestra, R. Caliò, and C. F. Perno. 2002. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J. Med. Virol. 68:479-488. [DOI] [PubMed] [Google Scholar]
- 2.Aquaro, S., E. Balestra, A. Cenci, M. Francesconi, R. Caliò, and C. F. Perno. 1997. HIV infection in macrophage: role of long-lived cells and related therapeutical strategies. J. Biol. Regul. Homeost. Agents 11:69-73. [PubMed] [Google Scholar]
- 3.Aquaro, S., R. Caliò, J. Balzarini, M. C. Bellocchi, E. Garaci, and C. F. Perno. 2002. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 55:209-225. [DOI] [PubMed] [Google Scholar]
- 4.Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623-631. [DOI] [PubMed] [Google Scholar]
- 5.Binette, J., and E. A. Cohen. 2004. Recent advances in the understanding of HIV-1 Vpu accessory protein functions. Curr. Drug Targets Immune Endocr. Metabol. Disord. 4:297-307. [DOI] [PubMed] [Google Scholar]
- 6.Bour, S., U. Schubert, K. Peden, and K. Strebel. 1996. The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release: a Vpu-like factor? J. Virol. 70:820-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deneka, M., A. Pelchen-Matthews, R. Byland, E. Ruiz-Mateos, and M. Marsh. 2007. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J. Cell Biol. 177:329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deora, A., and L. Ratner. 2001. Viral protein U (Vpu)-mediated enhancement of human immunodeficiency virus type 1 particle release depends on the rate of cellular proliferation. J. Virol. 75:6714-6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewart, G. D., K. Mills, G. B. Cox, and P. W. Gage. 2002. Amiloride derivatives block ion channel activity and enhancement of virus-like particle budding caused by HIV-1 protein Vpu. Eur. Biophys. J. 31:26-35. [DOI] [PubMed] [Google Scholar]
- 10.Ewart, G. D., N. Nasr, H. Naif, G. B. Cox, A. L. Cunningham, and P. W. Gage. 2004. Potential new anti-human immunodeficiency virus type 1 compounds depress virus replication in cultured human macrophages. Antimicrob. Agents Chemother. 48:2325-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewart, G. D., T. Sutherland, P. W. Gage, and G. B. Cox. 1996. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 70:7108-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gartner, S., and M. Popovic. 1990. Macrophage tropism of HIV-1. AIDS Res. Hum. Retroviruses 6:1017-1021. [DOI] [PubMed] [Google Scholar]
- 13.Gendelman, H. E., J. M. Orenstein, L. M. Baca, B. Weiser, H. Burger, D. C. Kalter, and M. S. Meltzer. 1989. The macrophage in the persistence and pathogenesis of HIV infection. AIDS 3:475-495. [DOI] [PubMed] [Google Scholar]
- 14.Gordon, S., and P. R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953-964. [DOI] [PubMed] [Google Scholar]
- 15.Haase, A. T., and T. W. Schacker. 1998. Potential for the transmission of HIV-1 despite highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]
- 16.Hout, D. R., E. R. Mulcahy, E. Pacyniak, L. M. Gomez, M. L. Gomez, and E. B. Stephens. 2004. Vpu: a multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1. Curr. HIV Res. 2:255-270. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. U. S. A. 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazdins, J. K., K. Woods-Cook, M. Walker, and E. Alteri. 1990. The lipophilic muramyl peptide MTP-PE is a potent inhibitor of HIV replication in macrophages. AIDS Res. Hum. Retroviruses 6:1157-1161. [DOI] [PubMed] [Google Scholar]
- 19.Lewin, S. R., M. Vesanen, L. Kostrikis, A. Hurley, M. Duran, L. Zhang, D. D. Ho, and M. Markowitz. 1999. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J. Virol. 73:6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldarelli, F., M. Y. Chen, R. L. Willey, and K. Strebel. 1993. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J. Virol. 67:5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldarelli, F., S. Palmer, M. S. King, A. Wiegand, M. A. Polis, J. Mican, J. A. Kovacs, R. T. Davey, D. Rock-Kress, R. Dewar, S. Liu, J. A. Metcalf, C. Rehm, S. C. Brun, G. J. Hanna, D. J. Kempf, J. M. Coffin, and J. W. Mellors. 2007. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 3:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh, M., K. Theusner, and A. Pelchen-Matthews. 2009. HIV assembly and budding in macrophages. Biochem. Soc. Trans. 37:185-189. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer, M. S., D. R. Skillman, P. J. Gomatos, D. C. Kalter, and H. E. Gendelman. 1990. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu. Rev. Immunol. 8:169-194. [DOI] [PubMed] [Google Scholar]
- 24.Montal, M. 2003. Structure-function correlates of Vpu, a membrane protein of HIV-1. FEBS Lett. 552:47-53. [DOI] [PubMed] [Google Scholar]
- 25.Murray, J. M., S. Emery, A. D. Kelleher, M. Law, J. Chen, D. J. Hazuda, B. Y. Nguyen, H. Teppler, and D. A. Cooper. 2007. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 21:2315-2321. [DOI] [PubMed] [Google Scholar]
- 26.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, W. A., A. Namazi, H. Kalhor, S. H. Mao, J. A. Zack, and I. S. Chen. 1994. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J. Virol. 68:1258-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perno, C. F., E. Balestra, M. Francesconi, D. Abdelahad, R. Caliò, J. Balzarini, and S. Aquaro. 2004. Antiviral profile of HIV inhibitors in macrophages: implications for therapy. Curr. Top. Med. Chem. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 29.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 30.Raposo, G., M. Moore, D. Innes, R. Leijendekker, A. Leigh-Brown, P. Benaroch, and H. Geuze. 2002. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic 3:718-729. [DOI] [PubMed] [Google Scholar]
- 31.Schubert, U., S. Bour, A. V. Ferrer-Montiel, M. Montal, F. Maldarell, and K. Strebel. 1996. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol. 70:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert, U., K. A. Clouse, and K. Strebel. 1995. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 69:7699-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuitemaker, H. 1994. Macrophage-tropic HIV-1 variants: initiators of infection and AIDS pathogenesis? J. Leukoc. Biol. 56:218-224. [DOI] [PubMed] [Google Scholar]
- 34.Sharova, N., C. Swingler, M. Sharkey, and M. Stevenson. 2005. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 24:2481-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen, R., H. E. Richter, R. H. Clements, L. Novak, K. Huff, D. Bimczok, S. Sankaran-Walters, S. Dandekar, P. R. Clapham, L. E. Smythies, and P. D. Smith. 2009. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 83:3258-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song, E., S. K. Lee, D. M. Dykxhoorn, C. Novina, D. Zhang, K. Crawford, J. Cerny, P. A. Sharp, J. Lieberman, N. Manjunath, and P. Shankar. 2003. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J. Virol. 77:7174-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonza, S., H. P. Mutimer, R. Oelrichs, D. Jardine, K. Harvey, A. Dunne, D. F. Purcell, C. Birch, and S. M. Crowe. 2001. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 15:17-22. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, K., B. P. Craddock, T. Kano, and R. T. Steigbigel. 1993. Colorimetric reverse transcriptase assay for HIV-1. J. Virol. Methods 41:21-28. [DOI] [PubMed] [Google Scholar]
- 39.Vadiveloo, P. K. 1999. Macrophages—proliferation, activation, and cell cycle proteins. J. Leukoc. Biol. 66:579-582. [DOI] [PubMed] [Google Scholar]
- 40.Van Damme, N., and J. Guatelli. 2008. HIV-1 Vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, Gag-containing endosomes. Cell. Microbiol. 10:1040-1057. [DOI] [PubMed] [Google Scholar]
- 41.van de Bovenkamp, M., H. S. Nottet, and C. F. Pereira. 2002. Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1-associated dementia. Eur. J. Clin. Invest. 32:619-627. [DOI] [PubMed] [Google Scholar]
- 42.Westervelt, P., H. E. Gendelman, and L. Ratner. 1991. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc. Natl. Acad. Sci. U. S. A. 88:3097-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westervelt, P., T. Henkel, D. B. Trowbridge, J. Orenstein, J. Heuser, H. E. Gendelman, and L. Ratner. 1992. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J. Virol. 66:3925-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu, T. 2002. HIV-1 in peripheral blood monocytes: an underrated viral source. J. Antimicrob. Chemother. 50:309-311. [DOI] [PubMed] [Google Scholar]