Abstract
Raltegravir concentrations and human immunodeficiency virus type 1 (HIV-1) RNA levels in semen samples from 10 treatment-experienced HIV-1-infected patients were measured after 24 weeks of raltegravir-based highly active antiretroviral therapy (HAART). Semen and plasma HIV-1 RNA levels were below 100 copies/ml and 50 copies/ml, respectively, in all samples. The median raltegravir concentrations in semen samples (n = 10) and in plasma samples (n = 9) drawn simultaneously were 345 (range, 83 to 707) ng/ml and 206 (range, 106 to 986) ng/ml, respectively. The median semen-to-plasma ratio of raltegravir concentration was 1.42 (range, 0.52 to 6.66), indicating good although variable levels of drug penetration of raltegravir in the seminal compartment.
Raltegravir is the first approved human immunodeficiency virus type 1 (HIV-1) integrase inhibitor which demonstrated potent antiviral efficacy in plasma samples from both treatment-experienced and treatment-naive HIV-infected patients (3, 11, 17). The male genital tract, however, represents a separate compartment, or “sanctuary site,” in which viral replication may persist in patients receiving combination antiretroviral therapy, despite a complete inhibition of HIV replication in blood (10, 15, 16, 18). HIV present in seminal plasma is responsible for sexual transmission of the virus, and an antiretroviral drug concentration within the male genital tract is therefore a potentially important factor affecting HIV replication and its sexual transmission (10, 18). No data are yet available on penetration of raltegravir in semen. Our aim was to assess the concentrations of raltegravir in seminal fluid and blood samples from 10 HIV-infected males enrolled in the EASIER-ANRS 138 trial.
EASIER-ANRS 138 is an open-label, multicenter, randomized clinical trial that demonstrated the noninferior antiviral efficacy at 24 weeks of a switch from enfuvirtide to raltegravir among treatment-experienced patients, with suppression of plasma HIV-1 RNA levels below 400 copies/ml under an enfuvirtide-based regimen (4). Ten male patients already enrolled in the EASIER-ANRS 138 trial gave written informed consent to participate in this semen substudy. Plasma and semen samples were collected after 24 weeks of raltegravir treatment (400 mg twice a day) given in combination with other antiretroviral drugs, so that steady-state conditions were ensured. Plasma samples were collected 5 h after the morning intake of raltegravir. Single semen samples were obtained at the same time by masturbation after a recommended 3-day period of sexual abstinence. All samples were rapidly centrifuged, and blood and seminal plasmas were stored at −80°C until analysis. The measures of HIV-1 RNA levels in blood and semen were performed with an adapted Cobas AmpliPrep/Cobas TaqMan HIV-1 assay (Roche, Meylan, France), with lower limits of detection of 50 and 100 copies/ml in plasma and semen, respectively (12). Raltegravir in plasma and semen was assayed by using validated liquid chromatography assays coupled with UV (320 nm) or mass tandem detection after liquid/liquid extraction (buffered plasma, pH 4, and dichloromethane/hexane extraction) and protein precipitation, respectively (7, 22). The limits of quantification of the assay were 10 ng/ml and 2.5 ng/ml in plasma and semen, respectively. The coefficients of variation of intra- and interassay precision and accuracy were below 15%. All results are presented as median (range).
The median age of the patients was 46.4 (range, 39.7 to 49.3) years, and their median CD4 cell count was 373 (range, 241 to 551) cells/mm3. In addition to raltegravir, 9 patients received a ritonavir-boosted protease inhibitor (PI) (darunavir for 5 patients, tipranavir for 2 patients, lopinavir for 1 patient, and a combination of lopinavir and fosamprenavir for 1 patient), 6 patients received 2 nucleoside (or nucleotide) reverse transcriptase inhibitors (NRTIs) (abacavir for 1 patient, tenofovir for 4 patients, emtricitabine for 2 patients, lamivudine for 3 patients, stavudine for 1 patient, and didanosine for 1 patient), 2 patients received 3 NRTIs (abacavir-tenofovir-emtricitabine), and 1 patient received didanosine-abacavir-tenofovir-efavirenz.
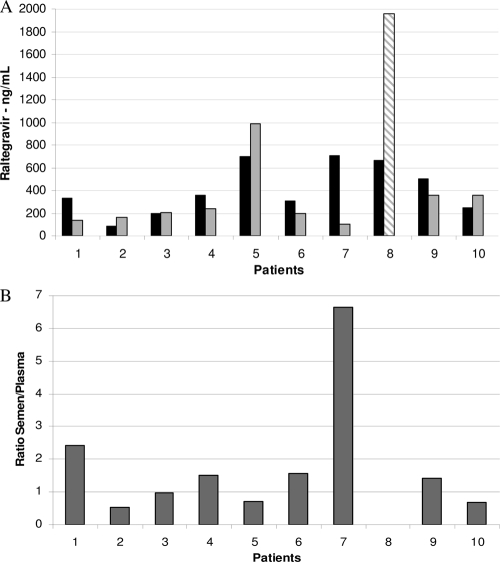
Raltegravir concentrations in plasma and semen samples obtained simultaneously at 5 h following drug intake on week 24 of treatment are shown in Fig. 1. A wide interpatient variability was observed for both compartments. Of note, raltegravir was detected in all seminal plasma samples analyzed, with medians of 345 (range, 83 to 707) ng/ml for semen samples (n = 10) and 206 (range, 106 to 986) ng/ml for the 9 plasma samples drawn at the same time, at 5 h postdosing. The semen-to-plasma ratios ranged from 0.52 to 6.66, with a median of 1.42 (P = 0.21; Wilcoxon signed-rank test). The HIV-1 RNA levels were below the detection levels in both semen and plasma in all samples.
FIG. 1.
(A) Raltegravir concentrations in plasma and semen samples obtained from 10 HIV-infected patients enrolled in the EASIER-ANRS 138 trial at week 24, 5 hours following drug intake. Semen (grey bars) and plasma (black bars) samples were drawn at 5 h postdosing. A plasma sample from patient 8 was drawn at 9 h postdosing (hatched bars). (B) Individual semen-to-plasma ratios of raltegravir concentrations. The ratio is not calculated for patient 8.
This study demonstrated that semen concentrations of raltegravir were on average higher than those in plasma in 9 treatment-experienced patients after 24 weeks of a raltegravir-based antiretroviral regimen. A wide interpatient variation in raltegravir concentration in seminal plasma was observed, however, which should be due to the variability observed in blood plasma concentrations and/or to the heterogeneity of ejaculate fractions. Despite this variability, and assuming that 83% of the drug is inactive due to protein binding, seminal concentrations remained close to or above the raltegravir 95% inhibitory concentration (IC95) for wild-type HIV-1 strains (14.6 ng/ml) (8).
We also found a median semen-to-plasma ratio of raltegravir concentrations of 1.42, indicating good penetration of the drug in the seminal compartment and suggesting passive diffusion from plasma to semen. Indeed, drug diffusion into semen depends on a number of factors, such as lipid solubility, ionization, plasma protein binding, and substrate of uptake or efflux transporters (1, 20). Raltegravir is expected to be mostly ionized in plasma, but ionization in the whole ejaculate is predicted to be negligible on the basis of pH-pKa partitioning, as previously described for other antiretroviral drugs (1). However, slight lipophilicity and pharmacokinetic properties (83% bound to plasma proteins) could support passive diffusion from blood to semen. Raltegravir was demonstrated to be a substrate of the efflux transporter MDR1 (2); however, limited information is available on the presence of this transporter within the male genital tract (1, 20).
This good penetration of raltegravir in semen could also explain the lack of HIV-1 RNA detection in this reservoir, although the concentration-efficacy relationship is difficult to assess in this study, since patients had plasma HIV-1 RNA levels below 400 copies/ml before starting raltegravir and received multiple antiretroviral agents in addition to raltegravir, some of which usually achieve very good semen penetration. Concentrations of nonnucleoside reverse transcriptase inhibitors (NNRTIs) in seminal plasma are consistently measurable, although lower than those in blood plasma (14, 21). Penetration of PIs in semen is poor, and seminal concentrations of PIs seem to be dependent on the degree of protein binding, with the weakest-bound drugs (amprenavir and indinavir) having the highest concentrations (5, 13, 19, 20, 23). In contrast to those observed for PIs, NRTI seminal plasma concentrations are similar to or higher than concentrations in blood plasma (5, 6, 13, 21).
One limitation of our study is its small size and the fact that only one semen sample was available from each patient. However, the good penetration of raltegravir in the semen reported here is in agreement with the high raltegravir concentrations also found in cervicovaginal fluids of healthy women (9), suggesting that raltegravir has good diffusion properties in several compartments and tissues.
In conclusion, the finding that raltegravir concentrations in seminal plasma are high, and close to or above the IC95 of wild-type viruses in most patients, is good news and has potential relevance for sexual transmission of HIV-1. Combination of raltegravir with other antiretroviral drugs with good penetration into this reservoir is therefore likely to reduce residual sexual transmission of HIV-1 among patients receiving antiretroviral therapy.
Acknowledgments
We thank M. Leruez-Ville and C. Rouzioux, Necker Hospital, Paris, France, for their assistance in HIV-1 RNA semen quantification.
We thank the departments of Clinical Immunopathology, Internal Medicine, and Infectious Diseases, Saint-Louis Hospital, Paris, France, for inclusion of patients in this study.
Footnotes
Published ahead of print on 7 December 2009.
REFERENCES
- 1.Cao, Y. J., and C. W. Hendrix. 2008. Male genital tract pharmacology: developments in quantitative methods to better understand a complex peripheral compartment. Clin. Pharmacol. Ther. 83:401-412. [DOI] [PubMed] [Google Scholar]
- 2.Cianfriglia, M., M. L. Dupuis, A. Molinari, A. Verdoliva, R. Costi, C. M. Galluzzo, M. Andreotti, A. Cara, R. Di Santo, and L. Palmisano. 2007. HIV-1 integrase inhibitors are substrates for the multidrug transporter MDR1-P-glycoprotein. Retrovirology 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croxtall, J. D., and S. J. Keam. 2009. Raltegravir: a review of its use in the management of HIV infection in treatment-experienced patients. Drugs 69:1059-1075. [DOI] [PubMed] [Google Scholar]
- 4.De Castro, N., J. Braun, I. Charreau, G. Pialoux, L. Cotte, C. Katlama, F. Raffi, L. Weiss, J. L. Meynard, Y. Yazdanpanah, C. Delaugerre, I. Madelaine-Chambrin, J. P. Aboulker, and J. M. Molina. 2009. Switch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: a randomized open-label trial. Clin. Infect. Dis. 49:1259-1267. [DOI] [PubMed] [Google Scholar]
- 5.Ghosn, J., M. L. Chaix, G. Peytavin, J. L. Bresson, J. Galimand, P. M. Girard, F. Raffi, I. Cohen-Codar, J. F. Delfraissy, and C. Rouzioux. 2008. Absence of HIV-1 shedding in male genital tract after 1 year of first-line lopinavir/ritonavir alone or in combination with zidovudine/lamivudine. J. Antimicrob. Chemother. 61:1344-1347. [DOI] [PubMed] [Google Scholar]
- 6.Ghosn, J., M. L. Chaix, G. Peytavin, E. Rey, J. L. Bresson, C. Goujard, C. Katlama, J. P. Viard, J. M. Treluyer, and C. Rouzioux. 2004. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS 18:1958-1961. [DOI] [PubMed] [Google Scholar]
- 7.Goldwirt, L., A. Barrail-Tran, A. M. Taburet, and V. Furlan. 2009. Quantification of raltegravir (MK 0518) in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. [Epub ahead of print.] doi: 10.1016/j.jchromb.2009.10.029. [DOI] [PubMed]
- 8.Iwamoto, M., L. A. Wenning, A. S. Petry, M. Laethem, M. De Smet, J. T. Kost, S. A. Merschman, K. M. Strohmaier, S. Ramael, K. C. Lasseter, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293-299. [DOI] [PubMed] [Google Scholar]
- 9.Jones, A. E., J. A. Talameh, K. B. Patterson, N. L. Rezk, H. A. Prince, and A. D. M. Kashuba. 2009. First-dose and steady-state pharmacokinetics (PK) of raltegravir (RAL) in the genital tract (GT) of HIV uninfected women, abstr. O_06. Tenth Int. Workshop Clin. Pharmacol. HIV Ther., Amsterdam, The Netherlands, 15 to 17 April 2009.
- 10.Kashuba, A. D., J. R. Dyer, L. M. Kramer, R. H. Raasch, J. J. Eron, and M. S. Cohen. 1999. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 43:1817-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennox, J. L., E. DeJesus, A. Lazzarin, R. B. Pollard, J. V. Madruga, D. S. Berger, J. Zhao, X. Xu, A. Williams-Diaz, A. J. Rodgers, R. J. Barnard, M. D. Miller, M. J. DiNubile, B. Y. Nguyen, R. Leavitt, and P. Sklar. 2009. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 374:796-806. [DOI] [PubMed] [Google Scholar]
- 12.Pasquier, C., C. Souyris, N. Moinard, L. Bujan, and J. Izopet. 2006. Validation of an automated real-time PCR protocol for detection and quantitation of HIV and HCV genomes in semen. J. Virol. Methods 137:156-159. [DOI] [PubMed] [Google Scholar]
- 13.Pereira, A. S., L. M. Smeaton, J. G. Gerber, E. P. Acosta, S. Snyder, S. A. Fiscus, R. R. Tidwell, R. M. Gulick, R. L. Murphy, and J. J. Eron, Jr. 2002. The pharmacokinetics of amprenavir, zidovudine, and lamivudine in the genital tracts of men infected with human immunodeficiency virus type 1 (AIDS clinical trials group study 850). J. Infect. Dis. 186:198-204. [DOI] [PubMed] [Google Scholar]
- 14.Reddy, Y. S., S. K. Gotzkowsky, J. J. Eron, J. Y. Kim, W. D. Fiske, S. A. Fiscus, L. Petch, M. S. Cohen, and A. D. Kashuba. 2002. Pharmacokinetic and pharmacodynamic investigation of efavirenz in the semen and blood of human immunodeficiency virus type 1-infected men. J. Infect. Dis. 186:1339-1343. [DOI] [PubMed] [Google Scholar]
- 15.Reddy, Y. S., A. Kashuba, J. Gerber, and V. Miller. 2003. Roundtable report: importance of antiretroviral drug concentrations in sanctuary sites and viral reservoirs. AIDS Res. Hum. Retrovir. 19:167-176. [DOI] [PubMed] [Google Scholar]
- 16.Saksena, N. K., and S. J. Potter. 2003. Reservoirs of HIV-1 in vivo: implications for antiretroviral therapy. AIDS Rev. 5:3-18. [PubMed] [Google Scholar]
- 17.Steigbigel, R. T., D. A. Cooper, P. N. Kumar, J. E. Eron, M. Schechter, M. Markowitz, M. R. Loutfy, J. L. Lennox, J. M. Gatell, J. K. Rockstroh, C. Katlama, P. Yeni, A. Lazzarin, B. Clotet, J. Zhao, J. Chen, D. M. Ryan, R. R. Rhodes, J. A. Killar, L. R. Gilde, K. M. Strohmaier, A. R. Meibohm, M. D. Miller, D. J. Hazuda, M. L. Nessly, M. J. DiNubile, R. D. Isaacs, B. Y. Nguyen, and H. Teppler. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339-354. [DOI] [PubMed] [Google Scholar]
- 18.Tachet, A., E. Dulioust, D. Salmon, M. De Almeida, S. Rivalland, L. Finkielsztejn, I. Heard, P. Jouannet, D. Sicard, and C. Rouzioux. 1999. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS 13:823-831. [DOI] [PubMed] [Google Scholar]
- 19.Taylor, S., D. J. Back, S. M. Drake, J. Workman, H. Reynolds, S. E. Gibbons, D. J. White, and D. Pillay. 2001. Antiretroviral drug concentrations in semen of HIV-infected men: differential penetration of indinavir, ritonavir and saquinavir. J. Antimicrob. Chemother. 48:351-354. [DOI] [PubMed] [Google Scholar]
- 20.Taylor, S., and A. S. Pereira. 2001. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex. Transm. Infect. 77:4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor, S., R. P. van Heeswijk, R. M. Hoetelmans, J. Workman, S. M. Drake, D. J. White, and D. Pillay. 2000. Concentrations of nevirapine, lamivudine and stavudine in semen of HIV-1-infected men. AIDS 14:1979-1984. [DOI] [PubMed] [Google Scholar]
- 22.ter Heine, R., C. G. Alderden-Los, H. Rosing, M. J. Hillebrand, E. C. van Gorp, A. D. Huitema, and J. H. Beijnen. 2007. Fast and simultaneous determination of darunavir and eleven other antiretroviral drugs for therapeutic drug monitoring: method development and validation for the determination of all currently approved HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 21:2505-2514. [DOI] [PubMed] [Google Scholar]
- 23.van Praag, R. M., G. J. Weverling, P. Portegies, S. Jurriaans, X. J. Zhou, M. L. Turner-Foisy, J. P. Sommadossi, D. M. Burger, J. M. Lange, R. M. Hoetelmans, and J. M. Prins. 2000. Enhanced penetration of indinavir in cerebrospinal fluid and semen after the addition of low-dose ritonavir. AIDS 14:1187-1194. [DOI] [PubMed] [Google Scholar]