Abstract
Topical microbicides for prevention of sexually transmitted diseases (STDs) would be especially useful for women who are not able to persuade their partner(s) to take precautions. Many topical microbicides are in various stages of development, based on a variety of active ingredients. We investigated the in vitro activity of an engineered antimicrobial peptide (WLBU2) and a lipid (3-O-octyl-sn-glycerol [3-OG]) which could potentially be used as active ingredients in such a product. Using commercially available cytotoxicity reagents [Alamar Blue, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and lactate dehydrogenase (LDH)], we first determined the toxicity of WLBU2 and 3-OG to the host cells in our assay procedure and excluded toxic concentrations from further testing. To determine activity against Chlamydia trachomatis, we used an assay previously developed by our laboratory in which chlamydial elementary bodies (EBs) were exposed to microbicides prior to contact with epithelial cells: the minimum (microbi)cidal concentration (MCC) assay. To further simulate conditions of transmission, we carried out the same assay in the presence of a simulated vaginal fluid, a simulated seminal fluid, human serum albumin, and a range of pH values which might be found in the human vagina at the time of exposure. Last, we tested WLBU2 and 3-OG in combination to determine if adding them together resulted in synergistic activity. We found that WLBU2 and 3-OG both have excellent activity in vitro against C. trachomatis and significantly more activity when added together. The simulated fluids reduced activity, but the synergy seen is good evidence that they would be effective when combined in a microbicide formulation.
Because vaccines are not available for most sexually transmitted diseases (STDs), alternative prevention methods are needed. Topical microbicides are STD prevention products currently under development that would be applied before sex, similar to a spermicide. Initial microbicide candidates included commercially available spermicides that contained nonoxynol-9 (non-9) as an active ingredient, but it has become clear that non-9 can actually increase a woman's risk of contracting HIV infection due to the lesions it causes when used frequently (10, 43). Thus, surfactants (like non-9) are no longer leading candidates for formulated products. Other compounds, such as lipids and peptides, have received recent attention as possible topical microbicide candidates (26, 49, 50). In this study, we focused on the antichlamydial activity of the antimicrobial peptide WLBU2 and lipid 3-O-octyl-sn-glycerol (3-OG). WLBU2 is an engineered cationic amphipathic 24-residue peptide that contains only arginine, valine, and tryptophan, whose sequence (RRWVRRVRRWVRRVVRVVRRWVRR) is optimized for formation of an amphipathic helix conformation (9). 3-OG is a synthetic lipid that is modeled after antibacterial lipids found in human breast milk (18, 23). Both WLBU2 and 3-OG have already been found to have excellent activity against many Gram-negative and Gram-positive bacteria (9, 30, 31). WLBU2 has been found to be highly active against herpesvirus and HIV (22; unpublished data). 3-OG has also been found to be active against HIV (21, 34). In our previous published and unpublished research, we found that lipids similar to 3-OG and peptides similar to WLBU2 have good activity against Chlamydia trachomatis specifically (1, 26). A combination microbicide with dual modes of action has the potential to be more potent and less likely to induce resistant strains than one containing a single active ingredient. Synergistic activity between lipids and peptides has already been demonstrated against the herpesvirus using a lipid that is similar to 3-OG and peptides that are similar to WLBU2 (19, 22). Thus, in this study we not only evaluated the activities of these two compounds individually but also examined their combined effect in vitro.
C. trachomatis is the most commonly reported bacterial STD in the United States, and the incidence continues to grow each year. In 2007, more than 1,100,000 new Chlamydia infections were reported to the CDC (5). Antibiotics can be used to cure Chlamydia infections, but many are asymptomatic and go unnoticed. If left untreated, these infections can lead to many serious complications, especially in women (5). C. trachomatis is an obligate intracellular parasite with a unique biphasic developmental cycle. The infectious form, the elementary body (EB), is fairly resistant to changes in environmental conditions. EBs are found in genital secretions and would be exposed to microbicides during transmission. Antibiotics, meant to cure existing infections, must be able to enter the host cell and target the metabolically active and vulnerable reticulate bodies (RBs). Topical microbicides are designed to prevent infection, and thus, we utilized our minimum (microbi)cidal concentration (MCC) assay, previously developed to mimic the order of events in the human vagina during exposure to C. trachomatis, to examine topical microbicide activity directly on extracellular EBs before infection occurs. In this study, we have also modified our MCC assay to simulate the presence of vaginal fluids, seminal fluids, and blood, body fluids that a topical microbicide might come into contact with during a transmission event.
We have found that WLBU2 peptide and 3-OG lipid show excellent potential as active ingredients in a topical microbicide formulation. Both are very active against multiple serovars of C. trachomatis in vitro and are somewhat resistant to simulated body fluids and changes in pH. Additionally, when tested in combination, they act synergistically to inhibit C. trachomatis more than these compounds do individually. This synergistic activity makes them especially desirable for use in a topical microbicide product because it reduces the amount of each compound needed to provide protection, decreasing costs, potential toxicity to the user, and potential for inducing resistant strains of C. trachomatis.
MATERIALS AND METHODS
Cell culture.
McCoy mouse fibroblast cells (ATCC CRL 1696) were maintained in antibiotic-free Eagle's minimal essential medium (M0769; Sigma) supplemented with 10% fetal calf serum, 0.017 M glucose, 0.02 M HEPES, 0.084% sodium bicarbonate, and 2 mM l-glutamine (CMGH). The McCoy cells were tested once per month for mycoplasma contamination by PCR using previously published primers (44, 45) and/or using the Stratagene Mycoplasma Plus PCR primer set (catalog no. 302008).
Inoculum.
C. trachomatis serovars D (UW-3/Cx), E (UW-5/Cx), and L2 (434/Bu) were propagated in McCoy cells and purified as previously described (1). All purified isolates were tested for mycoplasma contamination using the methods described above. A plate typing method was used to verify the serotype of each isolate (38). Directly prior to use, an aliquot of purified inoculum was thawed and diluted to the appropriate concentration in sucrose-phosphate-glutamate buffer (SPG) (0.219 M sucrose, 0.0038 M KH2PO4, 0.0086 M Na2HPO4, 0.0049 M l-glutamic acid, adjusted to pH 7.5 using 10 N NaOH).
Peptide.
WLBU2 was synthesized at the University of Pittsburgh Peptide Synthesis Facility by F-moc chemistry on an automated peptide synthesizer, purified by reverse-phase high-performance liquid chromatography (HPLC), characterized by mass spectrometry, and assayed by quantitative ninhydrin assays, all as previously described (9). The WLBU2 peptide was provided to us as a lyophilized powder and was rehydrated in sterile phosphate-buffered saline (PBS) (0.14 M NaCl, 0.0027 M KCl, 0.0101 M Na2HPO4, 0.0018 M KH2PO4), aliquoted, and stored at −20°C until use.
Lipid.
3-O-octyl-sn-glycerol lipid was designed by Charles Isaacs and synthesized by either Deva Biotech (Hatboro, PA) or Genzyme Pharmaceuticals (Switzerland). Pure 3-OG was diluted to an appropriate stock concentration in dimethyl sulfoxide (DMSO), aliquoted, and stored at 4°C until use.
Simulated environments.
Simulated vaginal fluid (VFS) was made using a recipe previously published by Owen and Katz (33). Simulated seminal fluid (SFS) was also prepared using a published recipe (32) except that we omitted zinc in order to eliminate problems with precipitation. Both simulated fluids were aliquoted and stored at −20°C until use. Human serum albumin (HSA) (A9511; Sigma) was mixed to a concentration of 10% in SPG, filter sterilized using a low-protein binding syringe filter, and stored at 4°C for a maximum of 2 days. SPG was made following our standard recipe as described above but was adjusted to pHs 4, 5.5, 7, and 8 using 10 N NaOH or 10 N glacial acetic acid. SPG was stored at 4°C, and pH was confirmed directly prior to use.
Controls.
Penicillin G (PEN-K; Sigma) and polymyxin B (P4932; Sigma) were used as negative and positive inhibition controls, respectively, in the MCC assay. An inoculum control, in which no drug was added, was included for each C. trachomatis serovar for each time point and simulated environmental condition. Percent inhibition of inclusion formation was calculated using this inoculum control. A cell control (no drug, no inoculum) was included in order to monitor untreated McCoy cell morphology. SPG and Triton X-100 (T8787; Sigma) were used as negative and positive toxicity controls, respectively, in the cytotoxicity assays.
Cytotoxicity assays.
Prior to the MCC assay, we measured the cytotoxicity of WLBU2 and 3-OG to the McCoy cells in the assay procedure. The assay, as described below, was followed except that no inoculum was added to the compounds. WLBU2 and 3-OG were instead diluted in half with SPG to imitate the dilution when an inoculum was added. After incubating the cells at 37°C for 48 h, the manufacturer's instructions were followed for the Alamar Blue (DAL 1100; Invitrogen), (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (V-13154; Molecular Probes), and lactate dehydrogenase (LDH) (11644793001; Roche) assays. The SPG with various pHs, SFS, VFS, HSA, and WLBU2 and 3-OG in combination with each other were also tested for cytotoxicity to McCoy cells in the same manner. A test compound was considered “nontoxic” if it displayed less than 10% toxicity to McCoy cell monolayers for all cytotoxicity testing methods.
Standard MCC assay.
We used our previously published preinoculation assay (26) to test the antichlamydial activities of WLBU2 and 3-OG. In brief, 96-well tissue culture (TC) plates were seeded with 5 × 104 mycoplasma-free McCoy cells in 0.2 ml CMGH per well the day prior to the assay and incubated at 37°C in 5% CO2 overnight. On the day of the assay, 10 2-fold dilutions of WLBU2, 3-OG, polymyxin B, and penicillin G (starting at 100 μM, 50 mM, 1.4 mM [2 mg/ml], and 5.4 mM [2 mg/ml], respectively) were made in SPG. PBS and DMSO were also tested at the concentrations at which they were present as diluents for WLBU2 and 3-OG, respectively. Chlamydial EBs were diluted in SPG to a concentration of 5 × 106 inclusion-forming units (IFU)/ml. The remainder of the assay was performed as previously published (26). Because the compounds were diluted 1:1 with inoculum, the actual concentrations that EBs were exposed to were 50 μM, 25 mM, 0.7 mM (1 mg/ml), and 2.7 mM (1 mg/ml) for WLBU2, 3-OG, polymyxin B, and penicillin G, respectively. An effective topical microbicide will be fast acting, and thus, a 5-min incubation period was chosen to demonstrate the ability of the compounds to quickly inhibit chlamydial EBs before they bind to and enter host cells. A 120-min time point was chosen as a long exposure to gauge the “maximum” activity of the compounds when acting alone against chlamydial EBs. The purpose of this assay is to determine the direct activity of the compounds on chlamydial EBs before they enter host cells. Purified EBs were exposed to test compounds for a defined period of time, the mixture was diluted 1:40 in SPG in order to effectively eliminate the activity of the compound, and the remaining viable EBs were then inoculated onto host cells. Following the appropriate incubation period for Chlamydia growth (36 to 48 h depending on the serovar), cell monolayers were fixed with 100% methanol and stained with a primary antibody to C. trachomatis lipopolysaccharide (LPS) generated in mice (E6-H1; provided by Harlan Caldwell). Secondary staining was done using an anti-mouse IgG fluorescein isothiocyanate-conjugated antibody (Sigma F-9006) diluted 1:250 in Evans Blue counterstain (0.5% Evans Blue, 5% sodium azide, and 94.5% PBS). For each test, plating was done in triplicate, three fields per well were counted, and the IFU counts between triplicates were averaged. Percent inhibition of inclusion formation was calculated with the following formula: {[(average IFU in the inoculum control) − (average IFU in the test)]/(average IFU in the inoculum control)} × 100 = percent inhibition of C. trachomatis IFU formation. All tests were performed three times, on different days. The results of the three independent tests were averaged, and the standard deviations (SD) between the three tests at each concentration were calculated. Results were reported as percent inhibition ± SD of C. trachomatis inclusion formation compared to results for a no-drug control. The standard minimum (microbi)cidal concentration (MCC) was defined as the lowest concentration of a test compound that completely inhibited C. trachomatis inclusion formation.
MCC assay in simulated environments.
We modified the previously described assay to examine the effects of simulated environmental conditions (the presence of vaginal fluid, seminal fluid, and human blood). Ninety-six-well plates were seeded with McCoy cells as described previously. On the day of the assay, five 4-fold dilutions of WLBU2, 3-OG, polymyxin B, and penicillin G (starting at concentrations of 120 μM, 60 mM, 1.4 mM [2 mg/ml], and 5.4 mM [2 mg/ml], respectively) were made in SPG. The C. trachomatis inoculum was diluted in either 100% VFS, 100% SFS, or 10% HSA to a concentration of 5 × 106 IFU/ml. The remainder of the assay was performed as described previously. After a 1:1 dilution of the test compound with inoculum, the final concentrations of VFS, SFS, and HSA in the assay were 50%, 50%, and 5%, respectively, and the final concentrations of WLBU2, 3-OG, polymyxin B, and penicillin G were 60 μM, 30 mM, 2.7 mM (1 mg/ml), and 0.7 mM (1 mg/ml), respectively. Percent inhibition of inclusion formation was calculated compared to a no-drug control in the same environment at the same exposure time.
MCC assay with pH adjustment.
The standard MCC assay procedure was followed as described previously except that WLBU2, 3-OG, and the inoculum were all diluted in pH 4, 5.5, 7, or 8 SPG. The pH remained constant in the exposure portion of the assay because the test compound and the inoculum were both diluted in SPG with the same pH. In the 1:40 dilution step after exposure, all tests and controls were diluted in pH 7.5 SPG. The percent inhibition of inclusion formation was calculated compared to the value for a no-drug control at the same pH and same exposure time.
WLBU2 and 3-OG tested in combination against serovar L2.
In addition to testing the peptide and lipid against C. trachomatis separately, we tested them in multiple concentration combinations against serovar L2 in the MCC assay to determine if adding them together resulted in higher than expected (synergistic) activity.
Determination of synergy.
Synergy was calculated in two ways. First, we reported synergy for a particular combination if the inhibition of chlamydial inclusion formation was greater than what would be expected if the effect was additive under Bliss' independence theory using the following formula: Ii = IWLBU2 + I3OG − (IWLBU2 × I3OG), where Ii is the predicted fractional response of the drug combination if there was no interaction between WLBU2 and 3-OG (additive effect) (3). IWLBU2 and I3OG are the actual fractional responses of each drug acting alone in the assay. In a second approach, synergy was calculated using the fractional inhibitory concentration (FIC) of each compound (MCCcombination/MCCalone). If an MCC was not achieved within the tested concentrations, the highest tested concentration was used. Synergy was determined based on the FIC index, which is defined as the sum of the FICs for the two compounds (24): FIC index = (MCCWLBU2 with 3-OG/MCCWLBU2 alone) + (MCC3-OG with WLBU2/MCC3-OG).
The combination of WLBU2 and 3-OG was considered to be synergistic if the FIC index was <1, additive if the FIC index equalled 1, and antagonistic if the FIC index was >1.
Statistical analysis.
For the simulated environment assays, two-way analysis of variance (ANOVA) was performed to test for the independent effects of environment, concentration, and the interaction between them on average percent inhibition of IFU formation. Significance was reported if the P value was ≤0.05. If it was determined that activity of the peptide or lipid was dependent on a simulated environmental fluid, Tukey's honestly significant difference (HSD) tests (47) were performed to compare each simulated environment to plain SPG at each concentration, and significance was reported if the P value was ≤0.05. The same analysis was performed with the pH assays, comparing the activities of the peptide and lipid in SPG at various pHs to the activity in pH 7 SPG. For all of these analyses (and the comparison of serovars, below), only the data for the first three test concentrations were used, because the lower concentrations of peptide (≤0.94 μM) and lipid (≤0.46 mM) had little activity in any buffer, thus making comparisons meaningless.
The susceptibilities of the three different serovars to WLBU2 peptide and 3-OG lipid were compared by performing a two-way ANOVA analysis of the independent effects of the serovar, the concentration, and the interaction between them on the average percent inhibition of IFU formation in each experiment. Significance was reported if the P value was ≤0.05. If it was determined that activity of the peptide or lipid was significantly different depending on the particular serovar, Tukey HSD tests (47) were performed to compare each serovar to the others and a significant difference was reported if the P value was ≤0.05.
For the synergy assays, a one-way ANOVA with Bonferroni's correction (Pcorr = 0.0004) was performed to determine if there was a significant difference between actual and expected activities. A two-sided paired t test was then performed to determine if the actual activity for each concentration combination was significantly different from the expected activity. Significance was reported at the alpha = 0.05 and 0.1 levels.
RESULTS
Cytotoxicity assays.
WLBU2 and 3-OG were tested for cytotoxicity to McCoy cells in the MCC assay using the LDH, Alamar Blue, and MTT assays. We found that WLBU2 could be used in our assay at concentrations of ≤60 μM without causing toxicity and 3-OG could be used at concentrations of ≤30 mM (data not shown). Per our assay procedure, compounds were diluted 1:40 before addition to McCoy cells, so the highest concentrations of WLBU2 and 3-OG that were nontoxic to the McCoy cell monolayers directly were 1.5 μM and 0.75 mM, respectively. WLBU2 and 3-OG in combination with each other were also tested for cytotoxicity. In general, the combinations were nontoxic, though the MTT assay did detect slight toxicity (≤16% ± 5%) for 60 μM WLBU2 paired with 7.5 mM, 3.75 mM, and 1.88 mM 3-OG (data not shown). These same combinations were nontoxic in the LDH assay, but the Alamar Blue assay detected slight toxicity (12% ± 8%) for the 60 μM WLBU2-7.5 mM 3-OG combination.
MCC assay.
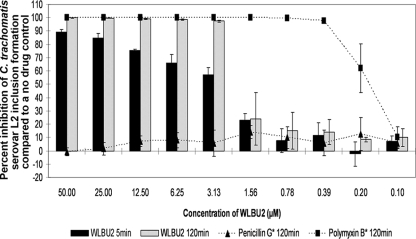
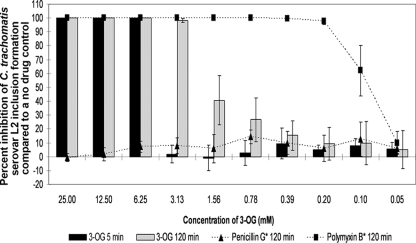
We screened the WLBU2 peptide and the 3-OG lipid for activity against C. trachomatis serovar L2 using the standard minimum (microbi)cidal concentration (MCC) assay, and both showed excellent dose-dependent activity. Fifty micromolar WLBU2 was 89.35% ± 1.86% inhibitory after 5 min and 99.94% ± 0.10% inhibitory after 120 min (Fig. 1). WLBU2 activity remained at >97% for all other concentrations of ≥3.13 μM after 120 min of exposure and >56% at the same concentrations after 5 min. 3-OG was 100% inhibitory after 5 min of exposure for concentrations of ≥6.25 mM (Fig. 2). A 3.13 mM concentration of 3-OG was 98.19% ± 1.63% inhibitory after 120 min. All other tested concentrations of WLBU2 and 3-OG were minimally active.
FIG. 1.
The activity of WLBU2 (50 to 0.1 μM) against C. trachomatis serovar L2 in our standard MCC assay after 5 and 120 min of exposure. Ten 2-fold dilutions of the negative and positive inhibition controls (penicillin G and polymyxin B, respectively) were also run in this assay. *, the highest test concentrations for penicillin G and polymyxin B were 2.69 mM and 0.72 mM, respectively. Percent inhibition of inclusion formation was calculated as a percentage based on the number of inclusions in the no-drug control. Each test was performed in triplicate, and the results are reported as the average of results from those three tests. Standard deviations (SD) of the results are indicated on the graph with error bars.
FIG. 2.
The activity of 3-OG (25 to 0.05 mM) against C. trachomatis serovar L2 in our standard MCC assay after 5 and 120 min of exposure. The remainder of the assay conditions are described in the legend for Fig. 1.
Simulated environment MCC assay.
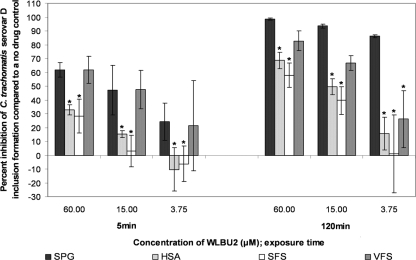
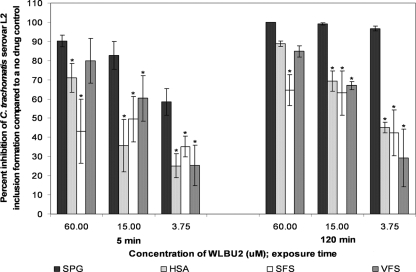
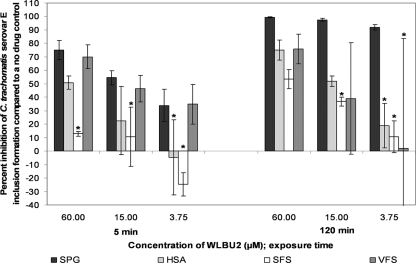
Five 4-fold dilutions of WLBU2 (starting at 60 μM) and 3-OG (starting at 30 mM) were tested in the standard MCC assay containing VFS, SFS, or HSA. All three simulants reduced the antichlamydial activity of WLBU2 against serovars D, E, and L2 (Fig. 3 to 5, respectively). VFS did not reduce the activity of WLBU2 against serovar D or E compared to results with SPG alone after 5 min (Fig. 3 and 4, respectively), but it did significantly reduce the activity of 3.75 μM WLBU2 against both serovars after 120 min (Tukey's test; P ≤ 0.05). VFS significantly reduced the activity of 15 μM and 3.75 μM WLBU2 against serovar L2 after 5 and 120 min (Tukey's test; P ≤ 0.05) (Fig. 5). HSA and SFS both reduced the activity of WLBU2 after 5 and 120 min for all serovars (Tukey's test; P ≤ 0.05) (Fig. 3 to 5).
FIG. 3.
Activity of WLBU2 (60 to 3.75 μM) against C. trachomatis serovar D in our simulated environment assay. The conditions for the standard MCC assay were followed except for the addition of 50% VFS, 50% SFS, or 5% HSA. Percent inhibition was calculated as for Fig. 1 but based on a no-drug control with the same simulated environment. Experiments were performed in triplicate, and results that are statistically different from those for SPG alone (Tukey's test; P ≤ 0.05) are indicated with an asterisk.
FIG. 5.
Activity of WLBU2 (60 to 3.75 μM) against C. trachomatis serovar L2 in our simulated environment assay. The remainder of the assay conditions are described in the legend for Fig. 3.
FIG. 4.
Activity of WLBU2 (60 to 3.75 μM) against C. trachomatis serovar E in our simulated environment assay. The remainder of the assay conditions are described in the legend for Fig. 3.
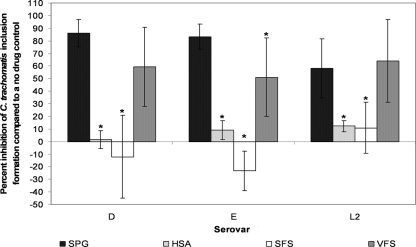
3-OG activity against C. trachomatis was fairly resistant to changes when the various simulated environments were present. However, when the inoculum was diluted in SFS or HSA rather than SPG, the MCC for 3-OG increased from 7.5 mM to 30 mM for the 5-min exposure for all serovars (Table 1) and the activity of 1.88 mM 3-OG was significantly reduced (Tukey's test; P ≤ 0.05) (Fig. 6). VFS had no effect on 3-OG activity against serovars D and L2 at either time point but did significantly reduce the activity of 1.88 mM 3-OG against serovar E at the 120-min time point (Tukey's test; P ≤ 0.05) (Fig. 6).
TABLE 1.
MCC of 3-OG against C. trachomatis serovars D, E, and L2 in the presence of various environmental exposures
| Environmental exposure | MCC (mM)a |
|
|---|---|---|
| 5 min | 120 min | |
| SPG only | 7.5 | 7.5 |
| VFS | 7.5 | 7.5 |
| SFS | 30 | 7.5 |
| HSA | 30 | 7.5 |
The MCC is the same for all serovars (D, E, and L2).
FIG. 6.
Activities of 1.88 mM 3-OG against C. trachomatis serovars D, E, and L2 in the presence of plain SPG, HSA, VFS, and SFS for 120 min of exposure. Percent inhibition and SD were calculated as for Fig. 1 but based on a no-drug control in the same simulated environment. An asterisk indicates that the activity of 3-OG is significantly different from the activity in the presence of plain SPG (Tukey's test; P ≤ 0.05).
MCC assay with pH adjustment.
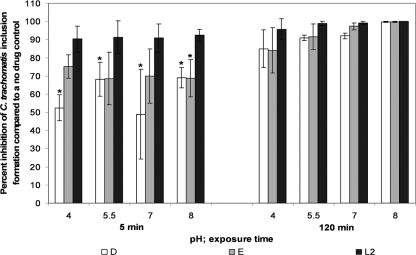
Five 4-fold dilutions of WLBU2 and 3-OG (starting at 60 μM and 30 mM, respectively) were tested against C. trachomatis serovars D, E, and L2 in SPG at different pH values. There was a slight trend toward decreased activity of 60 μM WLBU2 at lower pH, though this result was not statistically significant (Tukey's test; P > 0.05) (Fig. 7). After 120 min, WLBU2 was significantly less active in pH 4 SPG than in pH 7 SPG against serovars L2 and D at 3.75 μM and at 15 μM for serovar L2 (Tukey's test; P ≤ 0.05; data not shown).
FIG. 7.
Activities of 60 μM WLBU2 against C. trachomatis serovars D, E, and L2 in our pH assay. The conditions for the standard MCC assay were followed except that they were carried out in the presence of SPG at various pHs (4, 5.5, 7, and 8). Percent inhibition and SD were calculated as for Fig. 1 but based on a no-drug control with the same pH. An asterisk indicates that the activity is significantly different against serovar D or E than the activity against L2 (Tukey's test; P ≤ 0.05).
We found that pH adjustment did not alter the MCC of 3-OG at either time point. The only significant difference in activity seen was that 1.88 mM 3-OG was more active at pHs 4, 5.5, and 8 than pH 7 for serovars D and E at 120 min (Tukey's test; P ≤ 0.05; data not shown).
Comparison of activities against multiple serovars.
In the simulated environment and pH assays, we tested WLBU2 and 3-OG against three different serovars of C. trachomatis. Overall, in the pH assays and simulated environment assays, serovars D and E were significantly less susceptible to WLBU2 than L2 after 5 min of exposure and serovar D was significantly less susceptible to L2 at 120 min (Tukey's test; P ≤ 0.05; this effect can be seen by referencing Fig. 3 to 7). When broken down by concentration and pH, serovar L2 was significantly more susceptible to 60 μM WLBU2 than serovar D at all pHs for the 5-min exposure (Tukey's test; P ≤ 0.05) (Fig. 7). A similar trend was seen for serovar E, but the results were significant only for pH 8 (Fig. 7). All serovars had approximately the same sensitivity to 3-OG (two-way ANOVA; P > 0.05; data not shown).
WLBU2 and 3-OG tested in combination against serovar L2.
Overall, when tested in combination, WLBU2 and 3-OG had significantly greater activity in our standard MCC assay than would be expected if the activity resulting from the mixture was additive based on Bliss' independence theory (one-way ANOVA; P < 0.0001). The actual and expected activities for each concentration combination for the 5- and 120-min exposures are reported in Tables 2 and 3, respectively. In addition, the FIC index for WLBU2 and 3-OG in combination was 0.56 ± 0 and 0.45 ± 0.14 for the 5- and 120-min exposures, respectively, indicating that the combination is synergistic against Chlamydia in vitro. The FIC of WLBU2 was calculated using the highest concentration of WLBU2 tested (60 μM) because an MCC with WLBU2 was not achieved. Thus, the calculated FIC index likely understates the level of synergy.
TABLE 2.
Activity of WLBU2 and 3-OG in combination against C. trachomatis serovar L2 inclusion formation after 5 min of exposurea
| WLBU2 concn (μM) | Activity (%) at 3-OG concn (mM) ofb: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 7.5 | 3.75 | 1.88 | 0.94 | 0.47 | 0.23 | 0.12 | 0.00 | |
| 60 | 100.000 | †100.000 | ‡99.995 | ‡98.658 | †97.672 | ‡94.983 | 94.082 | 92.486 |
| 100.000 | 96.303 | 92.222 | 91.932 | 92.742 | 92.502 | 92.348 | ||
| 30 | 100.000 | †100.000 | †99.934 | †98.080 | †97.410 | 95.679 | 94.440 | 91.048 |
| 100.000 | 94.708 | 89.472 | 89.013 | 92.911 | 92.404 | 92.408 | ||
| 15 | 100.000 | †100.000 | †99.695 | †95.652 | †96.028 | 93.222 | 91.057 | 87.300 |
| 100.000 | 91.601 | 83.724 | 83.200 | 91.348 | 90.879 | 90.817 | ||
| 7.5 | 100.000 | †100.000 | †99.260 | †93.674 | ‡92.414 | 89.042 | 86.281 | 79.663 |
| 100.000 | 87.573 | 75.375 | 74.524 | 84.473 | 83.700 | 83.549 | ||
| 3.75 | 100.000 | 100.000 | 96.985 | 85.390 | 81.966 | 78.976 | 75.924 | 69.552 |
| 100.000 | 84.874 | 69.132 | 66.651 | 70.954 | 69.606 | 69.322 | ||
| 1.88 | 100.000 | †99.952 | †93.778 | †80.721 | 71.437 | 67.217 | 58.116 | 49.782 |
| 100.000 | 68.898 | 47.623 | 48.006 | 40.026 | 36.334 | 39.646 | ||
| 0.00 | 100.000 | 43.229 | -1.387 | -1.465 | -1.637 | -6.235 | -5.050 | 0.000 |
The data are presented as the average actual activity (percent inhibition) of the combinations. Directly underneath stated activity is the average expected percent inhibition of the combinations if the activity was additive. SDs are not reported in this table, but the “†” symbol indicate the actual activity is significantly different from the expected activity at the alpha = 0.05 level (two-sided paired t test). The “‡” symbol indicates the actual activity is significantly different from the expected activity at the alpha = 0.1 level (two-sided paired t test). The activities of the compounds singly are represented in boldface in the bottom row (3-OG) and the last column (WLBU2).
Activity reported for each compound alone at each of various concentrations (bottom row and last column) is the average of results from all experiments performed with that concentration. For technical reasons, some concentrations of each compound were tested in more than three experiments. The expected activity for each concentration combination that is reported within the table was calculated based on the activity of each compound alone in the same experiments.
TABLE 3.
Activities of WLBU2 and 3-OG in combination on C. trachomatis serovar L2 inclusion formation after 120 min of exposurea
| WLBU2 concn (μM) | Activity (%) at 3-OG concn (mM) of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 7.5 | 3.75 | 1.88 | 0.94 | 0.47 | 0.23 | 0.12 | 0.00 | |
| 60 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 99.997 | 99.994 | 99.932 |
| 100.000 | 100.000 | 99.960 | 99.939 | 99.955 | 99.950 | 99.950 | ||
| 30 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 99.991 | 99.811 |
| 100.000 | 100.000 | 99.872 | 99.877 | 99.860 | 99.844 | 99.846 | ||
| 15 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 99.991 | 99.994 | 99.738 |
| 100.000 | 100.000 | 99.769 | 99.828 | 99.867 | 99.853 | 99.853 | ||
| 7.5 | 100.000 | 100.000 | ‡100.000 | 100.000 | 100.000 | 99.997 | 99.967 | 99.415 |
| 100.000 | 100.000 | 99.534 | 99.586 | 99.662 | 99.624 | 99.627 | ||
| 3.75 | 100.000 | 100.000 | 100.000 | 100.000 | 99.982 | 99.927 | 99.817 | 99.045 |
| 100.000 | 100.000 | 99.077 | 99.807 | 99.277 | 99.202 | 99.199 | ||
| 1.88 | 100.000 | 100.000 | 100.000 | 99.965 | 99.958 | 99.910 | 99.523 | 87.898 |
| 100.000 | 100.000 | 88.225 | 84.781 | 78.581 | 77.660 | 75.163 | ||
| 0.00 | 100.000 | 100.000 | 38.879 | 32.155 | 10.946 | 1.185 | 1.019 | 0.000 |
See the footnotes for Table 2.
DISCUSSION
In vitro testing is much simpler and less expensive than in vivo testing, and the more closely an in vitro topical microbicide assay can mimic real conditions, the more relevant it is. Owen and Katz previously developed a simulated vaginal fluid and a simulated seminal fluid, which physically and chemically resemble real human vaginal fluid and semen (32, 33). These fluids have been used in experiments determining their effect on rheological properties and coating flow of microbicide gels (25), gel layer erosion, and drug release (15). The vaginal fluid simulant has also been used to determine its effect on the physical-chemical characteristics and anti-HIV activities of microbicide formulations containing 3-OG and UC-781 (34). A topical microbicide product must remain stable at various pHs because the pH of the human vagina can range from 4 to 8 depending on a variety of factors, including infection and the presence of semen (33, 41).
Blood is another body fluid that is often present in times of intercourse either due to menstruation, microabrasion, or other tissue damage. HSA is a component of blood that has been shown to inactivate some lipids and peptides (8, 30) and is often used as a substitute for whole blood during in vitro testing. In this study, we were able to use VFS, SFS, and HSA to simulate potential inhibiting factors that might be present while a topical microbicide product is in use. This information will be useful for determining the best product formulation. Clearly, these simulated fluids cannot provide the full effect of real vaginal fluid, seminal fluid, and whole human blood, because there are many other proteins and chemicals that are not present in the simulants. However, bodily fluids vary from person to person and require human subjects' approval before collection. Thus, for a preliminary study such as ours, these simulants are a good substitute; they are relatively cheap and easy to make, do not require human subjects' approval, and provide a reliable fluid with a consistent chemical makeup for experimental purposes.
Current topical microbicide research is, for the most part, focused on HIV prevention because of the severity and irreversibility of the disease. There are many products currently in various stages of development, with many different modes of action. Detergents, such as Savvy, act by disrupting the outer coat or lipid membrane of viruses and bacteria, which also makes them potentially cytotoxic to human tissue (28). Carrageenan (the active ingredient in Carraguard), cellulose sulfate, and PRO 2000 (a sulfated polyanion) prevent HIV from binding to the surfaces of target cells (4, 36, 40). Despite strong evidence of protection in vitro, in vivo results with these candidates have been disappointing. PRO 2000 was reported to reduce the risk of HIV infection by 30% at a recent retrovirus meeting, but this result was not statistically significant (6). Carraguard was found to be safe but not active against HIV in a randomized, double-blind, placebo-controlled trial (37). Most alarmingly, there is good evidence that cellulose sulfate might actually increase susceptibility to HIV (39, 42). Reverse transcriptase inhibitors (12) and dendrimers are also being investigated for use in topical microbicide products. One product containing the dendrimer SPL7013 (VivaGel) is active against herpes simplex virus (HSV) in vitro (14) and simian-human immunodeficiency virus (SHIV) in nonhuman primates (29). Glycerol monolaurate (GML) is a fatty acid monoester that inhibits growth and exotoxin production of vaginal pathogens and cytokine production by vaginal epithelial cells (35), and it has recently been found to prevent simian immunodeficiency virus (SIV) transmission (27).
While HIV prevention is clearly a priority, other STDs, such as chlamydia, gonorrhea, and herpes, should be addressed. There are many serious health problems that can result from such infections (5), and there is strong evidence that individuals suffering from STDs are actually more susceptible to acquiring or transmitting HIV (2, 11, 13, 46). The human host defense system is comprised of many factors, including naturally occurring peptides and lipids. These molecules and their derivations could be used as active ingredients in topical microbicides. In our previous research, we demonstrated that some lipids adapted from those found in human breast milk and a number of cecropin peptides have good activity against C. trachomatis (1, 26). A number of naturally occurring and synthetic peptides were previously found by Yasin et al. to be active against C. trachomatis (49), most notably the alpha-helical peptide novispirin G10 (48).
Ideally, a topical microbicide product would be nontoxic, inexpensive, easy to obtain and use, and effective against a number of STD pathogens without disrupting the normal vaginal or rectal flora. We found that 3-OG had an MCC of 6.25 mM against C. trachomatis EBs. It has already been shown that 3-OG is active against Neisseria gonorrhoeae (MCC, 2.5 to 5.0 mM), Haemophilus ducreyi (MCC, 1.25 to 2.5 mM), and Streptococcus agalactiae (MCC, 5.0 to 10 mM) at concentrations that are at the low range of or lower than those required to inactivate vaginal Lactobacillus (MCC, 5.0 to 7.5 mM, 5.0 mM, 5.0 to 7.5 mM, and >10.0 mM for L. crispatus, L. jensenii, L. iners, and L. vaginalis, respectively) (30). Both 3-OG and WLBU2 are active against HIV (21, 34; T. A. Mietzner and R. C. Montelaro, unpublished data). Using our MCC assay, we demonstrated that WLBU2 and 3-OG are both excellent candidates for use in a topical microbicide formulation that targets C. trachomatis. Individually, they have strong activity against multiple serovars of C. trachomatis at various pHs. The activity of 3-OG is decreased in the presence of SFS and HSA, but activity still remains high at concentrations of ≥15 mM, and there is no increase in the MCC in the presence of VFS. WLBU2 activity is reduced in the presence of all the simulated fluids and possibly at acidic pH, which should be compensated for in the formulation design. However, previous studies have indicated that WLBU2 retains substantial in vitro antibacterial activity in human serum and whole blood (8) and that WLBU2 can be used to effectively treat Pseudomonas aeruginosa bacteremia in a murine model (7).
We found that when tested in combination, WLBU2 and 3-OG have excellent synergistic activity against C. trachomatis. A combination microbicide would have less potential of inducing resistant Chlamydia strains, particularly if the active ingredients have different modes of action. We plan to examine the mechanisms of action of WLBU2 and 3-OG against Chlamydia, since they are currently unknown. However, lipids typically interact with and disrupt the lipid bilayers in bacterial and viral membranes (20). In our previously published work, we examined preparations of chlamydial EBs exposed to lipids similar to 3-OG by electron microscopy and found that the lipids disrupted the chlamydial inner membrane, allowing leakage of the cytoplasmic contents (26). Cationic peptides typically interact electrostatically with LPS in the bacterial membrane and enter the bacterial cell, where they can interact with the cytoplasmic membrane or other organelles (16, 17). However, it has been shown that the mechanism of action of cationic peptides often depends on the concentration tested; at high enough concentrations, most cationic peptides can cause major membrane damage even if the mode of action at or around the minimal effective bactericidal concentration is more specific (16). A combination microbicide which acts synergistically would be preferable because the amount of each compound needed in the final product is reduced. Limiting the amount of each compound not only reduces the costs associated with production; it reduces the chance of toxic effects to the user. Further experiments are under way to place these compounds in an actual formulation in the form of a gel or cream and determine safety and antichlamydial activity in vitro and in vivo. It will also be important to test the activities of these compounds against other STD pathogens and normal vaginal and rectal flora.
Acknowledgments
We thank Harlan Caldwell for providing the E6-H1 antichlamydial LPS antibody, Max Gordon and Inyoung Chong for their technical assistance, Lisa Rohan for her guidance on technical issues, faculty and students from the Statistical Consulting Program at the University of Washington for help with the statistical analysis, and Kazi Islam and the University of Pittsburgh Peptide Synthesis Facility for production of the WLBU2 peptide.
This work was supported by grant AR21 AI076020 from the National Institutes of Health.
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Ballweber, L. M., J. E. Jaynes, W. E. Stamm, and M. F. Lampe. 2002. In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 46:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman, S. M., and M. S. Cohen. 2006. STD treatment: how can it improve HIV prevention in the South? Sex. Transm. Dis. 33:S50-S57. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, C. I., and D. F. Mexico. 1939. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26:585-615. [Google Scholar]
- 4.Buck, C. B., C. D. Thompson, J. N. Roberts, M. Müller, D. R. Lowy, and J. T. Schiller. 2006. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2:e69. [DOI] [PMC free article] [PubMed]
- 5.CDC. 2009. Sexually transmitted disease surveillance, 2007. Department of Health and Human Services, Atlanta, GA.
- 6.Cohen, J. 2009. Retrovirus meeting. HIV/AIDS researchers reach for high-hanging fruit. Science 323:996-997. [DOI] [PubMed] [Google Scholar]
- 7.Deslouches, B., I. A. Gonzalez, D. DeAlmeida, K. Islam, C. Steele, R. C. Montelaro, and T. A. Mietzner. 2007. De novo-derived cationic antimicrobial peptide activity in a murine model of Pseudomonas aeruginosa bacteraemia. J. Antimicrob. Chemother. 60:669-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deslouches, B., K. Islam, J. K. Craigo, S. M. Paranjape, R. C. Montelaro, and T. A. Mietzner. 2005. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob. Agents Chemother. 49:3208-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deslouches, B., S. M. Phadke, V. Lazarevic, M. Cascio, K. Islam, R. C. Montelaro, and T. A. Mietzner. 2005. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 49:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 11.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher, P., S. Harman, H. Azijn, N. Armanasco, P. Manlow, D. Perumal, M.-P. de Bethune, J. Nuttall, J. Romano, and R. Shattock. 2009. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 53:487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvin, S. R., and M. S. Cohen. 2004. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 14.Gong, E., B. Matthews, T. McCarthy, J. Chu, G. Holan, J. Raff, and S. Sacks. 2005. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antiviral Res. 68:139-146. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, K. M., S. R. Barnes, R. A. Tangaro, M. C. Roberts, D. H. Owen, D. F. Katz, and P. F. Kiser. 2007. Temperature and pH sensitive hydrogels: an approach towards smart semen-triggered vaginal microbicidal vehicles. J. Pharm. Sci. 96:670-681. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, R. E., and A. Rozek. 2002. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 206:143-149. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet i:156-164. [DOI] [PubMed]
- 18.Isaacs, C. E. 2001. The antimicrobial function of milk lipids. Adv. Nutr. Res. 10:271-285. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs, C. E., J. H. Jia, and W. Xu. 2004. A lipid-peptide microbicide inactivates herpes simplex virus. Antimicrob. Agents Chemother. 48:3182-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacs, C. E., K. S. Kim, and H. Thormar. 1994. Inactivation of enveloped viruses in human bodily fluids by purified lipids. Ann. N. Y. Acad. Sci. 724:457-464. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs, C. E., R. E. Litov, and H. Thormar. 1995. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. Nutr. Biochem. 6:362-366. [DOI] [PubMed] [Google Scholar]
- 22.Isaacs, C. E., L. Rohan, W. Xu, J. H. Jia, T. Mietzner, and S. Hillier. 2006. Inactivation of herpes simplex virus clinical isolates by using a combination microbicide. Antimicrob. Agents Chemother. 50:1063-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaacs, C. E., and H. Thormar. 1991. The role of milk-derived antimicrobial lipids as antiviral and antibacterial agents. Adv. Exp. Med. Biol. 310:159-165. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan, M. Y., E. J. Manning, and M. T. Collins. 2009. Effects of interactions of antibacterial drugs with each other and with 6-mercaptopurine on in vitro growth of Mycobacterium avium subspecies paratuberculosis. J. Antimicrob. Chemother. 64:1018-1023. [DOI] [PubMed] [Google Scholar]
- 25.Lai, B. E., Y. Q. Xie, M. L. Lavine, A. J. Szeri, D. H. Owen, and D. F. Katz. 2008. Dilution of microbicide gels with vaginal fluid and semen simulants: effect on rheological properties and coating flow. J. Pharm. Sci. 97:1030-1038. [DOI] [PubMed] [Google Scholar]
- 26.Lampe, M. F., L. M. Ballweber, C. E. Isaacs, D. L. Patton, and W. E. Stamm. 1998. Killing of Chlamydia trachomatis by novel antimicrobial lipids adapted from compounds in human breast milk. Antimicrob. Agents Chemother. 42:1239-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Q., J. D. Estes, P. M. Schlievert, L. Duan, A. J. Brosnahan, P. J. Southern, C. S. Reilly, M. L. Peterson, N. Schultz-Darken, K. G. Brunner, K. R. Nephew, S. Pambuccian, J. D. Lifson, J. V. Carlis, and A. T. Haase. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madan, R. P., M. J. Keller, and B. C. Herold. 2006. Prioritizing prevention of HIV and sexually transmitted infections: first-generation vaginal microbicides. Curr. Opin. Infect. Dis. 19:49-54. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy, T. D., P. Karellas, S. A. Henderson, M. Giannis, D. F. O'Keefe, G. Heery, J. R. A. Paull, B. R. Matthews, and G. Holan. 2009. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2:312-318. [DOI] [PubMed] [Google Scholar]
- 30.Moncla, B. J., K. Pryke, and C. E. Isaacs. 2008. Killing of Neisseria gonorrhoeae, Streptococcus agalactiae (group B Streptococcus), Haemophilus ducreyi, and vaginal Lactobacillus by 3-O-octyl-sn-glycerol. Antimicrob. Agents Chemother. 52:1577-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak, K. F., W. J. Diamond, S. Kirakodu, R. Peyyala, K. W. Anderson, R. C. Montelaro, and T. A. Mietzner. 2007. Efficacy of the de novo-derived antimicrobial peptide WLBU2 against oral bacteria. Antimicrob. Agents Chemother. 51:1837-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen, D. H., and D. F. Katz. 2005. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26:459-469. [DOI] [PubMed] [Google Scholar]
- 33.Owen, D. H., and D. F. Katz. 1999. A vaginal fluid simulant. Contraception 59:91-95. [DOI] [PubMed] [Google Scholar]
- 34.Sassi, A. B., C. E. Isaacs, B. J. Moncla, P. Gupta, S. Hillier, and L. C. Rohan. 2008. Effects of physiological fluids on physical-chemical characteristics and activity of topical vaginal microbicide products. J. Pharm. Sci. 97:3123-3139. [DOI] [PubMed] [Google Scholar]
- 35.Schlievert, P. M., K. L. Strandberg, A. J. Brosnahan, M. L. Peterson, S. E. Pambuccian, K. R. Nephew, K. G. Brunner, N. J. Schultz-Darken, and A. T. Haase. 2008. Glycerol monolaurate does not alter rhesus macaque (Macaca mulatta) vaginal lactobacilli and is safe for chronic use. Antimicrob. Agents Chemother. 52:4448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scordi-Bello, I. A., A. Mosoian, C. He, Y. Chen, Y. Cheng, G. A. Jarvis, M. J. Keller, K. Hogarty, D. P. Waller, A. T. Profy, B. C. Herold, and M. E. Klotman. 2005. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob. Agents Chemother. 49:3607-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skoler-Karpoff, S., G. Ramjee, K. Ahmed, L. Altini, M. G. Plagianos, B. Friedland, S. Govender, A. De Kock, N. Cassim, T. Palanee, G. Dozier, R. Maguire, and P. Lahteenmaki. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977-1987. [DOI] [PubMed] [Google Scholar]
- 38.Suchland, R. J., and W. E. Stamm. 1991. Simplified microtiter cell culture method for rapid immunotyping of Chlamydia trachomatis. J. Clin. Microbiol. 29:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao, W., C. Richards, and D. Hamer. 2008. Enhancement of HIV infection by cellulose sulfate. AIDS Res. Hum. Retroviruses 24:925-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teleshova, N., T. Chang, A. Profy, and M. E. Klotman. 2008. Inhibitory effect of PRO 2000, a candidate microbicide, on dendritic cell-mediated human immunodeficiency virus transfer. Antimicrob. Agents Chemother. 52:1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tevi-Bénissan, C., L. Bélec, M. Lévy, V. Schneider-Fauveau, A. S. Mohamed, M. C. Hallouin, M. Matta, and G. Grésenguet. 1997. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 4:367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Damme, L., R. Govinden, F. M. Mirembe, F. Guédou, S. Solomon, M. L. Becker, B. S. Pradeep, A. K. Krishnan, M. Alary, B. Pande, G. Ramjee, J. Deese, T. Crucitti, D. Taylor, and the C. S. Study Group. 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl. J. Med. 359:463-472. [DOI] [PubMed] [Google Scholar]
- 43.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiègne-Traoré, C. Uaheowitchai, S. S. Karim, B. Mâsse, J. Perriëns, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 44.Van Kuppeveld, F. J. M., K. E. Johansson, J. M. D. Galama, J. Kissing, G. Bölske, J. T. M. Van Der Logt, and W. J. G. Melchers. 1994. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl. Environ. Microbiol. 60:149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Kuppeveld, F. J. M., J. T. M. Van Der Logt, A. F. Angulo, M. J. Van Zoest, W. G. V. Quint, H. G. M. Niesters, J. M. D. Galama, and W. J. G. Melchers. 1992. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 58:2606-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 47.Wu, J. C. F., and M. Hamada. 2000. Experiments: planning, analysis, and parameter design optimization. J. Wiley & Sons, Inc., New York, NY.
- 48.Yasin, B., M. Pang, R. I. Lehrer, and E. A. Wagar. 2003. Activity of Novispirin G-10, a novel antimicrobial peptide against Chlamydia trachomatis and vaginosis-associated bacteria. Exp. Mol. Pathol. 74:190-195. [DOI] [PubMed] [Google Scholar]
- 49.Yasin, B., M. Pang, R. I. Lehrer, and E. A. Wagar. 2004. A cumulative experience examining the effect of natural and synthetic antimicrobial peptides vs. Chlamydia trachomatis. J. Pept. Res. 64:65-71. [DOI] [PubMed] [Google Scholar]
- 50.Yasin, B., M. Pang, E. A. Wagar, and R. I. Lehrer. 2002. Examination of Chlamydia trachomatis infection in environments mimicking normal and abnormal vaginal pH. Sex. Transm. Dis. 29:514-519. [DOI] [PubMed] [Google Scholar]