Abstract
Ceftobiprole is a new cephalosporin that exhibits a high level of affinity for methicillin-resistant Staphylococcus aureus PBP 2a. It was reported that ceftobiprole did not interact with a mutated form of the low-affinity protein Enterococcus faecium PBP 5 (PBP 5fm) that, when overexpressed, confers a β-lactam resistance phenotype to the bacterium. Our results show that ceftobiprole binds to unmutated PBP 5fm to form a stable acyl-enzyme and that ceftobiprole is able to efficiently kill a penicillin-resistant Enterococcus faecium strain that produces this protein.
β-Lactam antibiotics (penicillins, cephalosporins, carbapenems, and monobactams) are the most frequently prescribed antibacterial agents used to fight serious bacterial infections. They inactivate the membrane-bound d,d-transpeptidases essential for peptidoglycan synthesis by forming with them stable acyl-enzymes (9). This explains why these enzymes are generally designated penicillin-binding proteins (PBPs) (17, 19). In Gram-positive cocci, the resistance to β-lactam antibiotics is primarily conferred by the presence or the overproduction of a low-affinity PBP. In enterococci, among which is the opportunistic human pathogen Enterococcus faecium (3), the resistance to penicillin may be associated with overproduction of the intrinsic low-affinity protein PBP 5 (PBP 5fm) or to other alterations affecting PBP 5fm (15).
Ceftobiprole (BPR) (BAL9141) is a novel broad-spectrum cephalosporin that is active against Gram-positive and Gram-negative bacterial groups, including methicillin-resistant Staphylococcus aureus (MRSA) (2, 6, 10, 12) and Enterococcus faecalis (1). It has been demonstrated to be a good inhibitor of the S. aureus low-affinity protein PBP 2a (6, 8). A previous study reported that ceftobiprole had poor inhibitory activity against β-lactam-resistant E. faecium and that ceftobiprole did not bind to the mutated, low-affinity PBP 5fm protein isolated from such a strain (10). To better understand the difference between these two low-affinity PBPs with respect to ceftobiprole, we have characterized the interaction between an unmutated form of PBP 5fm and ceftobiprole.
To perform this study, we used the penicillin-sensitive Enterococcus faecium strain D63 (benzylpenicillin MIC = 5 μg/ml and ampicillin MIC = 15 μg/ml) and its laboratory-derived penicillin-resistant strain D63r (benzylpenicillin MIC = 70 μg/ml and ampicillin MIC = 125 μg/ml) (21). The PBP profile of the latter strain exhibits a 6-fold increase in quantity of PBP 5fm (21). The ceftobiprole MICs determined by the microdilution method (4) for E. faecium D63r and D63 were 8 and 2 μg/ml, respectively. These values contrasted with those reported by other workers, who have found that most ampicillin-resistant E. faecium clinical isolates were resistant to ceftobiprole (13). They concluded that ceftobiprole was ineffective against ampicillin-resistant enterococcal strains.
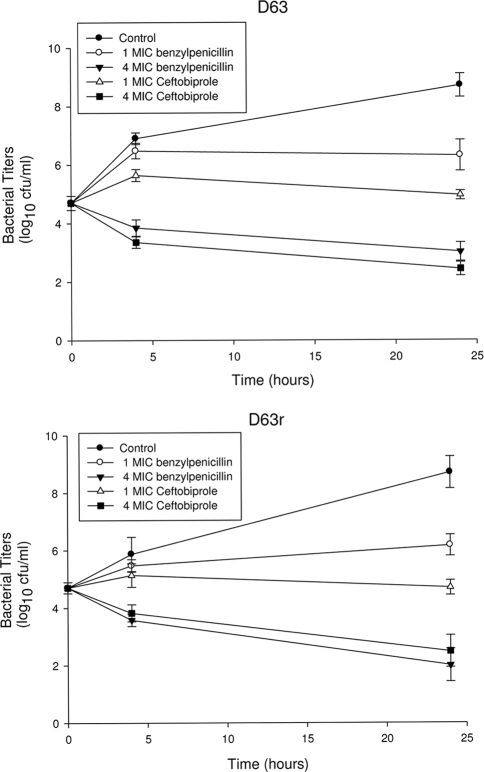
To further study the killing effects of benzylpenicillin and ceftobiprole on E. faecium D63r, we exposed exponentially growing cultures of both the sensitive and the resistant strains to increasing concentrations of antibiotic corresponding to 1 and 4 times the respective MICs (Fig. 1) (18). For concentrations higher than their MICs, benzylpenicillin and ceftobiprole show killing effects (Fig. 1).
FIG. 1.
Time-kill curves for resistant E. faecium D63r and susceptible E. faecium D63 in the presence of benzylpenicillin or ceftobiprole. The MICs for D63r were 70 μg/ml for benzylpenicillin and 8 μg/ml for ceftobiprole. The MICs for D63 were 5 μg/ml for benzylpenicillin and 2 μg/ml for ceftobiprole. The surviving bacteria were counted after 0, 4, and 24 h of incubation at 37°C by subculturing serial dilutions (at least 10-fold, to minimize drug carryover).
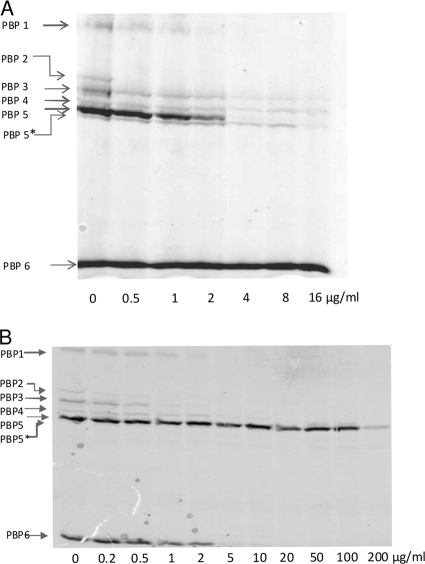
Our study was completed by the determination of the 50% inhibitory concentration (IC50) values for ceftobiprole, benzylpenicillin, cefepime, and ceftazidime for the different PBPs of E. faecium D63r by using purified membrane preparations and fluorescent ampicillin (20, 21). Membrane preparations (300 μg of proteins) were first incubated (20 min at 37°C) with increasing concentrations of ceftobiprole and next incubated (with a saturating concentration of 25 μM fluorescent ampicillin) for an hour at 37°C. The titration of the PBPs by ceftobiprole (Fig. 2 and Table 1) revealed that at a 2-fold MIC, all high-molecular-mass PBPs are inhibited by the antibiotic (Fig. 2) and that the low-molecular-mass protein PBP 6, which acts as a d,d-carboxypeptidase (7), is not affected. The high-molecular-mass protein PBP 2 is the most sensitive to ceftobiprole (IC50 = 0.2 μg/ml). PBPs 1 and 3 show similar IC50s (<1 μg/ml), whereas the low-affinity proteins PBP 5fm, PBP 4, and PBP 5* possess slightly higher values (≥1 μg/ml). This pattern of inhibition is completely different from that obtained with benzylpenicillin, for which the resistant protein PBP 5fm is the most insensitive PBP. The IC50 for ceftobiprole on purified soluble PBP 5fm (sPBP 5fm) gives results similar to those observed for the membrane preparations (0.7 μg/ml). Note that PBP 4 and PBP 5* exhibit very similar IC50 profiles (Table 1). A similar observation was made for PBP 4 and PBP 4* in Enterococcus hirae. PBP 4* was shown to be produced by a proteolytic cleavage of the 60 N-terminal amino acid residues of PBP 4 (11). It is very likely that, in the E. faecium membranes used here, PBP 5* was the result of an N-terminal truncation of PBP 4.
FIG. 2.
Relative affinities of ceftobiprole (A) and benzylpenicillin (B) for E. faecium D63r PBPs. The affinities of ceftobiprole and other β-lactams for PBPs were analyzed by a competition assay using fluorescent ampicillin. Membrane proteins were prepared from D63r and D63. Nonlabeled antibiotics were incubated with membrane proteins at 37°C for 20 min, followed by the addition of fluorescent ampicillin during 1-h incubation. The membrane fractions were subjected to SDS-PAGE and fluorography.
TABLE 1.
Inhibition of PBPs from E. faecium D63r and D63
| Strain | Antibiotic | IC50a (μg/ml) for PBP |
MIC (μg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 5* | 6 | |||
| D63r | Benzylpenicillin | 1.6 ± 0.4 | 0.06 ± 0.01 | 0.6 ± 0.3 | 8 ± 5 | 55 ± 15 | 8 ± 3 | 2 ± 1 | 70 |
| Cefepime | 1.7 ± 0.6 | 0.6 ± 0.3 | 0.5 ± 0.1 | >200 | >200 | >200 | >50 | >100 | |
| Ceftazidime | 0.9 ± 0.6 | 0.5 ± 0.3 | 0.04 ± 0.01 | >200 | >200 | >200 | >50 | >100 | |
| Ceftobiprole | 0.7 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 1.8 ± 0.2 | 1.0 ± 0.2 | 1.4 ± 0.5 | >16 | 8 | |
| D63 | Benzylpenicillin | 0.9 ± 0.7 | 0.1 ± 0.07 | 1.3 ± 0.6 | 10 ± 8 | 75 ± 25 | 6 ± 4 | 1.5 ± 1 | 5 |
| Ceftobiprole | 0.6 ± 0.2 | 0.2 ± 0.1 | 0.6 ± 0.2 | 1.5 ± 0.3 | 0.7 ± 0.1 | 1 ± 0.2 | >16 | 2 | |
Concentration of the β-lactam antibiotic that inhibits 50% of the fluorescent ampicillin in comparison to the level for a control containing no drug.
The kinetic parameters governing the acylation of PBP 5fm by ceftobiprole were determined by using sPBP 5fm (soluble PBP 5fm from which the N-terminal membrane anchoring peptide was removed). It was overproduced and purified as previously described, except that the molecular sieve was eliminated (16). The pseudo-first-order equation was applied (5) (Fig. 3). The opening of the ceftobiprole β-lactam ring was measured at 319 nm with a Specord 200 spectrophotometer (Analytik Jena, Germany) at 30°C in 10 mM phosphate buffer (pH 7.0) with 10 μM sPBP 5fm. ka (the observed rate constant) was estimated by fitting with 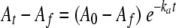 (where A0 is the initial absorbance, At is the absorbance at time t, and Af is the final absorbance). The slope allowed the determination of the value of the second-order rate constant k+2/K. A higher k+2/K value indicates faster acyl-enzyme formation, which means a faster inactivation of the PBP. The 110 ± 11 M−1 s−1 value reported for the second-order rate constant k+2/K obtained for ceftobiprole was 5 to 10 times higher than the value reported for benzylpenicillin (15 to 24 M−1 s−1), indicating that ceftobiprole inactivated sPBP 5fm faster than benzylpenicillin (21). However, this value is 70 times lower than the value obtained for S. aureus PBP 2a acylation (8,900 M−1 s−1), produced and purified in our laboratory (14). The sPBP 5fm-ceftobiprole adduct was very stable. Indeed, no free enzyme could be detected after 4 h of incubation at 37°C.
(where A0 is the initial absorbance, At is the absorbance at time t, and Af is the final absorbance). The slope allowed the determination of the value of the second-order rate constant k+2/K. A higher k+2/K value indicates faster acyl-enzyme formation, which means a faster inactivation of the PBP. The 110 ± 11 M−1 s−1 value reported for the second-order rate constant k+2/K obtained for ceftobiprole was 5 to 10 times higher than the value reported for benzylpenicillin (15 to 24 M−1 s−1), indicating that ceftobiprole inactivated sPBP 5fm faster than benzylpenicillin (21). However, this value is 70 times lower than the value obtained for S. aureus PBP 2a acylation (8,900 M−1 s−1), produced and purified in our laboratory (14). The sPBP 5fm-ceftobiprole adduct was very stable. Indeed, no free enzyme could be detected after 4 h of incubation at 37°C.
FIG. 3.
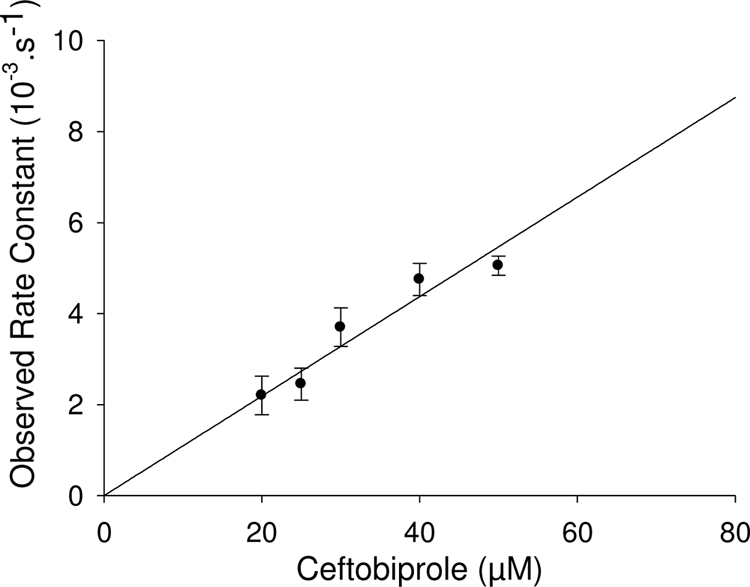
Variation of the pseudo-first-order rate constants (ka) of reaction of sPBP 5fm with ceftobiprole concentration. Upon reaction with β-lactam compounds, the active-site serine PBPs are immobilized in the form of a very stable acyl-enzyme. The kinetic model describing their interaction is 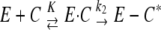 , where E is the PBP, C the β-lactam, E·C the noncovalent complex, and E − C* the acylated PBP. K is the dissociation constant of E·C, and k2 is the first-order rate constant characterizing the formation of the acyl-enzyme. The reaction obeys the equation E = E0·exp (−ka·t), in which ka is (k2/K)·C.
, where E is the PBP, C the β-lactam, E·C the noncovalent complex, and E − C* the acylated PBP. K is the dissociation constant of E·C, and k2 is the first-order rate constant characterizing the formation of the acyl-enzyme. The reaction obeys the equation E = E0·exp (−ka·t), in which ka is (k2/K)·C.
In conclusion, ceftobiprole efficiently inhibited the low-affinity protein E. faecium PBP 5 in our penicillin-resistant strain. It demonstrated bactericidal activity against this laboratory-derived ampicillin-resistant E. faecium mutant that overproduced an unmutated PBP 5 protein. This profile is different from that observed for most E. faecium clinical isolates bearing a mutant PBP 5 protein that had reduced affinity, where resistance is reported for all β-lactams, including ceftobiprole, suggesting that simple overexpression of PBP 5 is sufficient to elevate the MIC for ceftobiprole but that amino acid substitutions in the protein are necessary for high-level resistance. Finally, ceftobiprole is, up to now, the best tool for easily determining kinetic parameters of unlabeled β-lactams or for finding new inhibitors by high-throughput screening using the purified sPBP 5fm protein, because of its rapid acylation of the protein and the ability to directly follow the cleavage of its β-lactam ring at 319 nm.
Acknowledgments
We thank Karen Bush and Anne Marie Queenan from Johnson and Johnson Pharmaceutical Research and Development, LLC, for the gift of ceftobiprole.
This research was supported in part by the Belgian Program on Interuniversity Poles of Attraction, initiated by the Belgian State (grant P6/19); the Actions de Recherche Concertées (grant 03/08-297); the Fonds de la Recherche Fondamentale Collective (grants 6 2.4511.06 and 2.4524.03); the Fonds de la Recherche en Sciences Médicales (grant 7 3.4586.05); the European Union (EUR-INTAFAR [LSHM-CT-2004-512138] and COBRA [LSHM-CT-2003-503335, 6th PCRD] projects); and the R&D of the University of Liège.
Footnotes
Published ahead of print on 16 November 2009.
REFERENCES
- 1.Arias, C. A., K. V. Singh, D. Panesso, and B. E. Murray. 2007. Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates. Antimicrob. Agents Chemother. 51:2043-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, K., M. Heep, M. J. Macielag, and G. J. Noel. 2007. Anti-MRSA beta-lactams in development, with a focus on ceftobiprole: the first anti-MRSA beta-lactam to demonstrate clinical efficacy. Expert Opin. Investig. Drugs 16:419-429. [DOI] [PubMed] [Google Scholar]
- 3.Chenoweth, C., and D. Schaberg. 1990. The epidemiology of enterococci. Eur. J. Clin. Microbiol. Infect. Dis. 9:80-89. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Cornish-Bowden, A. 2004. Fundamentals of enzyme kinetics, 3rd ed. Portland Press, London, United Kingdom.
- 6.Davies, T. A., M. G. Page, W. Shang, T. Andrew, M. Kania, and K. Bush. 2007. Binding of ceftobiprole and comparators to the penicillin-binding proteins of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 51:2621-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.el Kharroubi, A., G. Piras, P. Jacques, I. Szabo, J. Van Beeumen, J. Coyette, and J. M. Ghuysen. 1989. Active-site and membrane topology of the DD-peptidase/penicillin-binding protein no. 6 of Enterococcus hirae (Streptococcus faecium) A.T.C.C. 9790. Biochem. J. 262:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghuysen, J. M. 1988. Bacterial active-site serine penicillin-interactive proteins and domains: mechanism, structure, and evolution. Rev. Infect. Dis. 10:726-732. [DOI] [PubMed] [Google Scholar]
- 10.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacques, P., A. el Kharroubi, J. Van Beeumen, G. Piras, J. Coyette, and J. M. Ghuysen. 1991. Mode of membrane insertion and sequence of a 32-amino acid peptide stretch of the penicillin-binding protein 4 of Enterococcus hirae. FEMS Microbiol. Lett. 66:119-123. [DOI] [PubMed] [Google Scholar]
- 12.Jones, M. E. 2007. In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 13(Suppl. 2):17-24. [DOI] [PubMed] [Google Scholar]
- 13.Jones, R. N., L. M. Deshpande, A. H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Chemother. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 14.Lemaire, S., Y. Glupczynski, V. Duval, B. Joris, P. M. Tulkens, and F. Van Bambeke. 2009. Activity of ceftobiprole and other cephalosporins against extracellular and intracellular (THP-1 macrophages and keratinocytes) forms of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2289-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice, L. B., L. L. Carias, R. Hutton-Thomas, F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauvage, E., F. Kerff, E. Fonzé, R. Herman, B. Schoot, J. P. Marquette, Y. Taburet, D. Prevost, J. Dumas, G. Leonard, P. Stefanic, J. Coyette, and P. Charlier. 2002. The 2.4-A crystal structure of the penicillin-resistant penicillin-binding protein PBP5fm from Enterococcus faecium in complex with benzylpenicillin. Cell. Mol. Life Sci. 59:1223-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauvage, E., F. Kerff, M. Terrak, J. A. Ayala, and P. Charlier. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234-258. [DOI] [PubMed] [Google Scholar]
- 18.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waxman, D. J., and J. L. Strominger. 1983. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu. Rev. Biochem. 52:825-869. [DOI] [PubMed] [Google Scholar]
- 20.Zhao, G., T. I. Meier, S. D. Kahl, K. R. Gee, and L. C. Blaszczak. 1999. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob. Agents Chemother. 43:1124-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]