Abstract
Clarithromycin is a 14-member lactone ring macrolide with potent activity against Haemophilus influenzae, including ampicillin-resistant strains. We evaluated the in vivo efficacy of clarithromycin at 40 mg/day and 100 mg/day for 3 days in the treatment of a murine model of pneumonia using a macrolide-resistant H. influenzae strain, which was also ampicillin resistant. The MIC of clarithromycin was 64 μg/ml. The viable bacterial counts in infected tissues after treatment with 100 mg clarithromycin/kg of body weight were lower than the counts obtained in control and 40-mg/kg clarithromycin-treated mice. The concentrations of macrophage inflammatory protein 2 (MIP-2) and interleukin 1β (IL-1β) in bronchoalveolar lavage fluid (BALF) samples from mice treated at both concentrations were lower than in the control group. Pathologically, following infection, clarithromycin-treated mice, particularly at a dose of 100 mg/kg, showed lower numbers of neutrophils in alveolar walls, and inflammatory changes had apparently improved, whereas large aggregates of inflammatory cells were observed within the alveoli of control mice. In addition, we demonstrated that clarithromycin has bacteriological effects against intracellular bacteria at levels below the MIC. Our results indicate that clarithromycin may be useful in vivo for macrolide-resistant H. influenzae, and this phenomenon may be related to the good penetration of clarithromycin into bronchoepithelial cells. We also believe that conventional drug susceptibility tests may not reflect the in vivo effects of clarithromycin.
Haemophilus influenzae is a Gram-negative bacillus that is a commensal inhabitant of the human nasopharynx and that can be isolated from most of the human population.
Nontypeable (NT) H. influenzae strains cause a variety of infections, including otitis media and acute exacerbation of chronic bronchitis, pneumonia, and meningitis, primarily in pediatric patients (7, 10, 18). The incidence of beta-lactamase-negative ampicillin (AMP)-resistant (BLNAR) strains of H. influenzae has recently exhibited a marked increase in some countries, particularly Japan (13, 28, 34). Macrolides are increasingly used for the treatment of respiratory tract infections (RTIs), and in the 1990s, the “new” macrolides clarithromycin (CAM) and azithromycin (AZM) were made available (30). The new macrolides have an expanded spectrum of activity, including fastidious Gram-negative bacilli, such as H. influenzae and Neisseria spp. (20, 23).
CAM is a 14-member lactone ring macrolide antibiotic that has been used for the treatment of various infectious diseases. The antimicrobial activities of macrolides are generally considered to be produced through the inhibition of microbial protein synthesis by acting on the 50S subunit of the 70S ribosome (36). In addition, macrolide antibiotics exert anti-inflammatory effects by inhibition of the production of proinflammatory cytokines (22, 33, 35). Moreover, the pharmacokinetics (PK) of macrolides is characterized by a combination of low serum concentrations and high tissue concentrations (40), with advanced-generation macrolides being highly concentrated within polymorphonuclear leukocytes. Following phagocytosis of pathogens at the infection site, these cells are exposed to very high intracellular concentrations of antibacterial agents.
Macrolide resistance in several pathogens was recently evaluated because of increases in worldwide macrolide consumption (14, 27). However, pharmacodynamics (PD) models and susceptibility breakpoints derived from studies with other classes of drugs, such as the beta-lactams and aminoglycosides, do not adequately explain the clinical utility of antibacterial agents that achieve high intracellular concentrations, such as macrolides and fluoroquinolones (1). Some authors have suggested that in vitro resistance is less useful for guiding clinical decisions. In addition, the mechanisms of airway epithelium invasion by H. influenzae have been reported (11, 16, 29), and the characteristics are thought to contribute to escape from antibiotics and to long-term persistence in the airway. We believe that macrolides also have good clinical effects in vivo against these intracellular bacteria due to their excellent tissue and intracellular penetration.
The focus of this study was to investigate the in vivo efficacies of CAM in an experimental pneumonia model using CAM-resistant H. influenzae.
MATERIALS AND METHODS
Bacteria.
The clinically isolated NT H. influenzae strain 4437, which had been stored in Trypticase soy broth with 10% glycerol stocks maintained at −80°C at Nagasaki University Hospital, was spread on chocolate agar plates (Nissui Pharmaceutical, Tokyo, Japan) and incubated overnight (18 to 24 h) at 37°C in 5% CO2.
Laboratory animals.
Six-week-old male ddY specific-pathogen-free mice (body weight, 16 to 20 g) were purchased from SLC Japan (Tokyo, Japan). All of the animals were housed in a pathogen-free environment and received sterile food and water in the Laboratory Animal Center for Biomedical Science at Nagasaki University (Nagasaki, Japan). The experimental protocols were approved by the Ethics Review Committee for Animal Experimentation at Nagasaki University.
A murine model of H. influenzae respiratory tract infection.
We used the intubation model of NT H. influenzae pneumonia in mice, as reported previously (26). Briefly, disposable, sterile, plastic cut-down intravenous catheters (3-Fr; 1.0-mm diameter; Atom, Tokyo, Japan) were used for tracheal intubation. The intubation procedure was performed under pentobarbital anesthesia. The blunted end of the inner needle of an intravenous catheter (Angiocath; Becton Dickinson Vascular Access, Sandy, UT) was inserted through the oral cavity, with the outer sheath and attached tube at the tip. The tube was advanced through the vocal cords into the trachea. The inner needle was then removed, and the outer sheath was gently pushed to place the plastic tube into the main bronchus. The organisms were instilled 7 days after intubation. H. influenzae was cultured on chocolate α agar plates (Nissui Pharmaceutical) and incubated overnight (18 to 24 h) at 37°C in 5% CO2, and the organisms were then suspended in normal saline. The final numbers of bacteria were approximately 2 × 109 CFU/ml, as determined by the optical density method. Infection was induced by intratracheal inoculation of 0.05 ml of a bacterial suspension under anesthesia with pentobarbital sodium.
Bacteriological and histopathological examination.
The mice were divided into 3 groups: control mice (no therapy), mice treated with 40 mg clarithromycin/kg of body weight twice a day (12, 25), and mice treated with 100 mg/kg clarithromycin twice a day (39). The mice were treated for 3 days and then sacrificed 12 h after the final treatment. The tubes were removed, and the lungs were excised under aseptic conditions. The lungs were homogenized in 1.0 ml of phosphate-buffered saline (PBS) and cultured quantitatively by serial dilution on chocolate α agar plates (Nissui Pharmaceutical), followed by incubation overnight (18 to 24 h) at 37°C in 5% CO2. For histopathological examination, lung specimens were fixed in 10% formalin-buffered solution.
BAL and cytokine ELISA.
Bronchoalveolar lavage (BAL) was performed as described previously (38). Briefly, mice were treated for 3 days and sacrificed 12 h after the final antibiotic administration. The chest was opened to expose the lungs and trachea, and a disposable sterile plastic cut-down intravenous catheter was inserted into the trachea. BAL was performed 3 times sequentially using 1.0 ml of saline each time. The recovered fluid fractions were pooled for each animal. Total cell counts were performed by Turk staining. For differential cell counts, cells were centrifuged at 850 rpm for 2 min onto slides, which were then stained with Diff-Quick stain. Differential cell counts were performed by counting 100 cells. Concentrations of macrophage inflammatory protein 2 (MIP-2) and interleukin 1β (IL-1β) in BAL fluid (BALF) were assayed using mouse cytokine enzyme-linked immunosorbent assay (ELISA) test kits (R&D Systems, Minneapolis, MN).
Antimicrobial agents.
CAM was kindly provided by Taisyotoyama Pharmaceutical Co., Ltd. (Tokyo, Japan). The CAM was dissolved in dimethyl sulfoxide prepared according to the manufacturer's instructions and frozen at −80°C until it was used.
Antibiotic susceptibility testing.
The susceptibility of H. influenzae to CAM was tested in duplicate for each isolate at 105 CFU/ml and was determined using the broth dilution method with Haemophilus Test Medium according to Clinical and Laboratory Standards Institute (CLSI) recommendations (6). Production of β-lactamase was confirmed by the nitrocefin test (Showa Chemical, Tokyo, Japan). Strains were classified according to CLSI AMP susceptibility criteria as susceptible strains (AMP MIC ≤ 1 μg/ml), intermediate strains (AMP MIC = 2 μg/ml), and resistant strains (AMP MIC ≥ 4 μg/ml).
Antibiotic examination.
At 24 h after challenge with H. influenzae, CAM was administered orally twice a day. Individual doses were either 40 mg/kg or 100 mg/kg, and treatment was administered for 3 days. Each group of mice was killed by cervical dislocation at 12 h after the final drug administration. Bacteriological examination and BALF analysis were performed using the methods described above.
Three-hour invasion assay with NCI-H292 cells.
The NCI-H292 epithelial cell line was obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured in RPMI 1640 medium with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were grown at 37°C under 5% CO2 in fully humidified air and were subcultured twice weekly. Cells were seeded in a 12-well plate at 5 × 105 cells/well. When confluent, the cells were incubated in RPMI 1640 medium containing 0.5% fetal bovine serum for 24 h. The cells were then rinsed with serum-free RPMI 1640 medium and exposed to bacteria. Bacterial suspensions at about 6 × 106 CFU/ml were inoculated at 10 μl/well. An invasion assay was performed as reported previously (17, 32). Briefly, cell monolayers were infected and incubated at 37°C under 5% CO2 for 3 h, washed 3 times with PBS, and treated with gentamicin (Sigma, Tokyo, Japan) at a concentration of 200 μg/ml for 2 h in order to kill extracellular bacteria. CAM at concentrations below the MIC (2 μg/ml to 64 μg/ml) was mixed with gentamicin. The cell monolayers were washed 3 additional times with PBS, and viable intracellular bacteria were released by incubation with 0.5 ml of 1% Triton X-100 (Sigma-Aldrich) in PBS for 15 min. Samples were harvested and vortex agitated for 1 min in order to lyse the cells. Viable bacteria were serially diluted and plated onto chocolate agar (Nissui, Tokyo, Japan) for colony counting.
Statistical analysis.
Data are expressed as means ± standard error of the mean (SEM). Differences between the numbers of viable bacteria in the lungs were evaluated by analysis of variance. P values of <0.05 were considered to be statistically significant.
RESULTS
In vitro susceptibility.
For H. influenzae clinical strain 4437, the MIC of CAM was 64 μg/ml. This strain was a BLNAR strain, and the AMP MIC was 16 μg/ml.
Changes in viable bacterial numbers over time.
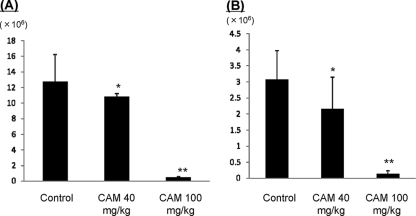
Respiratory infection occurred in all intubated mice with inoculation. The number of viable bacteria increased to 6.0 ± 1.2 log10 CFU/ml 3 days after inoculation in the control group, and there were no significant differences between the control group and the 40-mg/kg treatment group (5.8 ± 0.8 log10 CFU/ml) (Fig. 1). Conversely, the number of viable bacteria decreased significantly in the 100-mg/kg treatment group (4.6 ± 1.0 log10 CFU/ml). These results indicate that CAM has in vivo bacteriological effects against the macrolide-resistant strain, as determined by conventional in vitro drug susceptibility tests.
FIG. 1.
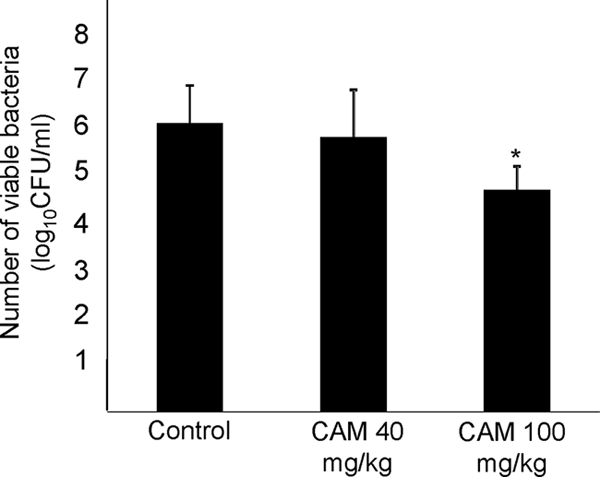
Numbers of viable organisms in lower respiratory organs. Mice were inoculated with 1 × 108 CFU/ml of H. influenzae strain 4437. On day 3 after infection, control mice and mice treated with CAM at 40 mg/kg and 100 mg/kg (oral administration twice a day) were compared. The numbers of viable bacteria were significantly lower in the 100-mg/kg treatment group. The data are expressed as means plus standard deviations (SD) for four experiments. *, P < 0.05 versus the control.
BALF analysis.
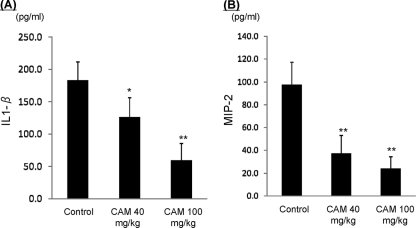
Total cell counts (Fig. 2A) and neutrophil counts (Fig. 2B) were significantly lower in the 40-mg/kg treatment group, and particularly in the 100-mg/kg treatment group. To estimate further effects of CAM, inflammatory cytokine levels in BALF were analyzed. IL-1β (Fig. 3A) and MIP-2 (Fig. 3B) were significantly decreased in both treatment groups, particularly in the 100-mg/kg treatment group. These data indicate that CAM has dose-dependent anti-inflammatory effects against the acute inflammation induced by macrolide-resistant H. influenzae.
FIG. 2.
Changes in the total cell counts in BALF and total cell counts (A) and neutrophils (B) between control mice and mice treated with CAM at 40 mg/kg and 100 mg/kg (oral administration twice a day). The cells were stained with Turk stain. The data are expressed as means plus SD for four experiments. *, P < 0.05, and **, P < 0.001 versus the control.
FIG. 3.
Changes in inflammatory cytokine levels in BALF, IL-1β (A), and MIP-2 (B) between control mice and mice treated with CAM at 40 mg/kg and 100 mg/kg (oral administration twice a day). The data are expressed as means plus SD for four experiments. *, P < 0.05, and **, P < 0.001 versus the control.
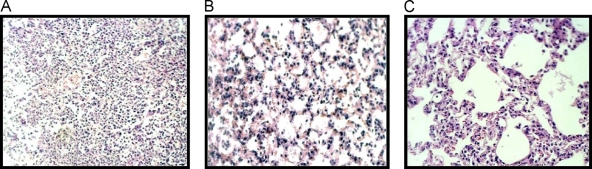
Histopathological examination.
In nontreated mice, the bronchioles and adjacent alveoli were filled with neutrophils, epithelial cells, and inflammatory cells (Fig. 4A). Conversely, in treated mice, although mild inflammatory changes were evident in the 40-mg/kg treatment group (Fig. 4B), inflammation had improved after 3 treatment days, particularly in the 100-mg/kg treatment group (Fig. 4C).
FIG. 4.
High-power magnification (×200; hematoxylin and eosin) of the lung after 3 days of treatment. (A) Control group, untreated. (B) 40-mg/kg treatment group (oral administration twice a day). (C) 100-mg/kg treatment group (oral administration twice a day). Inflammatory changes improved in both treatment groups, particularly in the 100-mg/kg treatment group.
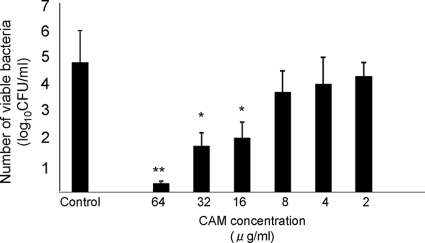
Intracellular bacteriological effects at various CAM concentrations.
In conventional drug susceptibility tests, such as the microdilution method, PK and PD are not reflected in the antimicrobial effects against the pathogen that has invaded the bronchoepithelial cells. We performed a 3-h invasion assay to investigate the intracellular bacteriological effects of CAM against macrolide-resistant H. influenzae, which was determined to be a highly resistant strain based on the CLSI judgment criteria. The number of intracellular H. influenzae organisms in the control group (nontreatment group) was 4.8 ± 1.2 log10 CFU/ml. We hypothesized that CAM shows bacteriological effects against macrolide-resistant H. influenzae at concentrations below the MIC, as CAM attains higher concentrations in lung tissue than in serum. As shown in Fig. 5, CAM reduced the number of viable bacteria at less than half the MIC. These data indicate that CAM can eradicate macrolide-resistant H. influenzae at levels below the MIC and that it may be able to prevent persistent and recurrent infection by the pathogen. In addition, the difference between our results and conventional drug susceptibility test results may be due to the good penetration of CAM into bronchoepithelial cells.
FIG. 5.
Intracellular bactericidal effects of various CAM concentrations against strain 4437. Intracellular viable bacterial numbers decreased significantly when the strain was treated with CAM at 1× MIC, 0.5× MIC, and 0.25× MIC. The data are expressed as means plus SD for three experiments. *, P < 0.05, and **, P < 0.001 versus the control.
DISCUSSION
Macrolides and beta-lactams are the antimicrobials most commonly prescribed to treat infection by H. influenzae. Antimicrobial resistance is a growing problem in H. influenzae. BLNAR H. influenzae was first observed in the 1980s (8, 21, 24) at very low frequency in the United States (3, 9, 13) but has rapidly become more common and accounts for 25 to 30% of isolates in Japan and other Asian countries (13, 28, 34). As safe and well-tolerated antibiotics, macrolides play a key role in the treatment of community-acquired RTIs against not only beta-lactam-susceptible strains, but also resistant strains, such as BLNAR H. influenzae. Their broad spectrum of activity against Gram-positive, Gram-negative, and atypical pathogens has led to the widespread use of macrolides for empirical treatment of RTIs.
CAM is a 14-member lactone ring macrolide antibiotic, and although its increased utility has been compromised by intrinsic and acquired resistance, treatment failures are uncommon. Generally, in vitro resistance is based on the results of using susceptibility breakpoints developed by the CLSI. These susceptibility data are considered useful for determining epidemiological trends of resistance, but in vitro resistance is less useful for guiding clinical decisions and does not necessarily indicate a lack of clinical efficacy.
The discrepancy may be based on the characteristic features of macrolides. It is known that macrolides are able to transfer and accumulate intracellularly and that they show intracellular bactericidal effects. Moreover, macrolides are readily taken up by phagocytes, lymphocytes, and epithelial cells (4, 30). The concentrations of macrolides in respiratory tract tissues and fluids have thus been shown to be higher than serum concentrations, resulting in the possibility of increased activity against organisms localized to these extraplasma sites. The PD parameter of CAM has not yet been fully studied. CAM is considered concentration dependent by some investigators and concentration independent by others (5). Tessier et al. (37) demonstrated that the area under the concentration-time curve (AUC)/MIC was the most reasonable predictor of CAM efficacy by using an experimental Streptococcus pneumoniae pneumonia model. However, they also showed that the time above the MIC and maximum concentration of the drug in serum (Cmax)/MIC were also important parameters correlated with the change of bacterial load. In the present study, although we did not demonstrate which method is better (1 dose or twice a day) in CAM administration, the 100-mg/kg treatment group showed improvement both in pathology and in the inflammatory mediators, indicating that CAM may be able to show dose- and concentration-dependent efficacy against H. influenzae pneumonia.
Moreover, macrolides also have anti-inflammatory properties that improve clinical outcomes via extramicrobial mechanisms, leading to improvements in symptoms and overall quality of life among patients with a variety of respiratory conditions. None of these benefits are reflected in in vitro drug susceptibility testing.
Drug concentrations in both the epithelial lining fluid (ELF) and alveolar macrophages (AM) are important in treating extracellular and intracellular bacteria. CAM is extensively concentrated in both the ELF and respiratory phagocytes, reaching levels that are between 1 and 3 orders of magnitude higher than in plasma (2, 23). Antibacterial potency is driven by the concentration of active agents at the site of infection, and thus, in the case of macrolides, efficacy may be increased substantially by the tissue penetration described previously. This aspect of macrolide pharmacology is not taken into account by traditional drug susceptibility tests, such as the microdilution method. Thus, the most likely rationale is that in vitro resistance MICs are misleading, leading to underestimation of the clinical efficacy of these therapeutic agents.
In the present study, to investigate the differences between the in vivo effects of CAM and the results of conventional drug susceptibility against macrolide-resistant H. influenzae, as determined by in vitro susceptibility testing, we experimentally demonstrated lower respiratory tract infection. The viable bacterial number was reduced significantly in the 100-mg/kg treatment group. In addition, CAM showed dose-dependent effects on improving the number of inflammatory cells and levels of inflammatory cytokines. Interestingly, however, CAM did not decrease viable bacterial numbers in the 40-mg/kg treatment group. CAM may be able to suppress the accumulation of neutrophils and other inflammatory cells through its immunomodulatory effects, thereby contributing to improvement of inflammation.
It has been shown that antimicrobial drug therapy based on PK and PD is necessary for the treatment of infectious diseases (7). β-Lactams, the most common antibiotics for treating respiratory infections, cannot eradicate bacteria that have invaded airway epithelial cells. Considering the pathology of airway infection by highly invasive H. influenzae strains, antimicrobial agents that readily penetrate the airway epithelium and to which bacteria show susceptibility are necessary. We performed a cell-invasive assay to estimate the effects of CAM against intracellular H. influenzae using a macrolide-resistant strain. Our data indicate that CAM can reduce the intracellular viable bacterial number at concentrations lower than the MIC, which was determined by the microdilution method. However, in this study, we did not measure intracellular CAM concentrations, and this effect may indicate intracellular concentrations higher than the MIC, thus contributing to the good in vivo effects against H. influenzae, even macrolide-resistant strains. Some authors have reported that CAM achieves high concentrations, not only in ELF, but also in the intracellular space. Rodvold et al. reported that the steady-state concentrations of CAM in ELF and AM obtained in intrapulmonary samples during bronchoscopy and bronchoalveolar lavage from 40 healthy, nonsmoking adult volunteers were analyzed, and CAM was extensively concentrated in the ELF (34.4 μg/ml at 4 h to 4.6 μg/ml at 24 h) and AM (480 μg/ml at 4 h to 99 μg/ml at 24 h) (31). However, we did not measure the intracellular concentrations of CAM at this time, and our result may reflect high penetration into the bronchial epithelial cells by CAM from the viewpoint of PK. We also consider that this information may be useful clinically, because H. influenzae is known to invade respiratory epithelial cells and tends to escape the effect of antibiotics.
Unfortunately, in vitro resistance data have a strong impact on the drug selection process, as macrolide therapy is largely empirical. This issue is not easy to resolve, as existing PD data use serum or plasma as an index for microbiological efficacy, which may not be appropriate for macrolides when they are used against organisms with higher MICs that reside in bodily fluids with drug concentration profiles that differ from those of serum. As time passes and more organisms become resistant and therefore have higher MICs, this situation will become more confused, and one can predict that the macrolide class of antibiotics will probably be replaced by the “respiratory” quinolones for empirical therapy of respiratory tract infections. This may relegate the macrolides to the role of adjunctive agents in the treatment or prophylaxis of infections believed to be caused by intracellular pathogens. This would be unfortunate if it is based upon erroneous resistance data and could deprive patients of an acceptable and somewhat unusual (due to the high tissue penetration and immunological properties) class of agents; of course, the entire situation is further complicated by the host immune function.
In conclusion, macrolide therapy remains a reasonable treatment for respiratory infection, even when macrolide-resistant H. influenzae is the causative pathogen. Advanced macrolides penetrate extensively within the respiratory tract and alveolar cells and have anti-inflammatory properties that improve clinical outcomes via extramicrobial mechanisms. None of these benefits are reflected by in vitro testing. Thus, conventional drug susceptibility tests do not sufficiently reflect the in vivo efficacy of macrolides.
Although we demonstrated the efficacy of CAM against CAM-resistant H. influenzae, the data are limited, because we did not analyze PK/PD, and these results are based on only an experimental H. influenzae pneumonia model. In fact, some papers that reported the clinical treatment failure of macrolide-resistant S. pneumoniae were published recently (15, 19). A randomized, prospective trial is needed to establish a causal relationship between in vitro resistance and clinical treatment in macrolides. We should treat macrolide-resistant H. influenzae carefully, and continuous monitoring of macrolide-resistant pathogens will also be important.
Footnotes
Published ahead of print on 30 November 2009.
REFERENCES
- 1.Amsden, G. W. 2001. Advanced-generation macrolides: tissue-directed antibiotics. Int. J. Antimicrob. Agents 18(Suppl. 1):S11-S15. [DOI] [PubMed] [Google Scholar]
- 2.Amsden, G. W., and C. L. Gray. 2001. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J. Antimicrob. Chemother. 47:61-66. [DOI] [PubMed] [Google Scholar]
- 3.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. Identification of β-lactamase-negative, ampicillin-resistant strains of Haemophilus influenzae with four methods and eight media. Antimicrob. Agents Chemother. 45:1585-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosnar, M., Z. Kelnerić, V. Munić, V. Eraković, and M. J. Parnham. 2005. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob. Agents Chemother. 49:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbon, C. 1998. Pharmacodynamics of macrolides, azalides, and streptogramins: effect on extracellular pathogens. Clin. Infect. Dis. 27:28-32. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing, 17th ed. Approved standard M100-S17. CLSI, Wayne, PA.
- 7.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A. 2001. The hidden impact of antibacterial resistance in respiratory tract infection. Re-evaluating current antibiotic therapy. Respir. Med. 95(Suppl. A):S12-S19, S26-S27. [DOI] [PubMed] [Google Scholar]
- 9.Doern, G. V., A. B. Brueggemann, G. Pierce, H. P. Holley, Jr., and A. Rauch. 1997. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob. Agents Chemother. 41:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felmingham, D., and R. M. Gruneberg. 2000. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J. Antimicrob. Chemother. 45:191-203. [DOI] [PubMed] [Google Scholar]
- 11.Forsgren, J., A. Samuelson, A. Ahlin, J. Jonasson, B. Rynnel-Dagöö, and A. Lindberg. 1994. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect. Immun. 62:673-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda, Y., K. Yanagihara, Y. Higashiyama, Y. Miyazaki, Y. Hirakata, H. Mukae, K. Tomono, Y. Mizuta, K. Tsukamoto, and S. Kohno. 2006. Effects of macrolides on pneumolysin of macrolide-resistant Streptococcus pneumoniae. Eur. Respir. J. 27:1020-1025. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa, K., K. Yamamoto, N. Chiba, R. Kobayashi, K. Nagai, M. R. Jacobs, P. C. Appelbaum, K. Sunakawa, and K. Ubukata. 2003. Diversity of ampicillin-resistance genes in Haemophilus influenzae in Japan and the United States. Microb. Drug Resist. 9:39-46. [DOI] [PubMed] [Google Scholar]
- 14.Hoban, D. J., and G. G. Zhanel. 2006. Clinical implications of macrolide resistance in community-acquired respiratory tract infections. Expert Rev. Anti Infect. Ther. 4:973-980. [DOI] [PubMed] [Google Scholar]
- 15.Kelley, M. A., D. J. Weber, P. Gilligan, and M. S. Cohen. 2000. Breakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycin. Clin. Infect. Dis. 31:1008-1011. [DOI] [PubMed] [Google Scholar]
- 16.Ketterer, M. R., J. Q. Shao, B. Hornick, B. Buscher, V. K. Bandi, and M. A. Apicella. 1999. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect. Immun. 67:4161-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunishima, H., H. Takemura, H. Yamamoto, K. Kanemitsu, and J. Shimada. 2000. Evaluation of the activity of antimicrobial agents against Legionella pneumophila multiplying in a human monocytic cell line, THP-1, and an alveolar epithelial cell line, A549. J. Infect. Chemother. 6:206-210. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman, D., F. Schlaeffer, I. Boldur, D. Lieberman, S. Horowitz, M. G. Friedman, M. Leiononen, O. Horovitz, E. Manor, and A. Porath. 1996. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax 51:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonks, J. R., J. Garau, L. Gomez, M. Xercavins, E. Ochoa, I. F. Gareen, P. T. Reiss, and A. A. Medeiros. 2002. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 35:556-564. [DOI] [PubMed] [Google Scholar]
- 20.Mandell, L. A. 1995. Community-acquired pneumonia: Etiology, epidemiology, and treatment. Chest 108:35S-41S. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz, S. M. 1980. Isolation of an ampicillin-resistant, non-beta-lactamase-producing strain of Haemophilus influenzae. Antimicrob. Agents Chemother. 17:80-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka, N., K. Eguchi, A. Kawakami, M. Tsebin, Y. Kawabe, T. Aoyagi, and S. Nagatani. 1996. Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clin. Exp. Immunol. 104:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarty, J. M. 2000. Clarithromycin in the management of community-acquired pneumonia. Clin. Ther. 22:281-294. [DOI] [PubMed] [Google Scholar]
- 24.Mendelman, P. M., D. O. Chaffin, T. L. Stull, C. E. Rubens, K. D. Mack, and A. L. Smith. 1984. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob. Agents Chemother. 26:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki, S., T. Fujikawa, T. Matsumoto, K. Tateda, and K. Yamaguchi. 2001. Efficacy of azithromycin, clarithromycin and beta-lactam agents against experimentally induced bronchopneumonia caused by Haemophilus influenzae in mice. J. Antimicrob. Chemother. 48:425-430. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, S., K. Yanagihara, Y. Morinaga, K. Izumikawa, M. Seki, H. Kakeya, Y. Yamamoto, S. Kamihira, and S. Kohno. 2009. In vivo efficacy of sitafloxacin in a new murine model of nontypable Haemophilus influenzae pneumonia by sterile intratracheal tube. Int. J. Antimicrob. Agents 34:210-214. [DOI] [PubMed] [Google Scholar]
- 27.Nuermberger, E., and W. R. Bishai. 2004. The clinical significance of macrolide-resistant Streptococcus pneumoniae: it's all relative. Clin. Infect. Dis. 38:99-103. [DOI] [PubMed] [Google Scholar]
- 28.Ohkuksu, K., A. Nakamura, and K. Sawada. 2000. Antibiotic resistance among recent clinical isolates of Haemophilus influenzae in Japanese children. Diagn. Microbiol. Infect. Dis. 36:249-254. [DOI] [PubMed] [Google Scholar]
- 29.Okabe, T., Y. Yamazaki, M. Shiotani, T. Suzuki, M. Shiohara, E. Kasuga, S. Notake, and H. Yanagisawa. 2010. An amino acid substitution in PBP-3 in Haemophilus influenzae associated with the invasion to bronchial epithelial cells. Microbiol. Res. 165:11-20. [DOI] [PubMed] [Google Scholar]
- 30.Piscitelli, S. C., L. H. Danziger, and K. A. Roldvold. 1992. Clarithromycin and azithromycin: new macrolide antibiotics. Clin. Pharm. 11:137-152. [PubMed] [Google Scholar]
- 31.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swords, W. E., B. A. Buscher, I. K. Ver Steeg, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama, Y., K. Yanagisawa, S. Tominaga, and S. Kitamura. 1999. Effects of long-term administration of erythromycin on cytokine production in rat alveolar macrophages. Eur. Respir. J. 14:1113-1116. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, K., T. Nishimura, and S. Baba. 2003. Current status of bacterial resistance in the otolaryngology field: results from the second nationwide survey in Japan. J. Infect. Chemother. 9:46-52. [DOI] [PubMed] [Google Scholar]
- 35.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 36.Taubman, S. B., N. R. Jones, F. E. Young, and J. W. Corcoran. 1966. Sensitivity and resistance to erythromycin in Bacillus subtilis 168: the ribosomal binding of erythromycin and chloramphenicol. Biochim. Biophys. Acta 123:438-440. [DOI] [PubMed] [Google Scholar]
- 37.Tessier, P. R., M. K. Kim, W. Zhou, D. Xuan, C. Li, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 46:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagihara, K., M. Seki, and P.-W. Cheng. 2001. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell Mol. Biol. 24:66-73. [DOI] [PubMed] [Google Scholar]
- 39.Yanagihara, K., K. Tomono, Y. Imamura, Y. Kaneko, M. Kuroki, T. Sawai, Y. Miyazaki, Y. Hirakata, H. Mukae, J. Kadota, and S. Kohno. 2002. Effect of clarithromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. J. Antimicrob. Chemother. 49:867-870. [DOI] [PubMed] [Google Scholar]
- 40.Zhanel, G. G., M. Dueck, D. J. Hoban, L. M. Vercaigne, J. M. Embil, A. S. Gin, and J. A. Karlowsky. 2001. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs 61:443-498. [DOI] [PubMed] [Google Scholar]