Abstract
We have evaluated the efficacy of posaconazole, amphotericin B, and itraconazole in a murine model of disseminated infection by Fonsecaea monophora. Of these three antifungal drugs tested, posaconazole prolonged survival significantly and reduced the fungal load in most of the organs tested. Bioassay studies demonstrated the relationship between posaconazole levels and dose escalation in serum and brain tissue. Posaconazole may have a clinical role in the treatment of disseminated infections by F. monophora.
The dematiaceous fungus Fonsecaea monophora is a causal agent of cerebral phaeohyphomycosis (10) and chromoblastomycosis (11-13). On the basis of molecular studies (3, 7), this fungus has recently been segregated from Fonsecaea pedrosoi, a traditionally well-known pathogen. Since very little is known about the pathogenicity and antifungal susceptibility of this novel fungus, the aim of this study was to develop a murine model of disseminated infection by F. monophora to evaluate its virulence and compare the therapeutic efficacies of amphotericin B (AMB), itraconazole (ITZ), and posaconazole (PSC).
Two clinical strains of Fonsecaea monophora were used: CBS 269.37 and CBS 117236. Their in vitro antifungal susceptibility tests (8) showed MICs of 1 μg/ml for AMB; 0.5 and 0.25 μg/ml for ITZ, respectively; and 0.25 and 0.12 μg/ml for PSC, respectively.
Male OF1 mice were immunosuppressed by a single intraperitoneal (i.p.) injection of 200 mg/kg of body weight of cyclophosphamide plus 5-fluorouracil at 150 mg/kg intravenously 1 day prior to the infection.
Development and characterization of an infection model.
For each strain, groups of 20 mice (10 for survival and 10 for tissue burden studies) were challenged with 2 × 105 CFU into the lateral tail vein. This was the lowest dose tested to produce an acute infection from which all the animals infected with strain CBS 117236 died within 15 days postinfection (data not shown). Animals were checked daily for 30 days. For the tissue burden study, mice were sacrificed on day 6 postinfection. Lungs, brain, spleen, liver, and kidneys were aseptically removed, and approximately half of each organ was weighed and homogenized in 1 ml of sterile normal saline. Dilutions of the homogenates were plated on potato dextrose agar (PDA), incubated at 30°C, and examined daily for 7 days. For the histopathology study, half of each organ was fixed, dehydrated, paraffin embedded, and sliced into 2-μm sections, which were stained with hematoxylin-eosin, periodic acid Schiff, or Grocott methamine silver. The Kaplan-Meier method and the log rank test were used for survival studies. When necessary, tissue burden studies were analyzed using the Kruskal-Wallis test, the Mann-Whitney U-test, and the Bonferroni correction. P < 0.05 was considered statistically significant.
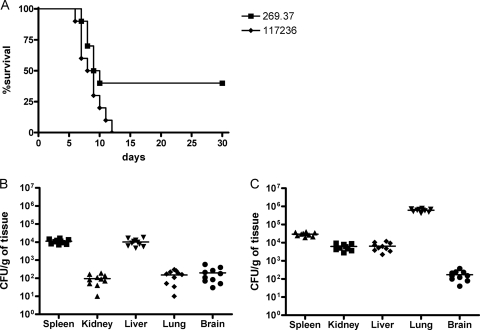
Virulence studies (Fig. 1) did not reveal significant differences in mortality rates between the isolates (P = 0.054). Fungi were present in all organs tested, and important differences were shown in the fungal loads of the different organs within and between both strains.
FIG. 1.
Cumulative mortality of mice infected with 2 × 105 CFU of F. monophora CBS 269.37 and CBS 117236 (A). Colony counts in spleen, kidney, liver, lung, and brain of mice infected with 2 × 105 CFU of F. monophora strain CBS 269.37 (B) and strain CBS 117236 (C). Horizontal lines indicate mean values.
Treatment studies.
Mice were challenged with 2 × 105 CFU of strain CBS 117236 or 1 × 106 CFU of strain CBS 269.37. Both inocula were chosen from previous studies and were able to produce acute infections, with all animals dying within 15 days postinfection. The drugs assayed were AMB (Fungizone), PSC (Noxafil), and ITZ (Canadiol). Their efficacy was evaluated by prolongation of survival and reduction of fungal load in kidneys, lungs, spleens, and brains. The procedures were performed as indicated above. The different groups (20 mice) were treated as follows: AMB at 1.5 mg/kg i.p. once daily; PSC at 10, 20, or 40 mg/kg orally once daily; and ITZ at 25 mg/kg orally twice daily. Control animals received no treatment. All treatments began 1 day after challenge, and the therapy lasted for 7 days. An additional group of 5 mice were similarly infected with strain CBS 269.37 and treated with the same doses used in the treatment study. These mice were used to determine, by bioassay, the level of each drug in serum and brain (1, 2, 4), 4 h after the last dosing on day 6 of therapy.
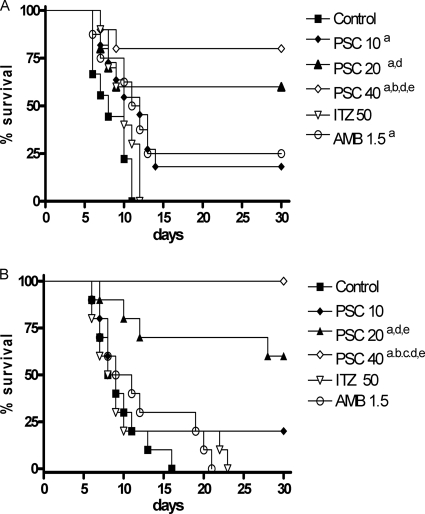
PSC at 20 mg/kg and 40 mg/kg significantly prolonged survival with respect to the control group and to the other therapies, although the lower dose prolonged survival only in mice infected by strain CBS 117236 (Fig. 2).
FIG. 2.
Cumulative mortality of mice infected with 2 × 105 CFU of F. monophora strain CBS 269.37 (A) and 1 × 106 CFU of strain CBS 117236 (B). a, P < 0.05 versus control; b, P < 0.05 versus 10 mg/kg PSC; c, P < 0.05 versus 20 mg/kg PSC; d, P < 0.05 versus 50 mg/kg ITZ; e, P < 0.05 versus 1.5 mg/kg AMB.
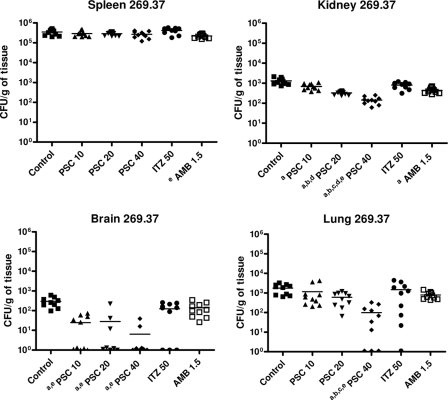
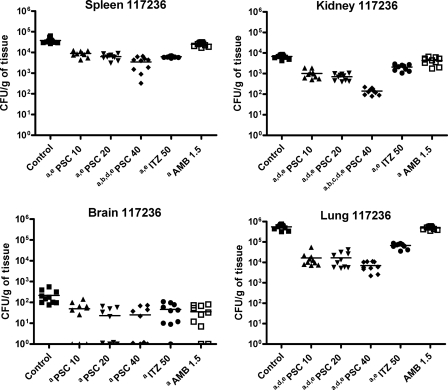
PSC showed a dose response efficacy in fungal load reduction (Fig. 3 and 4). PSC at 40 mg/kg was the most effective drug in reducing the fungal recovery in kidney for both strains and in lung for strain CBS 269.37 with respect to the other therapies. For strain CBS 117236, PSC at 10 or 20 mg/kg of body weight also improved fungal load in kidney and lung with respect to ITZ and AMB. All doses of PSC reduced significantly the fungal load in brain in both strains.
FIG. 3.
Effects of antifungal treatments on CFU counts in mice infected by CBS 269.37 in spleen, kidney, brain, and lung. a, P < 0.003 versus control; b, P < 0.003 versus 10 mg/kg PSC; c, P < 0.003 versus 20 mg/kg PSC; d, P < 0.003 versus 50 mg/kg ITZ; e, P < 0.003 versus 1.5 mg/kg AMB. Horizontal lines indicate mean values.
FIG. 4.
Effects of the antifungal treatments on CFU counts in mice infected by CBS 117236 in spleen, kidney, brain, and lung. a, P < 0.003 versus control; b, P < 0.003 versus 10 mg/kg PSC; c, P < 0.003 versus 20 mg/kg PSC; d, P < 0.003 versus 50 mg/kg ITZ; e, P < 0.003 versus 1.5 mg/kg AMB. Horizontal lines indicate mean values.
AMB only prolonged the survival of mice infected with strain CBS 269.37, and ITZ was not able to prolong the survival of mice in any case. The ability of AMB and ITZ to reduce tissue burden was considerably lower than that of PSC.
At day 6 of treatment, for all treatments administered, antifungal levels in serum and brain were above the corresponding MICs. PSC levels increased with dose escalation (Table 1).
TABLE 1.
Drug levels in serum and brain tissue measured by bioassay on day 6 of therapy and 4 h after last dosing
| Drug | Mean ± SD drug level in: |
|
|---|---|---|
| Serum (μg/ml) | Brain (μg/g) | |
| PSC | ||
| 10 mg/kg | 5.17 ± 0.99 | 2.02 ± 0.19 |
| 20 mg/kg | 6.35 ± 0.97 | 2.84 ± 0.74 |
| 40 mg/kg | 9.72 ± 0.57 | 6.73 ± 0.88 |
| ITZ (50 mg/kg) | 7.32 ± 3.44 | 6.88 ± 1.52 |
| AMB (1.5 mg/kg) | 6.45 ± 0.54 | 5.71 ± 0.43 |
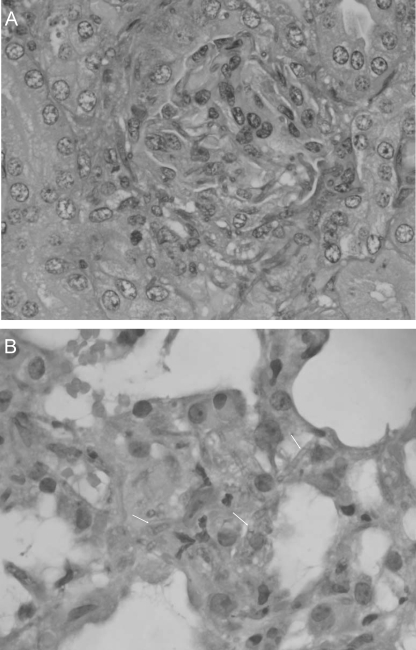
Histological studies only showed evidence of kidney and lung invasion by strain CBS 117236 (Fig. 5). Kidney sections showed glomerular and tubular invasion by hyphae. In lung, this isolate caused focal interstitial infiltration. None of the organs showed any inflammatory response.
FIG. 5.
(A) Kidney section showing hyphae and conidia of F. monophora CBS 117236 (periodic acid Schiff; ×400). (B) Lung section with hyphal invasion of F. monophora CBS 117236 with focal interstitial infiltration (hematoxylin-eosin; ×400).
In this study, PSC was the most effective drug at prolonging mouse survival and showed a dose-dependent efficacy in reducing tissue burden. These results correlated with drug levels in serum and brain obtained by bioassay. Although there is little clinical data on the use of these drugs in the treatment of phaeohyphomycosis (9), PSC has also shown efficacy in murine studies of disseminated infections by other dematiaceous fungi (5, 6). ITZ has also been recommended for the treatment of such infections (10). However, in our model the results obtained with this drug were very modest and clearly inferior to those of PSC.
F. monophora has been considered a neurotropic fungus (10). In our study, both strains, including an isolate from a brain abscess, were able to affect brain, although to a lesser degree than the other organs tested. Our data demonstrate a correlation between the in vitro activity of PSC, the brain concentration levels, and efficacy of this drug, even at low concentrations, in brain tissue burden.
In conclusion, this work confirms PSC as an alternative to ITZ for the treatment of phaeohyphomycoses.
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Bodet, C. A., III, J. H. Jorgensen, and D. J. Drutz. 1985. Simplified bioassay method for measurement of flucytosine or ketoconazole. J. Clin. Microbiol. 22:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen, K. J., E. M. Bernard, J. W. Gold, and D. Armstrong. 1985. Distribution and activity of amphotericin B in humans. J. Infect. Dis. 152:1037-1043. [DOI] [PubMed] [Google Scholar]
- 3.De Hoog, G. S., D. Attili-Angelis, V. A. Vicente, A. H. G. Gerrits van den Enden, and F. Queiroz-Telles. 2004. Molecular ecology and pathogenic potential of Fonsecaea species. Med. Mycol. 42:405-416. [DOI] [PubMed] [Google Scholar]
- 4.Fittler, A., B. Kocsis, I. Gerlinger, and L. Botz. 2009. Optimization of bioassay method for the quantitative microbiological determination of amphotericin B. Mycoses doi: 10.1111/j.1439-0507.2008.01660.x. [DOI] [PubMed]
- 5.Graybill, J. R., L. K. Najvar, E. Johnson, R. Bocanegra, and D. Loebenberg. 2004. Posaconazole therapy of disseminated phaeohyphomycosis in a murine model. Antimicrob. Agents Chemother. 48:2288-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariné, M., F. J. Pastor, and J. Guarro. 2009. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med. Mycol. 47:45-49. [DOI] [PubMed] [Google Scholar]
- 7.Najafzadeh, M. J., C. Gueidan, H. Badali, A. H. G. Gerrits van den Ende, and L. Xi. 2009. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med. Mycol. 47:17-25. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard, 2nd edition. Document M38-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 9.Negroni, R., S. H. Helou, N. Petri, A. M. Robles, A. Arechavala, and M. H. Bianchi. 2004. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin. Infect. Dis. 38:15-20. [DOI] [PubMed] [Google Scholar]
- 10.Surash, S., A. Tyagi, G. S. de Hoog, J. S. Zeng, R. C. Barton, and R. P. Hobson. 2005. Cerebral phaeohyphomycosis caused by Fonsecaea monophora. Med. Mycol. 43:465-472. [DOI] [PubMed] [Google Scholar]
- 11.Xi, L., C. Lu, J. Sun, X. Li, H. Liu, J. Zhang, Z. Xie, and G. S. de Hoog. 2009. Chromoblastomycosis caused by a meristematic mutant of Fonsecaea monophora. Med. Mycol. 47:77-80. [DOI] [PubMed] [Google Scholar]
- 12.Xi, L., J. Sun, C. Lu, H. Liu, Z. Xie, K. Fukushima, K. Takizawa, M. J. Najafzadeh, and G. S. de Hoog. 2009. Molecular diversity of Fonsecaea (Chaetothyriales) causing chromoblastomycosis in southern China. Med. Mycol. 47:27-33. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, J., L. Xi, C. Lu, X. Li, T. Xie, H. Zhang, Z. Xie, and G. S. de Hoog. 2009. Successful treatment for chromoblastomycosis caused by Fonsecaea monophora: a report of three cases in Guangdong, China. Mycoses 52:176-181. [DOI] [PubMed] [Google Scholar]