Abstract
Adsorption of gentamicin and netilmicin by new polyacrylonitrile and polyamide hemofiltration filters was studied over 4 h, using a single-compartment in vitro continuous venovenous hemofiltration model. After the first dose (16.6 mg of gentamicin, 19.3 mg of netilmicin), 14.9 mg of gentamicin and 19.2 mg of netilmicin were adsorbed to polyacrylonitrile filters in vitro. Adsorption by polyacrylonitrile filters was rapid and irreversible and could be increased by repeated dosing. Adsorption by polyamide filters was substantially less.
Previous in vitro data have demonstrated rapid adsorption of significant amounts of amikacin to polyacrylonitrile hemofilters (5). Adsorption of tobramycin and gentamicin may also occur (1-4); however, it is unclear whether adsorption to hemofilters is a property of aminoglycosides as a class. In view of this uncertainty, we carried out an in vitro study to determine the extent and time course of adsorption of gentamicin and netilmicin to polyacrylonitrile (PAN) and polyamide hemofilters.
The study used a previously described methodology involving a single-compartment in vitro model of continuous hemofiltration (5). Only changes to the methodology are given here. The blood-crystalloid mixture was made up of a unit of expired whole blood and heparinized (5,000 U heparin/liter) lactated Ringer's solution to a total volume of exactly 1,000 ml. Of this, 500 ml was placed in a glass reservoir where it was agitated, heated, and allowed to equilibrate for at least 10 min and until the temperature reached 36°C. A nominal dose of 14 mg gentamicin or 12 mg netilmicin was then infused into the mixing chamber over 10 min to obtain a circulating concentration comparable to the in vivo peak concentration (Table 1). The blood-crystalloid mixture was then pumped through a continuous venous-venous hemofiltration (CVVH) circuit. Samples of the blood-crystalloid mixture were taken from the mixing chamber immediately before the start of the drug infusion (0 min) for measurement of hemoglobin and then at 10 min (immediately after drug infusion, before starting the pump), 30, 60, and 90 min for measurement of drug concentration. The actual dose administered (the amount of aminoglycoside in commercial preparations is variable) and the amount of drug circulating at any given time point were calculated from the measured concentration at that time and the circulating volume (5). Adsorption was calculated as the difference between dose and circulating amount. At 90 min, the remaining 500 ml of the blood-crystalloid mixture was added to the mixing chamber. Another sample was taken after another hour. A second dose of drug (nominal 23 mg gentamicin or 20 mg netilmicin) was then added, and blood samples were taken 30, 60, and 90 min afterwards (Table 1). The second dose was also calculated to produce a clinically relevant concentration based on the assumption that the majority of the previous dose was adsorbed.
TABLE 1.
Actual doses and circulating concentrations in subgroupsa
| Drug | Hemofilter | Median (range) |
|||
|---|---|---|---|---|---|
| First dose (mg) | Circulating concn. after first dose (mg/liter) | Second dose (mg) | Total dose (mg) | ||
| Gentamicin | PAN | 16.6 (14.7-18.2) | 23.0 (20.5-25.3) | 28.1 (25.0-30.9) | 44.6 (39.7-49.1) |
| Polyamide | 15.5 (15.1-16.0) | 21.5 (20.9-22.2) | 26.2 (25.5-27.1) | 41.7 (40.6-43.1) | |
| Netilmicin | PAN | 19.3 (19.1-20.6) | 26.5 (26.4-28.4) | 32.6 (32.4-34.8) | 51.9 (51.5-55.4) |
| Polyamide | 19.8 (19.3-20.4) | 27.3 (26.5-28.1) | 33.6 (32.7-34.5) | 53.4 (52.0-54.9) | |
Circulating concentration was obtained from measured concentration at 10 min and adjusted for the priming volume of hemofilter and circuit.
Two types of filters, namely, a 0.6-m2 PAN hemofilter (Multiflow 60; Hospal, Meyzieu, France) and a 0.6-m2 polyamide hemofilter (Hemofilter 6S; Gambro, Hechingen, Germany), were used in the study, and the experiment was repeated four times for each hemofilter and each drug.
To test for spontaneous degradation or adsorption to the hemofilter tubing, a sham experiment was carried out. This was similar to the main experiment except that no hemofilter was used, there was no ultrafiltration, additional diluting fluid was not added, and a second dose of drug was not given. Blood samples were taken at 10, 120, and 240 min after the start of drug infusion. The sham experiment was repeated four times each for gentamicin and netilmicin.
Gentamicin concentration was measured with fluorescence polarization immunoassay technology (TDx/TDxFlx; Abbott Laboratories, Abbott Park, IL), and netilmicin concentration was measured with homogeneous enzyme immunoassay technology (Siemens Healthcare Diagnostics Inc., Newark, NJ). Samples were assayed once. Where necessary, samples were diluted to bring concentrations into the range of the assays.
Differences in adsorption and concentration were analyzed using the Mann-Whitney test (SPSS 10.0 for Windows). Differences were considered statistically significant when P < 0.05. Results are expressed as medians (ranges are shown in parentheses).
Both drugs were stable in the blood-crystalloid mixture and were not adsorbed by the circuit tubing. The circulating amount of drug at 10 min, 120 min, and 240 min was 13.7 (12.5 to 15.9) mg, 12.5 (8.9 to 15.3) mg, and 10.4 (8.3 to 14.8) mg, respectively (P > 0.05, 10 versus 240 min), for gentamicin and 9.1 (8.7 to 9.4) mg, 9.4 (9.1 to 9.4) mg, and 9.3 (9.2 to 9.6) mg, respectively (P > 0.05, 10 versus 240 min), for netilmicin.
Hemoglobin concentrations were similar for all experimental subsets: 6.5 (6.1 to 7.0) g/dl (gentamicin-PAN), 6.1 (4.7 to 6.4) g/dl (gentamicin-polyamide), 5.9 (5.4 to 6.6) g/dl (netilmicin-PAN), and 5.6 (5.0 to 6.5) g/dl (netilmicin-polyamide).
At clinically relevant concentrations, more than 90% of the first dose of gentamicin and 99% of the first dose of netilmicin were adsorbed by 20 min by PAN filters, but little drug was adsorbed by polyamide filters (Fig. 1 and 2). Adsorption to polyamide, but not PAN, filters was partially reversed by dilution. Administration of a second dose of drug resulted in a significant increase in adsorption of both drugs to PAN filters but only of netilmicin to polyamide. These findings are in keeping with previous data demonstrating similar in vitro adsorption characteristics for amikacin (5) and significant adsorption of tobramycin by PAN filters (3), suggesting that significant adsorption by PAN filters is a characteristic shared by aminoglycosides as a class.
FIG. 1.
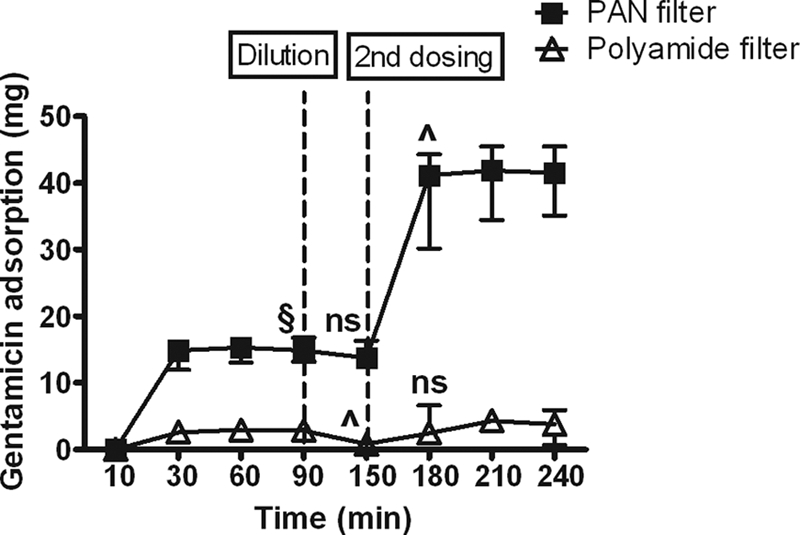
Cumulative adsorption of gentamicin by hemofilters against time. Data are shown as median and range. §, P < 0.05 versus polyamide; ns, no significant difference versus previous time point; ^, P < 0.05 versus previous time point.
FIG. 2.
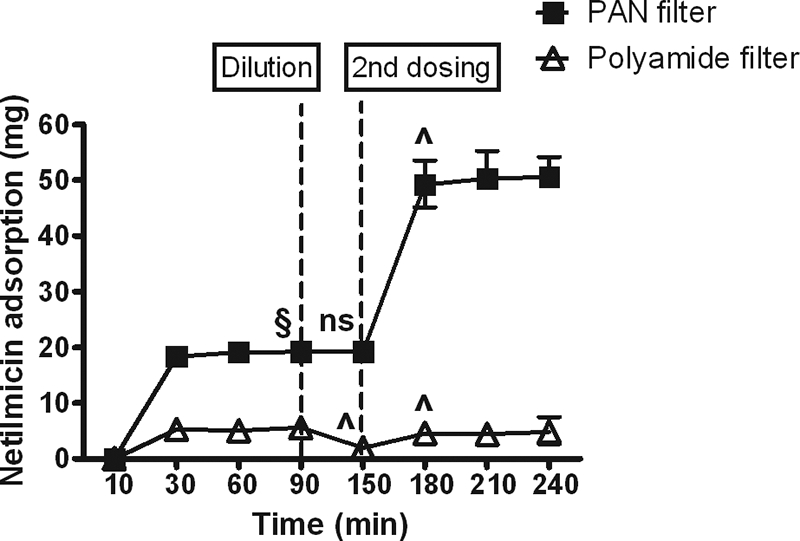
Cumulative adsorption of netilmicin by hemofilters against time. Data are shown as median and range. §, P < 0.05 versus polyamide; ns, no significant difference versus previous time point; ^, P < 0.05 versus previous time point.
It has previously been suggested that gentamicin adsorption is reversible (2). A reversal of adsorption with falling circulating concentrations might increase trough concentrations and hence toxicity. In our study, reversal of adsorption was demonstrated only in the polyamide subsets, and the amount of drug (2.1 mg gentamicin and 3.7 mg netilmicin) was small. Furthermore, in vivo, released drug may pass into the ultrafiltrate and thus be eliminated rather than be passed back into the blood.
Our study used an in vitro single-compartment model with a relatively low circulating volume. In vivo, drug will be distributed to other compartments, and this may reduce the adsorption of drug. In vitro amikacin adsorption is more closely related to the dose than circulating concentration (5); however, the low circulating volume of the model restricted the dose that could be given while achieving clinical relevant circulating concentrations. Other potential weaknesses of the study include the size of the second dose of drug, failure to measure albumin concentration, and the use of new hemofilters. Our assumption that the majority of the initial dose would be adsorbed proved invalid for polyamide filters, so the circulating concentration after the second dose would have been abnormally high. Despite this, the second dose had no effect on gentamicin adsorption and only a modest effect on netilmicin adsorption. It has been suggested that the length of time for which a filter has been used may affect adsorption (4), so we might have obtained different results had we carried out hemofiltration for a few hours before testing adsorption.
In conclusion, significant adsorption by new PAN hemofilters appears to be a property of aminoglycosides in general. The adsorption is rapid and not reversible by a fall in concentration.
Footnotes
Published ahead of print on 30 November 2009.
REFERENCES
- 1.Cigarran-Guldris, S., M. E. Brier, and T. A. Golper. 1991. Tobramycin clearance during simulated continuous arteriovenous hemodialysis. Contrib. Nephrol. 93:120-123. [DOI] [PubMed] [Google Scholar]
- 2.Kraft, D., and H. Lode. 1979. Elimination of ampicillin and gentamicin by hemofiltration. Klin. Wochenschr. 57:195-196. [DOI] [PubMed] [Google Scholar]
- 3.Kronfol, N. O., A. H. Lau, and M. M. Barakat. 1987. Aminoglycoside binding to polyacrylonitrile hemofilter membranes during continuous hemofiltration. ASAIO Trans. 33:300-303. [PubMed] [Google Scholar]
- 4.Rumpf, K. W., J. Rieger, R. Ansorg, B. Doht, and F. Scheler. 1977. Binding of antibiotics by dialysis membranes and its clinical relevance. Proc. Eur. Dial. Transplant Assoc. 14:607-609. [PubMed] [Google Scholar]
- 5.Tian, Q., C. D. Gomersall, M. Ip, P. E. Tan, G. M. Joynt, and G. Y. Choi. 2008. Adsorption of amikacin, a significant mechanism of elimination by hemofiltration. Antimicrob. Agents Chemother. 52:1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]


