Abstract
Host susceptibility to infection is controlled in large measure by the genetic makeup of the host. Spirochetes of the genus Borrelia include nearly 40 species of vector-borne spirochetes that are capable of infecting a wide range of mammalian hosts, causing Lyme disease and relapsing fever. Relapsing fever is associated with high-level bacteremia, as well as hematologic manifestations, such as thrombocytopenia (i.e., low platelet numbers) and anemia. To facilitate studies of genetic control of susceptibility to Borrelia hermsii infection, we performed a systematic analysis of the course of infection using immunocompetent and immunocompromised inbred strains of mice. Our analysis revealed that sensitivity to B. hermsii infections is genetically controlled. In addition, whereas the role of adaptive immunity to relapsing fever-causing spirochetes is well documented, we found that innate immunity contributes significantly to the reduction of bacterial burden. Similar to human infection, the progression of the disease in mice was associated with thrombocytopenia and anemia. Histological and fluorescence in situ hybridization (FISH) analysis of infected tissues indicated that red blood cells (RBCs) were removed by tissue-resident macrophages, a process that could lead to anemia. Spirochetes in the spleen and liver were often visualized associated with RBCs, lending support to the hypothesis that direct interaction of B. hermsii spirochetes with RBCs leads to clearance of bacteria from the bloodstream by tissue phagocytes.
The Borrelia genus is formed by a group of bacteria that are small flexible helical spirochetes. Lyme disease spirochetes, B. burgdorferi sensu lato (32), cause the most common arthropod-borne illness in the U.S. and are responsible for more than 20,000 reported cases per year (18a). Relapsing fever is another important worldwide infection that is caused by several species of the genus Borrelia and is typified by high levels of growth of spirochetes in the bloodstream. Epidemic relapsing fever is caused by Borrelia recurrentis and transmitted by body lice (20). Endemic relapsing fever is transmitted by ticks and caused by several species of Borrelia, such as B. hermsii, B. turicatae, and B. duttoni (22).
B. hermsii is the most important relapsing fever spirochete in the United States and is acquired by the bite of an infected Ornithodorus hermsi tick (21). The first manifestation of relapsing fever is associated with high-titer (106 to 108 spirochetes/ml) growth of the spirochete in blood. This infection is typified by recurrent febrile episodes, each of which corresponds to high-level bacteremia caused by antigenically distinct populations of bacteria (reviewed in references 8, 9, and 36). Antigenic switching is a consequence of the sequential expression of genes for serotype-specific major surface antigens known collectively as variable major proteins (Vmps). The mechanism of antigenic variation involves a gene rearrangement to localize a new variant vmp gene at a unique expression site (11). The spirochete encodes many silent vmp genes, and at least 25 antigenically distinct serotypes of this bacterium can be generated from a single bacterium (33, 38). Thus, a cycle of bacteremia, clearance, and outgrowth of antigenic variants can occur several times, giving rise to a relapsing illness. The high-titer growth of Borrelia in the bloodstream results in a wide range of symptoms that include fever, chills, and muscle and joint aches. After 2 to 9 days, these symptoms disappear, corresponding to the first clearance of bacteria from the blood, but the recurring nature of the bacteremia results in the reappearance of symptoms for several weeks, if left untreated. In addition, blood infection is associated with several striking hematological abnormalities. For example, thrombocytopenia, i.e., a low platelet count, is the most frequent laboratory manifestation of B. hermsii infection in humans, and normocytic anemia and leukocytosis are also common (14). Both of these manifestations might involve interactions between host cells and blood-borne bacteria, because B. hermsii has been demonstrated to bind to platelets and red blood cells (RBCs) (5, 26). In particular, not only were episodes of thrombocytopenia temporally and quantitatively correlated with episodes of bacteremia, but platelet-bacterium complexes were detected in infected mice (4).
Rodents are both natural hosts for relapsing fever spirochetes and provide an experimental model in which to investigate the pathogenesis of human infection. Murine infection recapitulates a number of pathophysiological aspects of the human disease, most notably the hallmark of recurrent episodes of severe (∼107 to 108/ml) bacteremia. In addition, hematological manifestations, such as leukocytosis, anemia, and thrombocytopenia, that are commonly observed in human patients are associated with the episodes of recurrent spirochetemia in mice. Thus, the groundbreaking discovery that immune evasion by relapsing fever spirochetes was due to antigenic variation resulting from genomic rearrangements relied upon murine infection to generate antigenic variants (8, 38). The murine model also provided a system in which to identify critical components of an adaptive immune response required for clearance of B. hermsii from the bloodstream. μMT−/− mice that lack B cells are completely susceptible to relapsing fever (19), and passive transfer experiments demonstrated that antibodies are essential effector molecules in protection from relapsing fever (6, 41). Interestingly, the B cells confer protection independently from T-cell help (1, 10, 31). T-cell-independent antibody responses occur with remarkable speed, consistent with the observation that each episode of spirochetemia lasts only two or three days. Finally, immune reconstitution of recombination-activating gene (rag)-deficient mice revealed that a subclass of T-independent B cells termed B1b cells was capable of conferring long-term immunity to B. hermsii infection (2).
Whereas the role of an adaptive immune response in the clearance of relapsing fever spirochetes has been the focus of significant scientific investigation, very little is known about the role of innate immune mechanisms and spirochetemia. Although mice deficient in adaptive immunity, such as rag and scid mutants, cannot resolve Borrelia infection completely, they possess some means of transiently controlling relapsing fever spirochetes. Thus, while infection of rag2−/− mice with B. hermsii leads to a rapid increase in the number of blood-borne bacteria that peaks at day 3 postinfection, in the ensuing days, the level of bacteremia in these mice diminishes significantly, indicating that innate immunity contributes to effective control of this bacterial infection. These observations prompt the question of how mice manage to control bacteremia even in the absence of an adaptive response.
Inbred strains of mice have been used extensively in the analysis of genetic control of infectious diseases (15). Several genes identified by genetic mapping have been shown to play an important role in the development and progression of human diseases. One of the most prominent examples is Nramp1/SLC11A1, which encodes a divalent cation transporter (13). Nramp1 was identified as a gene controlling susceptibility to a wide range of pathogens, such as Leishmania, Salmonella, and Mycobacterium bovis (39). Inbred strains of mice were used extensively for studies of genetic control of differences in sensitivity to diseases induced by B. burgdorferi infection. These studies identified several quantitative trait loci (QTL) that control B. burgdorferi-induced arthritis and levels of specific and total IgGs (34, 40). Subsequent analysis of 10 different phenotypic readouts identified up to 14 QTL that control various aspects of B. burgdorferi infection (34). To date, there are no studies of the contribution of genetic variation of the host to control of B. hermsii infection. In order to develop an experimental system to study the role of innate immunity in the control of blood-borne bacteremia, we performed a survey of common inbred mouse strains. We identified several significant differences in the course and outcome of B. hermsii infection in inbred strains of mice that indicate genetic control of both innate and adaptive response to this infection.
MATERIALS AND METHODS
Mice.
The mouse strains BALB/cByJ, C57BL/6ByJ, C3H/HeJ, DBA/1J, and SJL/J were purchased from Jackson Laboratories (Bar Harbor, ME). Information regarding the differences in the genetic backgrounds of these mouse strains is available on the Jackson website (http://www.informatics.jax.org/strains_SNPs.shtml). All wild-type mice were females between 4 and 6 weeks of age. Female rag2−/− mice in the BALB/c, C57BL/6, and C3H/HeN backgrounds were purchased from Taconic Farms (Germantown, NY). rag2−/− mice were also between 4 and 6 weeks of age. Additional male and female rag2−/− mice for this study were bred in the animal facility at the University of Massachusetts Medical School. Mice used in this study were housed in microisolator cages in the Department of Animal Medicine.
B. hermsii infections and blood sampling.
To generate standardized stocks of highly infectious bacteria, B. hermsii bacteria were harvested from the blood of infected secretory IgM-deficient (sIgM−/−) mice, which suffer high-level (∼108/ml) bacteremia, by cardiac puncture and frozen in 20% glycerol until needed. Five rag2−/− mice of BALB/c, C57BL/6, or C3H/HeN background were infected intravenously with 105 Borrelia hermsii strain DAH bacteria (27) in 100 μl of BSK-H medium (Sigma-Aldrich Co., St. Louis, MO). Blood sampling was carried out as previously described (4). Briefly, less than 1 mm of the tail was cut with surgical scissors, and 5 μl of blood was taken with a micropipette and placed in 45 μl of anticoagulant (100 mM citric acid, 100 mM sodium citrate in phosphate-buffered saline [PBS], pH 7.0). Bacteremia was monitored at 24-h intervals for 5 days for wild-type mice and at 12- or 24-h intervals for 14 days for rag2−/− mice. The levels of B. hermsii bacteria in these blood samples were evaluated by diluting 10 μl of the above-described blood sample into 40 μl of phosphate-buffered dextrose (2% dextrose in PBS, pH 7.0) for a final dilution of 1:50. Five-microliter amounts of the diluted blood samples were examined under dark-field microscopy (×400 magnification) to quantify the number of B. hermsii bacteria, as described previously (4). The limit of detection of B. hermsii bacteria by this approach is 1 × 105 bacteria/ml.
Flow cytometry.
One microliter of whole blood was diluted 1:10 in citrate anticoagulant. Phycoerythrin (PE)-Cy5-conjugated anti-mouse TER-119 (eBioscience) was diluted 1:10, and PE-conjugated anti-mouse CD61 antibody (PharMinGen) was diluted 1:200 in phosphate-buffered dextrose to label red blood cells and platelets, respectively. To measure the platelet and red blood cell counts by flow cytometry, 10 μl of SPHERO rainbow fluorescent polystyrene beads (Spherotech, Inc., Libertyville, IL) was added as an internal standard, as described previously (3). The numbers of red blood cells and platelets were measured by flow cytometry using a Becton-Dickinson FACSCalibur. All flow cytometry experiments were performed within 2 days of collection of blood samples.
Histology.
Tissues were harvested from infected mice at days 5 and 14 postinfection, fixed in 10% histological-grade buffered formalin, and then embedded in paraffin. Five-micrometer sections of spleen and liver were cut and then stained with hematoxylin and eosin (H&E).
FISH.
For fluorescence in situ hybridization (FISH) analysis, tissues were harvested from infected mice at days 5 and 14 postinfection and fixed in 3.7% histological grade formaldehyde with 50% ethanol in phosphate-buffered saline. Tissues were embedded in methacrylate and sectioned as described previously (30). A Borrelia genus-specific oligonucleotide probe, reBorr0 (5′-GCATGCTTAAGACGCACTGCC-3′), was designed and 5′ end labeled with Cy3 (indocarbocyanine) (Biomers, Ulm, Germany). To control for specificity of the probe, Borrelia garinii strain 1B29 (kindly provided by A. Schönberg, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany) and Treponema denticola (ATCC 35405) with two mismatches at the probe binding site were included as positive and negative controls, respectively, in each FISH experiment. Sections (3 μm) were hybridized as published previously (35), using a hybridization buffer containing 20% formamide. After incubation at 50°C for 2 h, slides were rinsed with double-distilled water, air dried, and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing the nonspecific nucleic acid stain DAPI (4′,6′-diamidino-2-phenylindole). For microscopy, an epifluorescence microscope (Axioplan2; Carl Zeiss, Jena, Germany) equipped with narrow-band filter sets (AHF; Analysentechnik, Tübingen, Germany) was used.
Statistical analysis.
Data were analyzed using GraphPad Prism. Comparison of multiple groups was performed using one-way analysis of variance (ANOVA), and the significance of differences was evaluated with Bonferroni's multiple comparison test. Statistical significance of difference between two groups was evaluated using two-tailed unpaired t tests. In all tests, P values below 0.05 were considered statistically significant. In all graphs, error bars represent standard deviations.
RESULTS
Immunocompetent BALB/c mice are relatively resistant to B. hermsii infection.
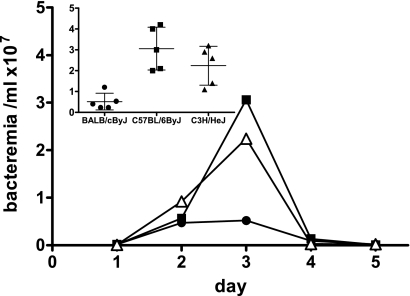
In order to test whether a phenotypic difference in resistance to B. hermsii exists among inbred strains of mice, we infected intraperitoneally (i.p.) mice of five different strains, BALB/cByJ, C57BL/6ByJ, C3H/HeJ, DBA/1J, and SJL/J (Jackson Laboratory, Bar Harbor, ME), with 105 B. hermsii strain DAH bacteria. These strains were chosen to match the progenitors of existing recombinant inbred mapping panels. For this initial survey, female mice were infected in groups of five, and bacteremia was evaluated daily by dark-field microscopy of diluted blood samples as previously described (4). Following the initial experiments, we established that an intravenous (i.v.) route of infection provides a more reproducible course of infection than i.p. injection. All subsequent experiments used i.v. infection for delivery of spirochetes to the bloodstream. Previous extensive study of infections in C57BL/6 mice (2, 5) indicated that peak bacteremia of B. hermsii strain DAH infection occurs on day 3 postinfection (5). Thus, to discern potential phenotypic differences among different mouse strains, we chose to compare bacteremia at day 3 postinfection. We found that BALB/cByJ was the most resistant strain, displaying a level of bacteremia 5-fold lower than that in C57BL/6ByJ mice and 4-fold lower than that in C3H/HeJ mice. DBA/1J and SJL/J mice had intermediate levels of bacteremia (data not shown). Two additional surveys with strains BALB/cByJ, C57BL/6ByJ, and C3H/HeJ revealed these phenotypic differences to be reproducible and significant between BALB/cByJ and C57BL/6ByJ (P < 0.01) and between BALB/cByJ and C3H/HeJ (P < 0.05) mice (Fig. 1, day 3).
FIG. 1.
Differences in control of B. hermsii infection among common inbred strains of mice. Wild-type female BALB/cByJ, C57BL/6ByJ, and C3H/HeJ mice were infected i.v. in groups of five with 105 B. hermsii strain DAH bacteria. Bacteremia was monitored daily by microscopic examination of blood smears. The limit of detection of spirochetes by this approach is 1 × 105 bacteria/ml of blood. Inset shows spirochete numbers in the bloodstreams of individual mice at day 3 postinfection, along with means and standard deviations for the groups. At this time point, there were significant differences in levels of bacteremia between BALB/cByJ and C57BL/6ByJ mice (P < 0.01) and between BALB/cByJ and C3H/HeJ mice (P < 0.05) as determined by one-way ANOVA with Bonferroni's multiple comparison test.
C57BL/6 rag2−/− mice are relatively resistant to B. hermsii infection.
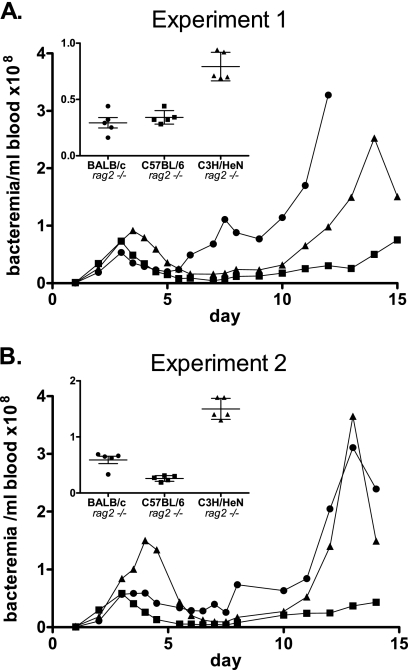
Initial observations in rag1−/− mice indicated that bacteremia peaked at day 3 or 4 postinfection, followed by a 10- to 20-fold decrease in bacterial load (1). Thus, some degree of control of bacteremia was evident even in the absence of the adaptive immune system, suggesting a substantial contribution of innate immunity in this process. To determine if differences in the response to B. hermsii infection observed with the immunocompetent wild-type mice described above might reflect genetic differences in the function(s) of the innate immune system, we analyzed the course and outcome of B. hermsii infection in rag2−/− mice of different strain backgrounds. Although rag2−/− mice suffer a persistent B. hermsii infection, they survive for approximately 12 to 21 days, allowing us to assess the role of the innate immune system in control of B. hermsii bacteremia. For these experiments, we selected rag2−/− counterparts of the three strains that showed the greatest differences at day 3 postinfection when the infection was performed in wild-type mice. BALB/c rag2−/−, C57BL/6 rag2−/−, and C3H/HeN rag2−/− mice were infected i.v. with 105 B. hermsii strain DAH bacteria and bled every 12 or 24 h for 14 days. Bacteremia was monitored daily by dark-field microscopy of blood smears.
The three strains examined exhibited significant differences in the level of bacteremia, and these differences are described below. Nevertheless, an overall triphasic pattern of infection was apparent for each of the three inbred strains (Fig. 2). First, the mice suffered a single peak of bacteremia that was then partially controlled by day 6 postinfection. Second, this was followed by a period of four to six days in which the bacteremia was moderate and relatively consistent. Finally, bacteremia increased, and in two of the three strains, this increase was dramatic, leading to morbidity and mortality.
FIG. 2.
The efficiency of innate immune control of B. hermsii bacteremia varies with mouse strain. Two independent surveys (A and B) reveal a genetically defined course of B. hermsii spirochetemia in rag2−/− mice. Female mice between 4 and 6 weeks of age were infected i.v. in groups of five with 105 B. hermsii strain DAH bacteria. Five microliters of blood per day was collected from each mouse for 14 days postinfection for microscopic examination of blood smears. Inset shows spirochete numbers in bloodstreams of individual mice at day 4 postinfection, along with means and standard deviations for the groups. At this time point, C3H/HeN rag2−/− mice demonstrated significantly (P < 0.001, one-way ANOVA) higher levels of bacteremia than BALB/c rag2−/− or C57BL/6 rag2−/− mice.
While the overall pattern of bacteremia showed some similarities among the three rag2−/− mouse strains, we were able to identify three periods over the course of the 14-day infection during which there were significant differences in bacteremia between the strains tested. First, at days 3.5 to 4 postinfection, we found that BALB/c rag2−/− mice suffered approximately 2.5-fold lower bacteremia than C3H/HeN rag2−/− mice, consistent with the relative susceptibilities of their wild-type counterparts, BALB/cByJ and C3H/HeJ mice, respectively. Interestingly, C57BL/6 rag2−/− mice were relatively resistant, suffering less than half the level of bacteremia (P < 0.01) seen in C3H/HeN rag2−/− mice (Fig. 2A, see inset), a finding in contrast to the results for wild-type C57BL/6 mice, which were relatively susceptible to B. hermsii infection at the first peak of bacteremia (Fig. 1). In addition, while none of the rag2−/− mice cleared B. hermsii infection, after the first peak of bacteremia, C57BL/6 rag2−/− mice were able to control bacteremia better and survived longer than either C3H/HeN rag2−/− or BALB/c rag2−/− mice.
Second, at approximately day 7 postinfection, BALB/c rag2−/− mice suffered an additional peak of bacteremia that was not observed with the other strains (Fig. 2A). Thus, at this time point, BALB/c rag2−/− mice had 6-fold (P < 0.001) and 10-fold (P < 0.001) higher numbers of blood-borne bacteria than C3H/HeN rag2−/− and C57BL/6 rag2−/− mice, respectively. By day 9 postinfection, BALB/c rag2−/− mice were able to establish partial (albeit temporary) control of bacteremia.
Finally, between days 12 and 14 postinfection, BALB/c rag2−/− mice displayed approximately 10-fold greater bacteremia than C57BL/6 rag2−/− mice (P < 0.05 at day 12 for all experiments). During this period of time, C3H/HeN rag2−/− mice had intermediate numbers of bacteria in the bloodstream. The increase in bacteremia in BALB/c rag2−/− and C3H/HeN rag2−/− mice corresponded to signs of clinical illness, such as listlessness and ruffled fur, prompting euthanasia of many of the mice (not shown). Thus, although it has been established that an antibody response is critical for clearing B. hermsii infection in wild-type mice, the variation in response to infection by different strains of rag2−/− mice indicates that innate components of the immune system also contribute to the clearance of B. hermsii from the bloodstream.
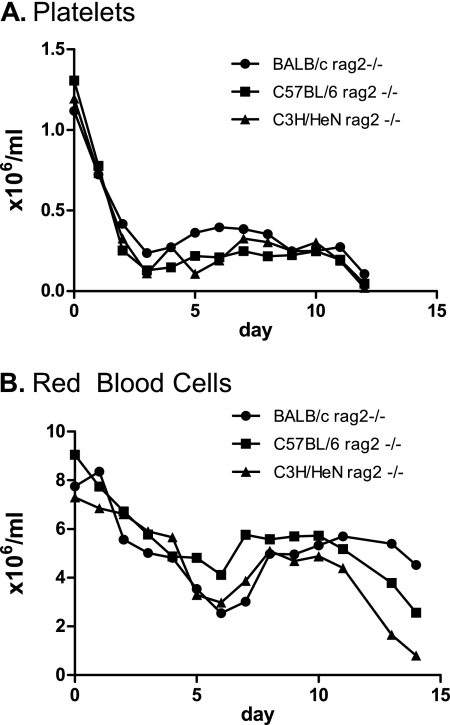
B. hermsii induced thrombocytopenia and anemia.
Associated with the episodes of spirochetemia in mice are anemia and thrombocytopenia, which are also commonly observed in human patients (5, 25). Thus, in addition to monitoring bacteremia, in the second strain survey of rag2−/− strains (Fig. 2B), we also followed RBC and platelet counts by flow cytometry (see Materials and Methods). Consistent with earlier analysis of B. hermsii infection in immunocompromised mice (5), all mice became thrombocytopenic. The kinetics of thrombocytopenia were similar across all mouse strains examined (Fig. 3A). Over the first three days, there was a rapid decrease in the number of platelets, with platelet counts reaching 10 to 20% of normal levels by day 3 postinfection, the time point of peak bacteremia. The platelet counts recovered very slightly over the next few days, but the mice remained severely thrombocytopenic. At day 12 postinfection, the average platelet count dropped even further. The platelet count profiles among all three strains of mice were highly similar, although the counts of BALB/c rag2−/− mice recovered slightly better than those of the other strains (P < 0.05 for day 5). We conclude that the genetic background of these mice has little influence on the development and course of thrombocytopenia during relapsing fever infection.
FIG. 3.
Thrombocytopenia and anemia in rag2−/− mice. Blood sampled from bacteremic rag2−/− mice was used for flow cytometry-based measurement of platelets (A) and RBCs (B). Rapid initial reduction in the numbers of RBCs and platelets was followed by a recovery period in all 3 strains of mice examined. The minimum in the platelet numbers corresponded to the peak bacteremia at day 4. The minimum in the number of RBCs corresponded to the minimum bacterial loads following the initial peak of bacteremia.
Measurement of RBC counts indicated that infection affects the numbers of RBCs, but not in a fashion synchronous with changes in platelet numbers. We discerned three phases of anemia in immunocompromised mice. In the first phase, RBC counts in infected mice began to decline rapidly early in infection, although less precipitously than platelet counts (Fig. 3B). Unlike the platelet numbers, for which the period of rapid decline was limited to the first three days of infection, RBC numbers continued to decline until day 6. At this time point, all three strains of mice had only 30% to 45% of their normal number of RBCs. Interestingly, this time point corresponded to the point at which bacteremia was at a minimum following the initial peak of bacteremia at day 3 to 4 (Fig. 2). In the second phase, after day 6, the RBC count partially recovered and held steady for approximately four to six days, until about day 11 postinfection. Finally, the RBC count diminished in all three mouse strains through day 14, at which point the experiment was terminated due to frank illness of the animals. This third phase corresponded to an increase in bacteremia in all three strains. However, the degree of anemia did not directly correlate with the degree of bacteremia. For example, at day 12 postinfection, while both C3H/HeN rag2−/− and BALB/c rag2−/− mice had very high levels of bacteremia (>2 × 108) (Fig. 2), C3H/HeN rag2−/− mice had approximately 12-fold-lower red blood cell counts than BALB/c rag2−/− mice (P < 0.001) (Fig. 3B). In addition, whereas the bacterial load in C57BL/6 rag2−/− mice at days 12 to 14 was considerably lower than that in C3H/HeN rag2−/− and BALB/c rag2−/− mice, the C57BL/6 mice displayed a level of anemia intermediate between the levels in the other two strains. Therefore, our results indicate that changes in the number of RBCs following B. hermsii infection (Fig. 3B) do not simply reflect bacterial burdens and thus appear to be under independent genetic control.
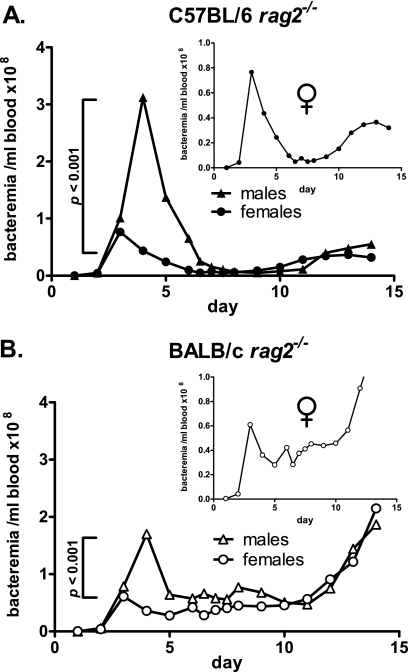
Sex-specific differences in innate control of B. hermsii infection.
The gender of the host often influences the course and outcome of infection. To determine if innate immune control of B. hermsii infection has a sex-specific component, we compared the courses of infection in male and female littermates. We infected five male and five female littermates of the BALB/c rag2−/− and C57BL/6 rag2−/− strains with 105 B. hermsii strain DAH bacteria via tail vein injection. In both strains examined, we found that at the early time point (days 3 to 4), male littermates suffered 2- to 4-fold higher levels of bacteremia than their female counterparts (Fig. 4). In addition, peak bacteremia occurred 12 to 24 h later in male littermates than in females. At the middle and late time points, however, differences in bacteremia between male and female littermates were not significant. These data indicate a strong influence of gender on resistance to B. hermsii infection, particularly in the kinetics and effectiveness of early suppression of bacteremia.
FIG. 4.
Male rag2−/− mice are more susceptible than females to B. hermsii infection in the early stages of infection. (A) BALB/c rag2−/− males suffered more severe bacteremia than females of the same strain at the early time point (day 4 postinfection). Peak bacteremia in males occurred 12 to 24 h later than that of female mice. (B) C57BL/6 rag2−/− males also suffered higher levels of bacteremia than female littermates at the early time point. However, bacteremia in C57BL/6 rag2−/− males was lower than that of BALB/c rag2−/− males at the same time point. Inset panels show peak bacteremia in the female cohorts only. All experiments, n = 4; comparisons by two-tailed t test.
Histopathology of infection.
To further characterize the course of B. hermsii infection in immunocompromised mice, we performed histopathological analysis of liver, spleen, and heart tissues from infected animals. The liver and spleen were selected on the basis of their role as primary sites of bacterial clearance. Additionally, in mice, liver and spleen are organs where ectopic hematopoiesis can occur. We also collected heart tissue, to determine if this organ is a site of spirochetal invasion, given that carditis is a prominent feature of Borrelia burgdorferi and relapsing fever Borrelia infections (17, 28).
Liver, spleen, and heart tissues were collected from animals infected with 105 CFU of DAH bacteria for various amounts of time. Hepatosplenomegaly has been previously observed during B. hermsii infection of immunocompetent mice (5). Indeed, necropsies of rag2−/− animals selected for tissue harvest revealed dramatic hepato- and splenomegaly at the late stages of infection in all strains examined. In addition, the spleens of BALB/c rag2−/− animals were more than two times larger than those of the C57BL/6 rag2−/− mice (data not shown).
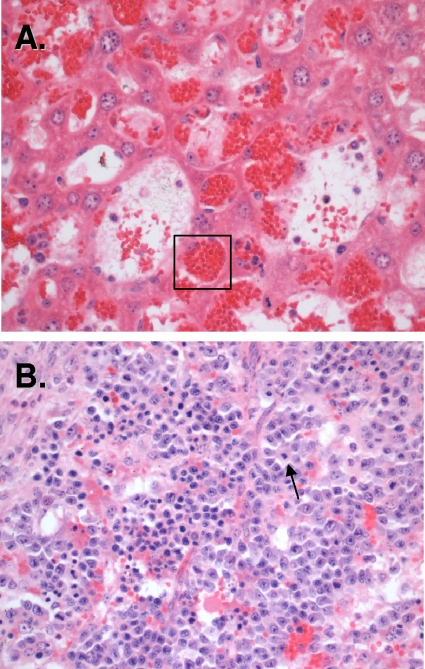
Our histopathological analysis revealed that B. hermsii infection leads to the development of three processes that could contribute to splenomegaly. There were no qualitative differences between spleens of rag2−/− mouse strains (data not shown), and to illustrate the results described below, we use representative images obtained using samples from BALB/c rag2−/− mice. First, analysis of H&E-stained sections revealed the expected influx of inflammatory cells in both livers and spleens of infected animals (Fig. 5). Second, spleens of infected animals had numerous megakaryocytes present (Fig. 5B, arrow), indicating extensive extramedullary hematopoiesis. Extramedullary hematopoiesis is often seen in chronic anemia and is consistent with the anemia of B. hermsii infection (Fig. 3A). Third and most striking, all sections examined demonstrated extensive erythrophagocytosis by resident tissue macrophages. Indeed, by day 6 postinfection, the majority of tissue macrophages contained large numbers of RBCs (Fig. 5A, box). Thus, one of the developments in the progression of B. hermsii infection in immunocompromised mice may be removal of RBCs by tissue-resident macrophages, leading to anemia. The extent of erythrophagocytosis in this nonquantitative assessment was similar in all inbred strains examined.
FIG. 5.
Histopathology of infected rag2−/− mice. Panels show H&E staining of liver (A) and spleen (B) sections of organs recovered from BALB/c rag2−/− animals infected with 105 CFU B. hermsii strain DAH bacteria. Numerous RBCs were found in tissue-resident macrophages (box), indicating extensive erythrophagocytosis. The presence of megakaryocytes in spleens of infected animals indicates ongoing extramedullary hematopoiesis.
Localization of spirochetes by fluorescent in situ hybridization.
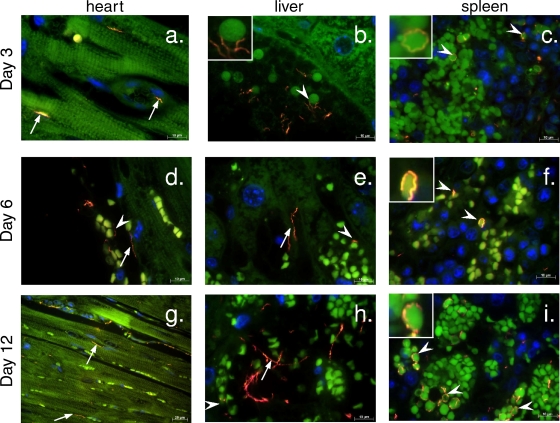
To determine the level of tissue invasion by spirochetes during infection and whether the erythrophagocytosis observed upon histological analysis might be reflected in specific associations between spirochetes and RBCs, we visualized spirochetes in tissues using FISH with a Borrelia genus-specific probe. FISH analysis revealed that by day 3 postinfection, spirochetes were found in all three of the tissues examined, i.e., heart, spleen, and liver. In the heart, spirochetes were visualized associated with blood vessel walls, as well as with cardiac muscle, oriented parallel to the muscle fiber (Fig. 6a, arrows). In the liver and spleen, RBCs were identified on the basis of their anuclear and highly autofluorescent morphology (see also stacked RBCs in the cardiac blood vessel, Fig. 6d), and many spirochetes were observed associated with these cells (Fig. 6a and b, arrowheads). By day 6, the numbers of spirochetes found in each tissue increased despite a reduction in numbers of blood-borne bacteria (Fig. 2). Spirochetes were readily seen associated with the blood vessel walls (Fig. 6d and e, arrows), and numerous spirochetes were observed wrapped around RBCs (Fig. 6d to f, arrowheads). By day 12 postinfection, B. hermsii numbers in the tissues increased further, correlating with the increased bacterial load in the blood and ultimately leading to severe infection and death of BALB/c rag2−/− and C3H rag2−/− animals. In the heart, spirochetes were observed within the heart muscle (Fig. 6g), and in the liver and, particularly, in the spleen, there were numerous spirochetes associated with RBCs (Fig. 6h and i). Thus, in addition to providing evidence that B. hermsii bacteremia is associated with significant invasion into tissues such as the heart, the results of our immunofluorescence examination indicate an association of spirochetes with RBCs, one that might underlie the extensive phagocytosis of red blood cells by tissue-resident macrophages.
FIG. 6.
FISH analysis of the time course of B. hermsii infection. B. hermsii spirochetes (orange [arrows and arrowheads]) could be detected in tissues (green background fluorescence) of infected BALB/c rag2−/− animals as early as 3 days after infection (a to c). By day 6, the spirochetes could be found associated with anuclear cells (d to f, arrowheads) and blood vessel walls (d and e, arrows). At the late stages of infection, numerous spirochetes were visible in cardiac muscle (g), liver (h), and spleen (i) tissue. DAPI nucleic acid stain (blue) reveals the host cell nuclei.
DISCUSSION
The genetic identity of the host plays a significant role in the control of a wide range of infectious diseases, from viral infections to colonization with parasites. The tick-borne spirochete B. hermsii is extremely well adapted to growth in the bloodstream of infected hosts and may therefore serve as a model in which to study the mechanisms of clearance of bacterial pathogens from the bloodstream. Although the analysis of B. hermsii infection has provided important insights into how blood-borne pathogens trigger a host immune response, particularly a robust antibody response, no studies of the contribution of the genetic component of the host to the course and outcome of B. hermsii infection have been performed. In the current study, by analyzing the course of B. hermsii infection in inbred strains of mice, we established that in fact, in the absence of a humoral response, the innate immune system is capable of partial control of B. hermsii infection. In addition, we found that the genetic background of the host contributes significantly to the severity of both bacteremia and anemia, prominent manifestations of infection.
To begin our analysis, we selected 5 common inbred commercially available mouse strains that have previously been demonstrated to display differential patterns of sensitivity to various pathogens (23). Our survey revealed significant strain-specific differences in the progression of infection, in that bacteremia was 6-fold more severe in C57BL/6ByJ than in BALB/cByJ animals at day 3 after infection (Fig. 1). The high statistical significance (P < 0.01) of this finding suggests that inbred mouse strains are sufficiently different in clearing B. hermsii from the bloodstream to allow for the identification of the genetic polymorphisms that likely account for this differential susceptibility.
It is well established that adaptive immunity, particularly a T-cell-independent antibody response, is critical for clearance of relapsing fever spirochetes. To determine if genetic differences in innate immunity contribute to the differences in susceptibility to infection that we observed in immunocompetent strains, we followed the course of B. hermsii infection in an analogous set of strains carrying a rag2 deletion. Rag2 knockout mice, like scid and rag1−/− animals, lack specific immunity, but their innate immunity is intact (37). We found that rag2−/− mice had higher levels of circulating spirochetes at the initial peak of bacteremia than their immunocompetent counterparts. This is likely due to a combined contribution of innate and adaptive immunity to the early response to B. hermsii infection in immunocompetent mice. Rapid response to B. hermsii early in the infection can be mediated by both marginal-zone (MZ) B and B1 cells (1, 12). These T-cell-independent B-cell subsets can differentiate into plasmablasts within 48 h after exposure to bacteria or bacterial products and are therefore capable of generating a significant specific-antibody response, required for controlling bacterial burden, by 3 days postinfection (7, 29).
Consistent with the results of our previous work with immunocompromised (rag1−/−) mice, none of the animals fully cleared the infection, but each was capable of diminishing the bacterial load from an early peak, indicating that innate immune mechanisms were functioning to control infection (Fig. 2) (2). In addition, consistent with the relative susceptibility of immunocompetent C3H mice, C3H rag2−/− mice did not control the initial peak of bacteremia as well as BALB/c rag2−/−mice, indicating that at least in part, the susceptibility of wild-type C3H rag2−/−mice reflects a relative defect in innate immunity. Interestingly, in direct contrast to the relative susceptibility of immunocompetent C57BL/6ByJ mice, female C57BL/6 rag2−/− mice were able to control the initial peak of bacteremia as well as BALB/c mice. Because these C57BL/6 mice are separated from their common progenitors by many generations, it is possible that the different patterns of response to B. hermsii infection of immunocompetent and immunocompromised mice are due to subline-specific genetic differences. In fact, we recently described C57BL/6 subline-specific differences in response to L. monocytogenes infection (24). Nevertheless, the detrimental effect of adaptive immune cells on innate immune function has been documented for the mouse model of L. monocytogenes infection (18). Therefore, it is possible that although the combined efforts of the innate and adaptive immune systems of wild-type C57BL/6 mice are capable of completely clearing B. hermsii infection, the innate immune response to B. hermsii by these mice is impaired at the early stages of infection.
The gender of the host can often influence susceptibility to infectious disease. In addition to the relevance of such sex-specific phenotypes for understanding the pathogenesis of infection, for genetic studies it is important to know if the phenotype can be analyzed using entire cross populations or if the animals have to be segregated according to sex. To test the effect of gender, we compared the progression of B. hermsii infection in male and female C57BL/6 rag2−/− and BALB/c rag2−/− animals. For both strains, males had significantly higher and somewhat delayed initial peaks of bacteremia compared to the initial peaks in female animals, indicating that the gender of the host plays a substantial role in control of B. hermsii bacteremia. While it would be difficult to test directly if similar gender-specific differences in susceptibility to relapsing fever exist in the human population, our observation provides a starting point for research in this direction.
In addition to bacteremia, relapsing fever patients commonly suffer from anemia and thrombocytopenia. The etiology of these changes in blood cell counts is not understood and could be multifactorial, but it is tempting to speculate that the proximity of these cells to high concentrations of bacteria in the blood promotes interactions that lead to host cell clearance. Indeed, episodes of thrombocytopenia correspond temporally with peaks of bacteremia, and the spirochetes bind to circulating platelets during infection (4). In addition, both Borrelia crocidurae and B. hermsii have been shown to bind to erythrocytes in vitro and in blood samples from infected animals (16, 26). Our examination of tissues provided data that support the hypothesis that the interaction of spirochetes with cellular components of blood influences the number of circulating cells. We found that liver-resident macrophages (Kupffer cells) contain significant numbers of phagocytosed and highly clustered RBCs (Fig. 5), and FISH analysis demonstrated that RBCs are frequently associated with spirochetes, some of which appear to wrap around the entire periphery of the cell (Fig. 6). These findings, along with the kinetics of anemia, are consistent with a speculative model in which RBC binding might contribute to the ability of animals to control bacteremia. According to this model, bacteremia is associated with spirochete-RBC interactions, leading to RBC clearance by erythrophagocytosis, either due to RBC damage or to “bystander” clearance of RBCs stably bound to spirochetes. The phagocytosis of spirochetes and RBCs leads to hepatosplenomegaly, as well as to a decrease in bacteremia by day 6 of infection. This is followed by a partial recovery in RBC count as hematopoiesis (including extramedullary hematopoiesis) occurs, and a period of relatively consistent anemia and bacteremia. However, late in infection, e.g., by day 11 or 12, perhaps as the erythropoietic and/or phagocytic capacities of infected animals become exhausted, decreasing numbers of circulating RBCs and increasing numbers of blood-borne spirochetes are observed, leading to the terminal phase of infection. Thus, one might imagine that RBCs play a role in the initial control of B. hermsii infection by acting as a “sink” for circulating bacteria. Bergstrom and colleagues have suggested that RBC binding by B. crocidurae promoted a delay in the development of an adaptive immune response (16), and these models are not mutually exclusive.
Our results indicate that innate immunity plays a significant role in the control of B. hermsii replication and that this contribution is different in inbred mouse strains. In addition, our study lays the groundwork for a future thorough, unbiased genetic analysis of differential responses to B. hermsii infection in inbred mouse strains that will identify critical control elements in the regulation of innate immunity. Analysis of these elements will improve our understanding of innate immune response to blood-borne pathogens. In addition, since innate immunity contributes to defense against a wide range of pathogens, these findings are likely to contribute to our understanding of general mechanisms of host-pathogen interactions.
Acknowledgments
The work was supported by NIH grants AI060991 to V.L.B. and AI37601 to J.M.L.
We thank David Garlick for help in interpretation of histopathology results.
The authors declare no competing financial interests.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 7 December 2009.
REFERENCES
- 1.Alugupalli, K. R., R. M. Gerstein, J. Chen, E. Szomolanyi-Tsuda, R. T. Woodland, and J. M. Leong. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819-3827. [DOI] [PubMed] [Google Scholar]
- 2.Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379-390. [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli, K. R., A. D. Michelson, M. R. Barnard, and J. M. Leong. 2001. Serial determinations of platelet counts in mice by flow cytometry. Thromb. Haemost. 86:668-671. [PubMed] [Google Scholar]
- 4.Alugupalli, K. R., A. D. Michelson, M. R. Barnard, D. Robbins, J. Coburn, E. K. Baker, M. H. Ginsberg, T. G. Schwan, and J. M. Leong. 2001. Platelet activation by a relapsing fever spirochaete results in enhanced bacterium-platelet interaction via integrin alphaIIbbeta3 activation. Mol. Microbiol. 39:330-340. [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli, K. R., A. D. Michelson, I. Joris, T. G. Schwan, K. Hodivala-Dilke, R. O. Hynes, and J. M. Leong. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102:2843-2850. [DOI] [PubMed] [Google Scholar]
- 6.Arimitsu, Y., and K. Akama. 1973. Characterization of protective antibodies produced in mice infected with Borrelia duttonii. Jpn. J. Med. Sci. Biol. 26:229-237. [DOI] [PubMed] [Google Scholar]
- 7.Balazs, M., F. Martin, T. Zhou, and J. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17:341-352. [DOI] [PubMed] [Google Scholar]
- 8.Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. [DOI] [PubMed] [Google Scholar]
- 9.Barbour, A. G. 1993. Linear DNA of Borrelia species and antigenic variation. Trends Microbiol. 1:236-239. [DOI] [PubMed] [Google Scholar]
- 10.Barbour, A. G., and V. Bundoc. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect. Immun. 69:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbour, A. G., S. L. Tessier, and H. G. Stoenner. 1982. Variable major proteins of Borrellia hermsii. J. Exp. Med. 156:1312-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell, J. M., S. Searle, H. Mohamed, and J. K. White. 2003. Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol. Lett. 85:197-203. [DOI] [PubMed] [Google Scholar]
- 14.Blevins, S. M., R. A. Greenfield, and M. S. Bronze. 2008. Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease. Cleve. Clin. J. Med. 75:521-530. [DOI] [PubMed] [Google Scholar]
- 15.Buer, J., and R. Balling. 2003. Mice, microbes and models of infection. Nat. Rev. Genet. 4:195-205. [DOI] [PubMed] [Google Scholar]
- 16.Burman, N., A. Shamaei-Tousi, and S. Bergstrom. 1998. The spirochete Borrelia crocidurae causes erythrocyte rosetting during relapsing fever. Infect. Immun. 66:815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadavid, D. 2006. The mammalian host response to borrelia infection. Wien. Klin. Wochenschr. 118:653-658. [DOI] [PubMed] [Google Scholar]
- 18.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203:933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Centers for Disease Control and Prevention. 15 June 2007. Lyme disease—United States, 2003-2005. MMWR Morb. Mortal. Wkly. Rep. 56:573-576. http://www.cdc.gov/mmwr/PDF/wk/mm5623.pdf. [PubMed] [Google Scholar]
- 19.Connolly, S. E., and J. L. Benach. 2001. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J. Immunol. 167:3029-3032. [DOI] [PubMed] [Google Scholar]
- 20.Cutler, S. J., J. Moss, M. Fukunaga, D. J. Wright, D. Fekade, and D. Warrell. 1997. Borrelia recurrentis characterization and comparison with relapsing-fever, Lyme-associated, and other Borrelia spp. Int. J. Syst. Bacteriol. 47:958-968. [DOI] [PubMed] [Google Scholar]
- 21.Dworkin, M. S., D. E. Anderson, Jr., T. G. Schwan, P. C. Shoemaker, S. N. Banerjee, B. O. Kassen, and W. Burgdorfer. 1998. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin. Infect. Dis. 26:122-131. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin, M. S., P. C. Shoemaker, C. L. Fritz, M. E. Dowell, and D. E. Anderson, Jr. 2002. The epidemiology of tick-borne relapsing fever in the United States. Am. J. Trop. Med. Hyg. 66:753-758. [DOI] [PubMed] [Google Scholar]
- 23.Festing, M. F. 9 April 1998, posting date. Inbred strains of mice. http://www.informatics.jax.org/external/festing/mouse/STRAINS.shtml.
- 24.Garifulin, O., Z. Qi, H. Shen, S. Patnala, M. R. Green, and V. Boyartchuk. 2007. Irf3 polymorphism alters induction of interferon beta in response to Listeria monocytogenes infection. PLoS Genet. 3:1587-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebbia, J. A., J. C. Monco, J. L. Degen, T. H. Bugge, and J. L. Benach. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Invest. 103:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyard, C., E. M. Chester, S. J. Raffel, M. E. Schrumpf, P. F. Policastro, S. F. Porcella, J. M. Leong, and T. G. Schwan. 2005. Relapsing fever spirochetes contain chromosomal genes with unique direct tandemly repeated sequences. Infect. Immun. 73:3025-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinnebusch, B. J., A. G. Barbour, B. I. Restrepo, and T. G. Schwan. 1998. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect. Immun. 66:432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Londono, D., Y. Bai, W. R. Zuckert, H. Gelderblom, and D. Cadavid. 2005. Cardiac apoptosis in severe relapsing fever borreliosis. Infect. Immun. 73:7669-7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 30.Moter, A., G. Leist, R. Rudolph, K. Schrank, B. K. Choi, M. Wagner, and U. B. Gobel. 1998. Fluorescence in situ hybridization shows spatial distribution of as yet uncultured treponemes in biopsies from digital dermatitis lesions. Microbiology 144(Pt. 9):2459-2467. [DOI] [PubMed] [Google Scholar]
- 31.Newman, K., Jr., and R. C. Johnson. 1984. T-cell-independent elimination of Borrelia turicatae. Infect. Immun. 45:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paster, B. J., F. E. Dewhirst, W. G. Weisburg, L. A. Tordoff, G. J. Fraser, R. B. Hespell, T. B. Stanton, L. Zablen, L. Mandelco, and C. R. Woese. 1991. Phylogenetic analysis of the spirochetes. J. Bacteriol. 173:6101-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Restrepo, B. I., T. Kitten, C. J. Carter, D. Infante, and A. G. Barbour. 1992. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol. Microbiol. 6:3299-3311. [DOI] [PubMed] [Google Scholar]
- 34.Roper, R. J., J. J. Weis, B. A. McCracken, C. B. Green, Y. Ma, K. S. Weber, D. Fairbairn, R. J. Butterfield, M. R. Potter, J. F. Zachary, R. W. Doerge, and C. Teuscher. 2001. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2:388-397. [DOI] [PubMed] [Google Scholar]
- 35.Schmiedel, D., H. J. Epple, C. Loddenkemper, R. Ignatius, J. Wagner, B. Hammer, A. Petrich, H. Stein, U. B. Gobel, T. Schneider, and A. Moter. 2009. Rapid and accurate diagnosis of human intestinal spirochetosis by fluorescence in situ hybridization. J. Clin. Microbiol. 47:1393-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 38.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 156:1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 41.Yokota, M., M. G. Morshed, T. Nakazawa, and H. Konishi. 1997. Protective activity of Borrelia duttonii-specific immunoglobulin subclasses in mice. J. Med. Microbiol. 46:675-680. [DOI] [PubMed] [Google Scholar]