Abstract
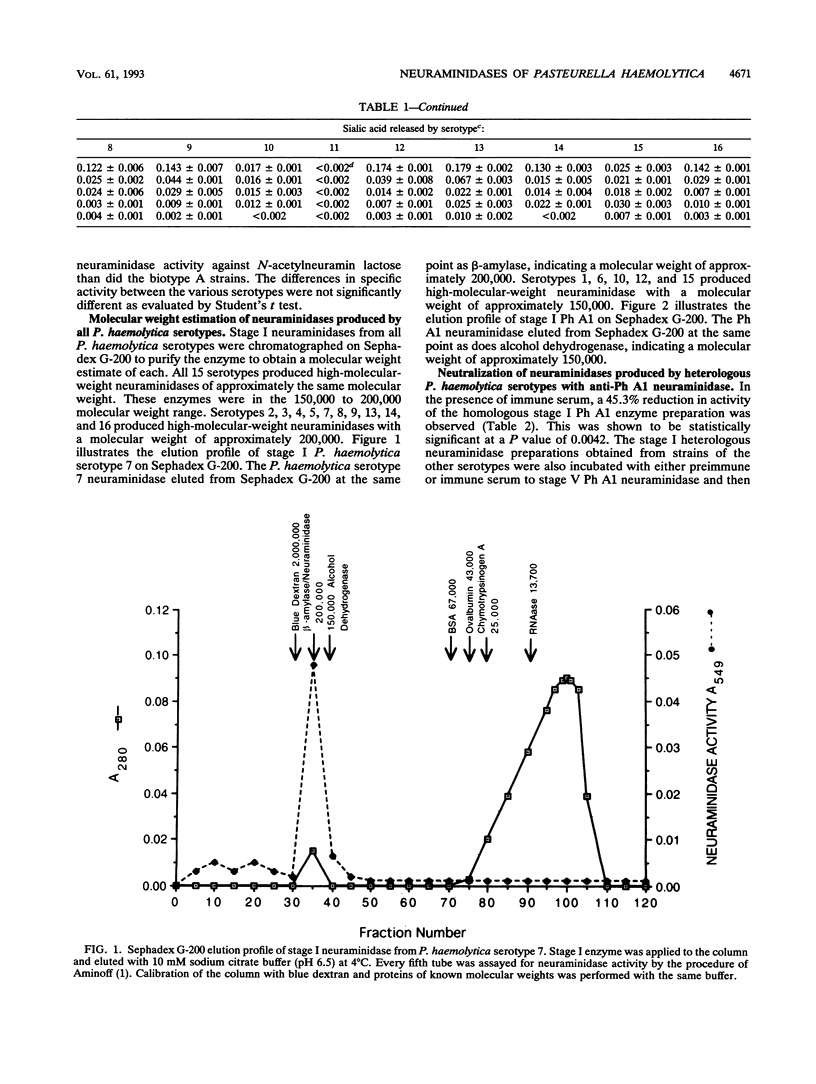
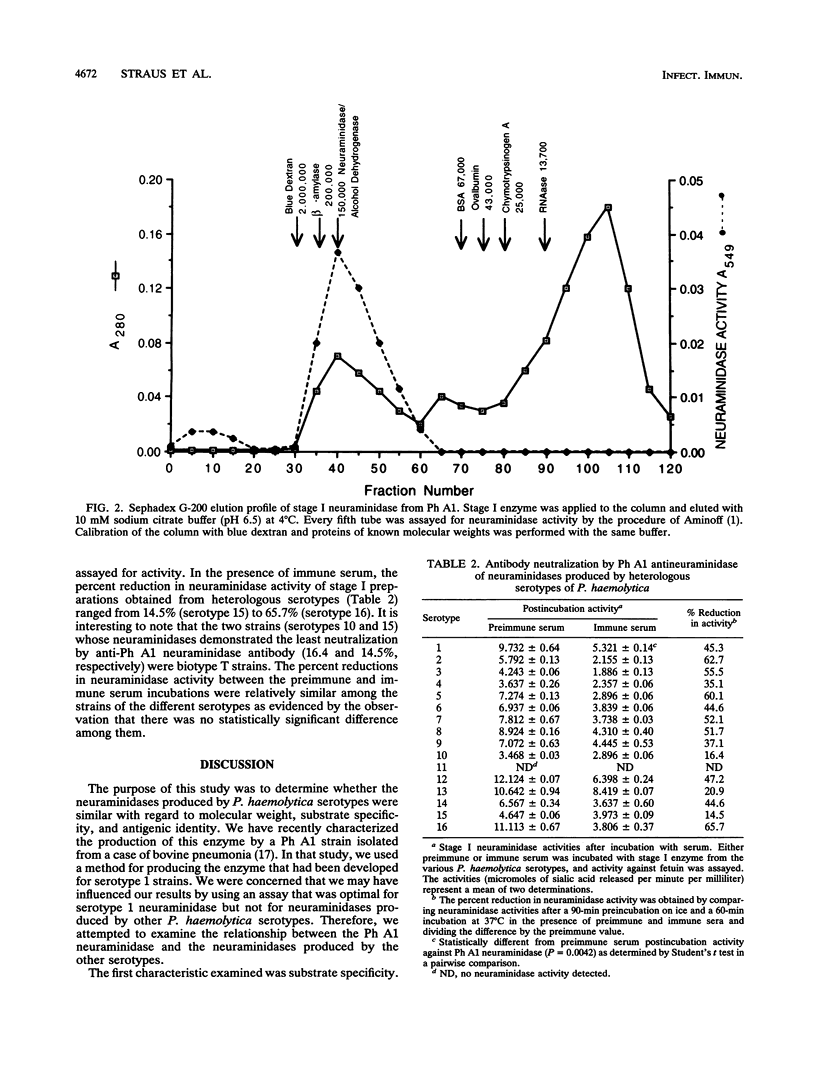
Neuraminidases produced by 16 strains of Pasteurella haemolytica (serotypes 1 to 16) were characterized by molecular weight, antigenic identity, and substrate specificity. After growth in a chemically defined medium, stage I (lyophilized) culture supernatants were assayed for activity with N-acetylneuramin lactose, human alpha-1-acid glycoprotein, fetuin, colominic acid, and bovine submaxillary mucin. Neuraminidase produced by P. haemolytica serotype A1 (Ph A1) was purified by a combination of salt fractionation, ion-exchange chromatography on DEAE-Sephacel, and gel filtration on Sephadex G-200. Purified Ph A1 neuraminidase was used to immunize rabbits, and the resultant antiserum reduced the activity of Ph A1 neuraminidase by 46%. This antiserum also reduced the activity of neuraminidase produced by the other serotypes by between 15 and 66%. Molecular weight estimates of the neuraminidases produced by the various serotypes were obtained by gel filtration chromatography on Sephadex G-200. Fifteen of the 16 serotypes examined produced a neuraminidase with a molecular weight of approximately 150,000 to 200,000. One serotype (serotype 11) produced no material with neuraminidase activity. In addition, all 15 high-molecular-weight neuraminidases showed similar substrate specificities. That is, they were all most active against N-acetylneuramin lactose and least active against bovine submaxillary mucin. On the basis of these results, it appears that the high-molecular-weight neuraminidases produced by the different P. haemolytica serotypes are quite similar.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. G., Straus D. C. Characterization of neuraminidases produced by various serotypes of group B streptococci. Infect Immun. 1987 Jan;55(1):1–6. doi: 10.1128/iai.55.1.1-6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzeniek R. Viral and bacterial neuraminidases. Curr Top Microbiol Immunol. 1972;59:35–74. doi: 10.1007/978-3-642-65444-2_2. [DOI] [PubMed] [Google Scholar]
- Flashner M., Wang P., Hurley J. B., Tanenbaum S. W. Properties of an inducible extracellular neuraminidase from an Arthrobacter isolate. J Bacteriol. 1977 Mar;129(3):1457–1465. doi: 10.1128/jb.129.3.1457-1465.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G. H., Tabatabai L. B. Neuraminidase activity of Pasteurella haemolytica isolates. Infect Immun. 1981 Jun;32(3):1119–1122. doi: 10.1128/iai.32.3.1119-1122.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. I. PURIFICATION AND PROPERTIES OF A NEURAMINIDASE AND A BETA-GALACTOSIDASE. ACTION ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1535–1543. doi: 10.1021/bi00898a025. [DOI] [PubMed] [Google Scholar]
- Kelly R. T., Greiff D., Farmer S. Neuraminidase activity in Diplococcus pneumoniae. J Bacteriol. 1966 Feb;91(2):601–603. doi: 10.1128/jb.91.2.601-603.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lillie L. E. The bovine respiratory disease complex. Can Vet J. 1974 Sep;15(9):233–242. [PMC free article] [PubMed] [Google Scholar]
- Moriyama T., Barksdale L. Neuraminidase of Corynebacterium diphtheriae. J Bacteriol. 1967 Nov;94(5):1565–1581. doi: 10.1128/jb.94.5.1565-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy C. W., Straus D. C., Livingston C. W., Jr, Foster G. S. Immune response to pulmonary injection of Pasteurella haemolytica-impregnated agar beads followed by transthoracic challenge exposure in goats. Am J Vet Res. 1990 Oct;51(10):1629–1634. [PubMed] [Google Scholar]
- Scharmann W., Drzeniek R., Blobel H. Neuraminidase of Pasteurella multocida. Infect Immun. 1970 Mar;1(3):319–320. doi: 10.1128/iai.1.3.319-320.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. C., Unbehagen P. J., Purdy C. W. Neuraminidase production by a Pasteurella haemolytica A1 strain associated with bovine pneumonia. Infect Immun. 1993 Jan;61(1):253–259. doi: 10.1128/iai.61.1.253-259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


