Abstract
Certain complement defects are associated with an increased propensity to contract Neisseria meningitidis infections. We performed detailed analyses of complement-mediated defense mechanisms against N. meningitidis 44/76 with whole blood and serum from two adult patients who were completely C2 or C5 deficient. The C5-deficient patient and the matched control were also deficient in mannose-binding lectin (MBL). The proliferation of meningococci incubated in freshly drawn whole blood was estimated by CFU and quantitative DNA real-time PCR. The serum bactericidal activity and opsonophagocytic activity by granulocytes were investigated, including heat-inactivated postvaccination sera, to examine the influence of antimeningococcal antibodies. The meningococci proliferated equally in C2- and C5-deficient blood, with a 2 log10 increase of CFU and 4- to 5-log10 increase in DNA copies. Proliferation was modestly decreased in reconstituted C2-deficient and control blood. After reconstitution of C5-deficient blood, all meningococci were killed, which is consistent with high antibody titers being present. The opsonophagocytic activity was strictly C2 dependent, appeared with normal serum, and increased with postvaccination serum. Serum bactericidal activity was strictly dependent on C2, C5, and high antibody titers. MBL did not influence any of the parameters observed. Complement-mediated defense against meningococci was thus dependent on the classical pathway. Some opsonophagocytic activity occurred despite low levels of antimeningococcal antibodies but was more efficient with immune sera. Serum bactericidal activity was dependent on C2, C5, and immune sera. MBL did not influence any of the parameters observed.
Systemic meningococcal disease evolves when pathogenic Neisseria meningitidis breach the pharyngeal mucosa and start proliferating in the circulation (36, 44). The majority of the patients develops low-grade bacteremia leading to meningitis with a comparatively low case-fatality rate if adequate antibiotic treatment is given early (44). A minority develops fulminant sepsis caused by massive bacterial proliferation in the circulation, resulting in a very high case-fatality rate (44). A number of genetic disorders and polymorphisms in the host that influence the clinical presentation and outcome have been implicated in the response to intruding meningococci (4, 9).
The complement system plays a crucial part in the host defense against systemic meningococcal disease (39). Acquisition of serum bactericidal antibodies correlates with protection (14, 16), whereas other mechanisms, primarily opsonophagocytosis, may also be important (1, 47). Deficiencies of the complement system affecting the alternative pathway, C3, and the terminal pathway have for a long time predominantly been associated with increased susceptibility to meningococcal disease (12, 13). Also, the rather common deficiency of mannose-binding lectin (MBL) has been associated with meningococcal disease, but only in early childhood (8, 11, 15, 19, 45). C2 deficiency, which apart from MBL deficiency is the most common inherited complement deficiency affecting about 1/20,000 of Caucasians (41), appears to be associated with a wide range of infections with encapsulated bacteria of which Streptococcus pneumoniae is the most frequent causative agent, whereas infections due to N. meningitidis occur less frequently (12, 25).
In the present study blood samples from two individuals being genetically completely deficient in complement factor 2 (C2) or complement factor 5 (C5) and MBL were used to examine details regarding the specific roles of different parts of the complement system in the protection against serogroup B meningococcal disease. Bacterial survival and proliferation was examined in freshly drawn whole blood. Opsonophagocytic activity (OPA) and serum bactericidal activity (SBA), as well as the role of antimeningococcal antibodies, were studied separately. Functionally active and highly purified complement components were used for reconstitution experiments both of whole blood and of serum in order to confirm the specific roles of these components.
MATERIALS AND METHODS
Patients and control individuals.
Whole blood and serum from a completely C2-deficient and a completely C5-deficient patient were used. The C2-deficient patient, an 18-year-old male, was diagnosed after recurrent respiratory tract infections and the C5-deficient patient, a 44-year-old female, was diagnosed after recurrent episodes of meningococcal disease. The bacteria from her first two systemic meningococcal infections were not serogrouped, whereas from the latter two infections serogroup C and Y organisms, respectively, were identified. Genetic analyses and structural and functional assays confirmed the complement deficiencies (29). Incidentally, the C5-deficient patient also proved to be lectin-pathway-deficient with a very low concentration of MBL (<50 μg/liter). For this reason an individual with a similar MBL deficiency (<50 μg/liter) but otherwise normal complement function was used as a control for the C5-deficient patient. An individual with normal complement function served as a control for the C2-deficient patient (29).
Bacteria.
In all experiments the international reference strain N. meningitidis 44/76 (also denoted H44/76) characterized as B:15:P1:7,16:L3,7,9 belonging to the multilocus sequence type (ST) 32/ET-5 clone was used (35). This is a representative strain belonging to a clone that has caused epidemics worldwide (5). For logistical reasons the bacteria had to be grown overnight for about 18 h in the whole-blood and OPA assays. Preliminary experiments indicated that there were no significant differences of the OPA responses when sera from vaccinees were analyzed against bacteria grown overnight (stationary phase) or for 4 h (log phase).
Antimeningococcal antibodies.
Quantification of IgG antibodies binding to live meningococci was done by an indirect immunofluorescence technique against live group B meningococci as described previously (2). The results are reported in arbitrary units (AU) against a reference postvaccination serum.
Bacterial survival and proliferation in whole blood.
Whole blood was collected by using lepirudin as anticoagulant from the respective individuals immediately before the experiments were performed (29, 30). Lepirudin was chosen since this anticoagulant is inert with respect to the complement system. An aliquot of blood from each of the complement-deficient patients was reconstituted with its respective lacking factor (29). Two separate experiments were performed on two consecutive days. Meningococci were grown on chocolate agar for about 18 h at 37°C in 5% CO2 before a suspension of meningococci (optical density of 0.25 at 625 nm) in Hanks balanced salt solution (HBSS; pH 7.2) with 0.1% bovine serum albumin was diluted to give the intended final concentration of 105 bacteria/ml of blood. The incubation was performed in sterile Vacutainer tubes at 37°C in a water bath, systematically mixing the contents thoroughly during the experiments. The inoculum and bacterial density at the start of the experiments and at predetermined time points 10 min, 60 min, 120 min, 180 min, 240 min, and 24 h were determined by plating 10-fold dilutions of whole blood and counting plates with 25 to 250 CFU after 24 h of growth. Samples were simultaneously frozen in −70°C for later bacterial genome DNA quantification with quantitative real-time PCR (LightCycler; Roche Diagnostics GmbH, Mannheim, Germany), as described previously (32).
OPA.
OPA was measured as the oxidative burst using live meningococci, as previously described (2). The bacteria were grown on Colombia horse blood agar for about 18 h at 37°C in 5% CO2. The effector cells were dihydrorhodamine 123-primed (Invitrogen, Carlsbad, CA) polymorphonuclear granulocytes (PMNs) from heparinized whole blood from a healthy donor, where the red cells were removed by ammonium chloride lysis. The OPA was analyzed by a flow cytometer (CyFlow ML, Partec GmbH, Münster, Germany), examining the samples for fluorescence within the PMN population. OPA titers were expressed as the reciprocal of the final serum dilution giving oxidative burst in ≥50% of the PMNs. Three different protocols were examined. (i) The first was twofold titration of test sera (complement-deficient, reconstituted, and control sera) to reveal the OPA of test sera depending on its own IgG and complement content. (ii) The second protocol entailed twofold titration of heat-inactivated test sera following the addition of 10% homologous serum passed through a protein G column (HiTrap protein G HP; GE Healthcare, Oslo, Norway) to remove IgGs to provide fixed amount of complement. The absorption with protein G was done on an ice bath by monitoring the process visually. The unbound fraction was eluted by using ice-cold HBSS. By using this approach, uncertainty about whether antibodies or complement was the limiting factor at higher dilutions was avoided, since the complement concentration was kept constant. (iii) The third protocol consisted of twofold titration of heat-inactivated serum from a subject (serum TKH) immunized with a N. meningitidis 44/76 outer membrane vesicle vaccine using each of the test sera preparations as the complement source (10%) after passing them through a protein G column. By this method, the antibody coating of the meningococci was standardized.
CD11b expression.
The CD11b expression on PMNs was measured in whole blood kept at 4°C and after preparation of the PMNs, as used in the OPA assay (30).
Serum bactericidal activity.
SBA was measured using meningococci grown overnight at 37°C in 5% CO2. The overnight growth was plated onto Colombia horse blood agar plates and incubated for 4 h before suspension in HBSS (pH 7.2) with 0.1% bovine serum albumin (3). Then, 25% human serum (from a complement-deficient patient or healthy individual with no SBA) was used as an exogenous source of human complement. Heat-inactivated sera known to contain specific antibodies against the target strain were diluted twofold in microtiter plates (starting at a serum dilution of 1:2) and incubated for 30, 60, and 90 min at 37°C in air with bacteria and complement. After plating onto agar plates and incubation overnight at 37°C, CFU were counted (Sorcerer colony counter; Perceptive Instruments, Suffolk, United Kingdom), and SBA antibody titers were expressed as the reciprocal of the final serum dilution giving ≥50% killing of inoculum compared to controls.
RESULTS
Concentration of antimeningococcal antibodies.
The concentrations of specific antibodies to group B meningococci in serum from each of the donors were quantified to interpret our results in relation to their antibody levels. In the C5-deficient serum, the concentration of antibodies was 34 AU/ml, which was similar to levels found in vaccinees after adequate immunization (results not shown). In the C2-deficient serum and in the two control sera, the antibody concentrations were 3 to 6 AU/ml, i.e., low levels typically found in nonimmunized persons.
Bacterial survival and proliferation in whole blood.
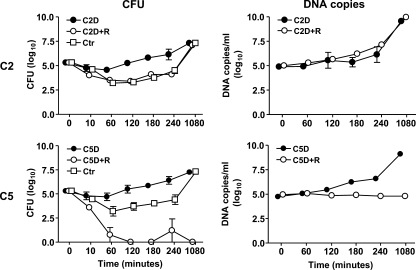
First, we examined the survival and proliferation of meningococci in freshly drawn whole blood from each of the donors with or without reconstitution of the lacking factors. In the C2-deficient whole blood, the number of meningococci increased throughout incubation. The final increase in CFU was ∼2 log10, while the final increase in DNA copies was 4 to 5 log10 (Fig. 1, upper panels). In the reconstituted C2-deficient blood and in blood from the C2-sufficient control individual, the number of CFU initially decreased ∼2 log10 and, after 2 h, it started to increase, ending up with a final number of CFU similar to that seen in the C2-deficient blood. The final number of DNA copies in the C2-reconstituted blood did not differ from the C2-deficient blood.
FIG. 1.
Survival and proliferation of N. meningitidis strain 44/76 in whole blood. The mean numbers of N. meningitidis quantified by CFU (left panel) and DNA copies (right panel) from the C2-deficient (C2D) and C5-deficient (C5D) patients and in their whole blood reconstituted with purified complement factor C2 (C2D+R) and C5 (C5D+R) is shown. CFU was also performed in blood from the two control individuals (C2Ctr and C5Ctr). Error bars indicate the range of two experiments.
In the C5-deficient whole blood, the number of meningococci increased throughout incubation. The final increase in CFU was ∼2 log10, while the final increase in DNA copies was 4 to 5 log10 (Fig. 1, lower panels). In the C5-reconstituted blood the number of CFU decreased 5 log10 within 2 h, implying that all live bacteria were killed. Correspondingly, no increase in the number of DNA copies was seen in the C5-reconstituted blood. In blood from the C5-sufficient but MBL-deficient control individual the number of CFU initially decreased ∼2 log10, but after 2 h the numbers started to increase, ending up with a final number of CFU similar to that seen in the C5-deficient blood. Thus, blood from the MBL-deficient control individual behaved similarly to the complement-sufficient control individual in the CFU assay. The control individuals were not examined for meningococcal DNA.
Opsonophagocytic activity.
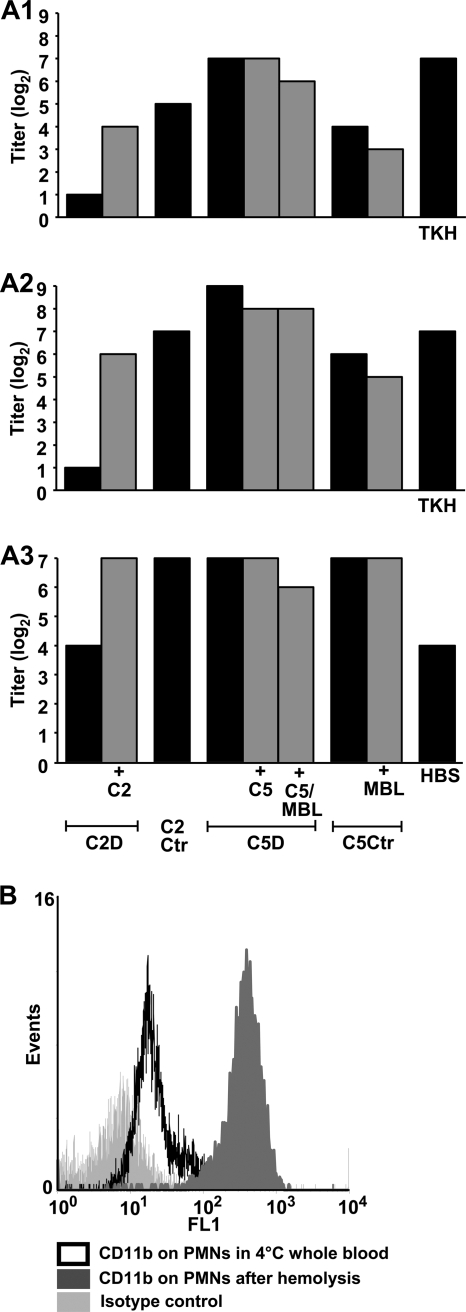
The influence of C2, MBL, C5, and specific antibodies on opsonophagocytosis was examined with sera from each of the donors. Twofold titrations of sera from the four individuals were done, and meningococci were added to the dilutions. This was followed by incubation with PMNs and measurement of OPA (Fig. 2A1). PMNs incubated with C2-deficient serum had no detectable OPA. Reconstitution with C2 restored OPA to the level of the C2 control. PMNs incubated with C5-deficient serum revealed high OPA. The activity was at the same level after reconstitution with C5 and MBL. OPA with postvaccination serum as a positive control serum (TKH) was similar to that seen with serum from the C5-deficient patient. With serum from the MBL-deficient C5 control individual the OPA was lower and similar to the reconstituted C2-deficient serum. OPA was unchanged after reconstitution of the MBL-deficient control serum with MBL.
FIG. 2.
Opsonophagocytosis of N. meningitidis strain 44/76 by polymorphonuclear granulocytes (PMNs). Sera were diluted twofold, and the results represent the highest titer giving oxidative burst in ≥50% of the PMNs. Black columns represent complement-deficient or control serum. Gray columns represent serum reconstituted with the lacking complement factors, as indicated below the graphs. (A1) Titration of test sera against meningococci. TKH, postvaccination serum from an individual immunized with a N. meningitidis group B 44/76-based outer membrane vesicle vaccine (positive control); (A2) titration of heat-inactivated test sera against meningococci, followed by the addition of IgG-depleted homologous sera as complement sources; (A3) titration of heat-inactivated postvaccination serum against meningococci, followed by the addition of IgG-depleted test sera as complement sources. HBSS, Hanks balanced buffered salt solution (negative control). The data from one of two experiments with similar results were shown. (B) Upregulation of CD11b on PMNs after hemolysis of whole blood prior to assay of opsonophagocytosis.
Heat-inactivated deficient and control sera were then twofold titrated against meningococci, followed by the addition of IgG-depleted homologous serum as the complement source to provide equal concentration of complement factors in all titrations from each donor (Fig. 2A2). The results proved similar to the previous experiment, where native deficient and control sera were titrated against meningococci, showing an absence of OPA only with the C2-deficient serum.
Next, heat-inactivated postvaccination serum (TKH) was twofold titrated against meningococci to provide equally high degree of antibody coating before the addition of IgG-depleted deficient and control sera as the complement sources (Fig. 2A3). The OPA titer was similar when C2-deficient serum was provided as the complement source as it was in the absence of complement (HBSS), which was, however, somewhat higher than the low OPA titer seen with C2-deficient serum in the presence of a low antibody level. After reconstitution with C2, the titer increased to high levels similar to those observed when the other test sera were used as the complement sources.
Upregulation of CD11b on PMNs.
Bacterial killing did not occur in C5-deficient whole blood, whereas opsonophagocytosis apparently was effective. The latter assay was based on PMNs from a normal donor. This led us to hypothesize that PMNs during the preparation procedure increased their expression of CD11b, combined with CD18 being the main CR3 phagocytosis receptor, and therefore could respond in this assay. Thus, we evaluated whether any upregulation of CD11b occurred on our PMNs caused by their handling ex vivo. Expression of CD11b on the surface of PMNs from normal blood was found to be markedly increased by the hemolysis procedure used for preparation of these cells (Fig. 2B).
Serum bactericidal activity.
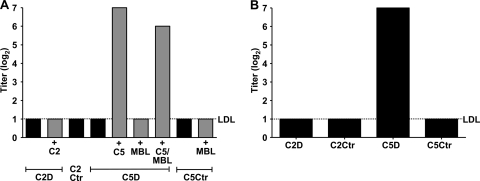
The influence of C2, MBL, C5, and specific antibodies on SBA was then examined with sera from each of the donors. Sera from the two complement-deficient and the two control individuals were twofold titrated and incubated with meningococci, followed by estimation of SBA after 30, 60, and 90 min of incubation (Fig. 3A). The titers at the different time points were identical. No SBA was detected in any of the sera without reconstitution. SBA was only observed after C5 reconstitution of the C5-deficient serum, whereas MBL reconstitution had no effect on SBA.
FIG. 3.
SBA against N. meningitidis strain 44/76. The titers given are reciprocal log2 dilutions resulting in ≥50% killing of inoculum. Black columns represent complement-deficient or control serum. Gray columns represent serum reconstituted with the lacking complement factors, as indicated below the graphs. The dotted lines indicate the lower detection limit (LDL) of the assay. The results after 60 min incubation are shown. (A) SBA of test sera before and after reconstitution making use of the intrinsic complement activity in the respective sera. (B) SBA of heat-inactivated test sera with a normal serum without bactericidal antibodies against meningococci as the complement source.
Heat-inactivated sera from the four individuals were then titrated against meningococci, followed by the addition of serum without SBA antibodies against meningococci from a complement sufficient donor as the complement source. SBA was seen only with serum from the C5-deficient patient (Fig. 3B).
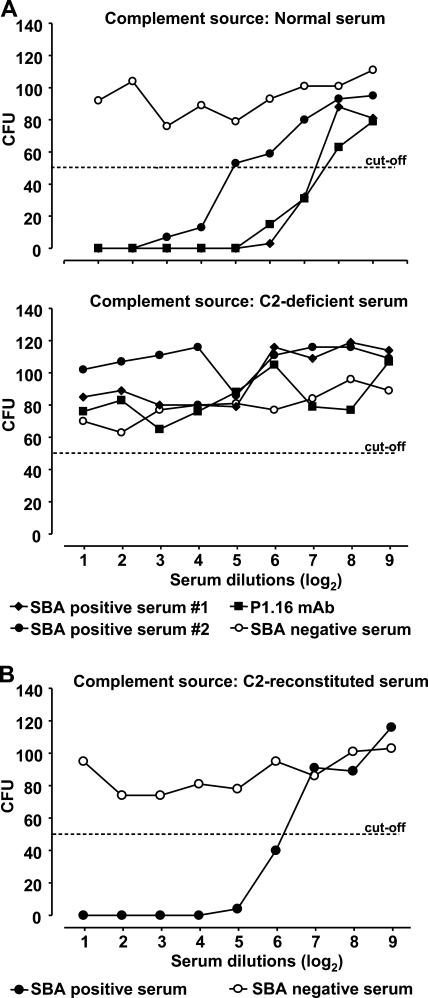
C2 bypass mechanisms of the classical pathway have been identified (27). With this in mind, we investigated the role of C2 in the lysis of meningococci. To obtain sufficient antibody coating, heat-inactivated postvaccination sera from two different donors and a monoclonal antibody (P1.16) against PorA in the meningococcal outer membrane were titrated against meningococci, followed by the addition of IgG-depleted normal serum and IgG-depleted C2-deficient serum, respectively (Fig. 4A), and reconstituted C2-deficient serum (Fig. 4B). SBA was observed only with normal serum and reconstituted C2-deficient serum and not in the C2-deficient serum, implying no significant complement activation by C2 bypass mechanisms.
FIG. 4.
Serum bactericidal activity against N. meningitidis strain 44/76 in postvaccination sera with known bactericidal activity against the target strain, or in the presence of a monoclonal antibody against the P1.16 epitope on PorA using normal, C2-deficient, or reconstituted sera as the complement sources. The results after 60 min incubation are shown. (A) SBA of two postvaccination sera denoted SBA positive serum 1 and 2 and the monoclonal antibody against the P1.16 epitope with normal serum or C2-deficient serum as the complement sources. A serum without bactericidal activity denoted SBA negative serum is shown as a negative control. (B) SBA of postvaccination serum denoted SBA positive serum using C2-deficient serum reconstituted with C2 as the complement source. A serum without bactericidal activity denoted SBA negative serum is shown as a negative control.
DISCUSSION
The present study is the first to use freshly drawn whole blood as well as serum from genetically deficient human subjects to study the effect of complement and antibodies on the survival, OPA, and SBA of N. meningitidis 44/76. Collectively, the data indicate a crucial role for antibodies, C2 and C5, but not MBL, in these defense mechanisms.
N. meningitidis exhibits the unique feature of being a pathogen strikingly inclined to cause disease in individuals with certain complement deficiencies (12, 28). The C5-deficient patient included in the present study represents a characteristic example, since she had suffered four episodes of serious systemic meningococcal disease, including meningitis and severe sepsis, between the ages of 7 and 42. It is therefore of particular interest to examine the interaction between this bacterium and complement to better understand the mechanisms involved in the development of systemic meningococcal disease, as well as to obtain insight into general principles of complement functions.
The growth experiments in whole blood demonstrated abolished the capacity to kill meningococci in C2-deficient blood. In contrast, proliferation of meningococci in reconstituted C2-deficient blood and blood from the control individual was impaired. This implies that C2-dependent complement activation is essential for the killing of meningococci. The classical and lectin pathways display similar activation of C2, but our data indicate no activation by the lectin pathway of any importance, since similar results were obtained in MBL-sufficient and MBL-deficient individuals. This is in accordance with previously published results (7, 21, 46). These results are also in accordance with the well-established role of antibodies to effectively protect against meningococcal disease (14, 16, 20). This does not fully exclude the lectin pathway activation, since MBL-independent activation by ficolins might occur.
The susceptibility of individuals with properdin or factor D deficiency to meningococcal disease, as well as the recently reported increased serum resistance by factor H binding on the surface of meningococci, support an essential role also of the alternative pathway in the killing of meningococci (12, 38, 43). Recently, the binding of properdin to various microbes, including Neisseria gonorrhoeae, followed by alternative pathway activation was reported (26). However, our experiments indicate that facing meningococci, the alternative pathway is not activated directly to any important degree. Most likely, the alternative pathway serves an important function by amplifying the complement activation initially triggered by the classical pathway (39). The contribution of alternative amplification to C5 activation has been reported to be as much as 80 to 90% when the initial activation was highly specific for the classical pathway (18, 24).
No OPA occurred with the C2-deficient serum, whereas some OPA was seen with reconstituted C2-deficient serum, as well as with serum from the matched control donor. The supplementary OPA experiments performed with titration of heat-inactivated homologous or postvaccination (TKH) sera and C2-deficient, reconstituted, or control sera as complement sources demonstrated that an intact complement system was needed for opsonophagocytosis to occur in the presence of low antibody levels. However, in the presence of a high antibody level by the use of postvaccination serum, some OPA was seen even when HBSS was added as the negative control, i.e., in the absence of complement. This probably occurred through the involvement of Fc receptors. In contrast, SBA was strictly dependent on the presence of both C2 and high antibody levels. Consequently, it seems that complement-mediated opsonophagocytosis of meningococci can occur even in the presence of low background levels of antimeningococcal antibodies, while higher levels of antimeningococcal antibodies obtained by immunization are needed for detectable SBA to occur (37). The specificity of antimeningococcal antibodies probably also determines whether opsonphagocytosis or bactericidal effects will be effective (1).
The number of live meningococci in reconstituted C2-deficient whole blood and in blood from the two control donors declined only temporarily before rising again. In accordance with our results from the OPA and SBA experiments, the observed decrease was probably mainly mediated by opsonophagocytosis. Consequently, the capacity of killing meningococci by opsonophagocytosis in these experiments was limited. This could be due to low levels of specific antibodies in relation to the rather large inoculum of 105 bacteria/ml, which presumably is much higher than encountered in the initial phase of meningococcemia. Thus, we speculate whether complement-mediated opsonphagocytosis represents the primary defense mechanism against intruding meningococci in individuals with low SBA, as in nonimmunized individuals. This is consistent with previous reports suggesting that opsonophagocytosis may protect against invasive meningococci when sufficient SBA is absent (16, 17). However, it cannot be excluded that the susceptibility of the meningococci to SBA was increased in the whole-blood experiments compared to the SBA experiments, because the bacteria used in the former were in the stationary phase, whereas those in the latter were in log phase. Thus, bactericidal killing may also have contributed to the killing of meningococci in the whole-blood experiments.
Interestingly, the ability to kill meningococci was also absent in C5-deficient whole blood. Obviously, no formation of the lytic terminal C5b-9 complement complex took place in the absence of C5. Our results indicate, however, that efficient killing by opsonophagocytosis did not occur in C5-deficient whole blood. Opsonophagocytosis of meningococci is initiated by the binding of C3 split products, mainly iC3b deposited onto the surface of bacteria, to CD11b/CD18 (complement receptor 3 [CR3]) on the phagocytes (42). The binding per se should not be impaired in C5-deficient blood, since C5 is downstream of C3 in the complement cascade. However, in a separate study performed with blood from the same C5-deficient patient published recently, we have demonstrated that CD11b upregulation on PMNs was induced by meningococci only after reconstitution with C5 and completely abolished after the addition of a C5a receptor antagonist to the C5 reconstituted blood (29). Furthermore, it was demonstrated that no oxidative burst occurred by stimulation with E. coli either in PMNs or in monocytes in the absence of C5. Reconstitution led to a marked increase of oxidative burst that was completely reversed by the C5a receptor antagonist (29). The C5a dependency of CD11b upregulation on PMNs have also been reported in other studies (30, 42). C5a was evidently not present in the C5-deficient blood, and thus our results correspond to an essential role of C5 for initiating efficient opsonophagocytosis by the formation of C5a. We were, however, unable to demonstrate impaired OPA with C5-deficient serum. This was most likely because of upregulation of CD11b on the PMNs during their preparation ex vivo, including hemolysis of whole blood, as we also demonstrated in a separate experiment. The PMNs were harvested from a complement-sufficient donor, and it is well known that some spontaneous activation of complement with formation of C5a easily occurs in vitro (31). The lack of protection against meningococci in the C5-deficient blood despite high levels of antimeningococcal antibodies implies that immunizing C5-deficient individuals, as well as patients treated with C5 inhibitors such as eculizumab (Soliris), may not be effective. However, it cannot be excluded that some protection by opsonophagocytosis may be offered in the presence of high antibody levels by the involvement of Fc receptors, since we found increased OPA in the presence of postvaccination serum even in the absence of complement. This protection mechanism may be more effective in the initial phase of invasive meningococcal disease when the concentration of bacteria is more limited than what we examined in our experiments. Platonov et al. have demonstrated that patients with late complement deficiencies may benefit from adequate vaccination (33). However, none of the patients in that study were C5 deficient, a very rare complement defect.
Even if meningococci in C2- and C5-deficient, as well as C2-reconstituted, blood to a large extent survived and proliferated, there was a substantial difference of 2 to 3 log10 in the total increase in CFU and number of DNA copies, which illustrates that most of the bacteria died after proliferation. The mechanism behind this is not known.
In sharp contrast to the modest killing of meningococci in the previously discussed blood samples was the efficient killing of all live bacteria observed in reconstituted blood from the C5-deficient patient. The high concentration of antimeningococcal antibodies she had increased the efficiency of opsonophagocytosis, as was also confirmed by the OPA experiments using heat-inactivated postvaccination serum as a source of antibodies and the reconstituted C5-deficient serum as a complement source. Thereby, it seemed that even if opsonophagocytosis occurred in the presence of low antibody levels, as in sera from nonimmunized individuals, it was increased by immunization. This is in accordance with previous studies performed with serum from immunized individuals (1, 34). The efficient killing of meningococci in the reconstituted C5-deficient whole blood was obviously also due to high SBA.
From clinical studies, MBL is suggested to play a protective role against meningococcal disease in early childhood, before a normal background antibody level is obtained (8, 11, 15, 19, 45). In the present study, proliferation of meningococci in whole blood from the MBL-deficient control was identical to the proliferation seen in reconstituted blood from the C2-deficient patient and the respective MBL-sufficient matched control. Since these three donors were comparable with respect to their equally low levels of antimeningococcal antibodies, this indicates that MBL did not play any important role in the killing of meningococci in our experiments.
The lack of effect of MBL in our experiments was confirmed in the OPA and SBA experiments, since neither of these processes was increased by reconstitution of MBL-deficient serum. In the case of reduced sialylation, however, which is supposed to occur by phase switching when meningococci breach the pharyngeal mucosa and enter into the circulation (6), a possible effect of MBL could potentially appear, since sialic acid is thought to hide MBL-binding targets on the bacterial surface (10, 22, 23). Such phase-dependent MBL-mediated effects could in turn also influence the relevance of C2 since it has been demonstrated that MBL can activate complement by a C2 bypass mechanism (40). This interesting observation sets up a possible theory explaining the strikingly modest disposition of individuals with C2 deficiency compared to individuals with other complement deficiencies to suffer systemic meningococcal disease (12).
When interpreting the results of the present study, one should be aware that different meningococcal strains may behave differently from strain 44/76 used in the present experiments, for example, due to different binding of complement regulatory proteins such as factor H (48).
In conclusion, the present study demonstrated a critical role of complement and antibodies in the defense against systemic meningococcal disease. OPA depends on classical complement activation through C3 and was present at a low antibody concentration. The effectiveness, however, increased markedly with higher levels of antibodies. SBA was critically dependent on higher antibody concentration and a functional classical complement pathway. The roles of C5 and C2 were equally important in the defense against proliferating meningococci, consistent with a role of C5a in the regulation of opsonophagocytosis. In our experiments, MBL played an insignificant role in these defense mechanisms.
Acknowledgments
We thank Reidun Øvstebø at Ullevål University Hospital, Oslo, Norway, for contributions to the methodological development of quantitative real-time PCR to quantify meningococcal DNA.
Financial support was provided from the Research Council of Norway, the Regional Health Organization Helse Sør-Øst, Medinnova SF, the Norwegian Council on Cardiovascular Disease, the Odd Fellows Foundation, and the Family Blix Foundation.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Aase, A., G. Bjune, E. A. Hoiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aase, A., L. M. Naess, R. H. Sandin, T. K. Herstad, F. Oftung, J. Holst, I. L. Haugen, E. A. Hoiby, and T. E. Michaelsen. 2003. Comparison of functional immune responses in humans after intranasal and intramuscular immunizations with outer membrane vesicle vaccines against group B meningococcal disease. Vaccine 21:2042-2051. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, R., I. S. Aaberge, G. F. Santos, T. L. Eudey, P. Oster, A. Glennie, J. Findlow, E. A. Hoiby, E. Rosenqvist, P. Balmer, and D. Martin. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 12:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer, M. C., J. de Gans, S. G. Heckenberg, A. H. Zwinderman, T. van der Poll, and D. van de Beek. 2009. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 9:31-44. [DOI] [PubMed] [Google Scholar]
- 5.Caugant, D. A., and M. C. Maiden. 2009. Meningococcal carriage and disease-population biology and evolution. Vaccine 27(Suppl. 2):B64-B70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries, F. P., A. van der Ende, J. P. van Putten, and J. Dankert. 1996. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drogari-Apiranthitou, M., C. A. Fijen, S. Thiel, A. Platonov, L. Jensen, J. Dankert, and E. J. Kuijper. 1997. The effect of mannan-binding lectin on opsonophagocytosis of Neisseria meningitidis. Immunopharmacology 38:93-99. [DOI] [PubMed] [Google Scholar]
- 8.Eisen, D. P., and R. M. Minchinton. 2003. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 37:1496-1505. [DOI] [PubMed] [Google Scholar]
- 9.Emonts, M., J. A. Hazelzet, R. de Groot, and P. W. Hermans. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3:565-577. [DOI] [PubMed] [Google Scholar]
- 10.Estabrook, M. M., D. L. Jack, N. J. Klein, and G. A. Jarvis. 2004. Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J. Immunol. 172:3784-3792. [DOI] [PubMed] [Google Scholar]
- 11.Faber, J., T. Schuessler, A. Finn, C. Murdoch, W. Zenz, P. Habermehl, C. U. Meyer, B. U. Zabel, H. Schmitt, F. Zepp, and M. Knuf. 2007. Age-dependent association of human mannose-binding lectin mutations with susceptibility to invasive meningococcal disease in childhood. Pediatr. Infect. Dis. J. 26:243-246. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fijen, C. A., E. J. Kuijper, M. T. te Bulte, M. R. Daha, and J. Dankert. 1999. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin. Infect. Dis. 28:98-105. [DOI] [PubMed] [Google Scholar]
- 14.Frasch, C. E., R. Borrow, and J. Donnelly. 2009. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 27:B112-B116. [DOI] [PubMed] [Google Scholar]
- 15.Garred, P., T. E. Michaelsen, G. Bjune, S. Thiel, and A. Svejgaard. 1993. A low serum concentration of mannan-binding protein is not associated with serogroup B or C meningococcal disease. Scand. J. Immunol. 37:468-470. [DOI] [PubMed] [Google Scholar]
- 16.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granoff, D. M. 2009. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine 27(Suppl. 2):B117-B125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harboe, M., and T. E. Mollnes. 2008. The alternative complement pathway revisited. J. Cell Mol. Med. 12:1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibberd, M. L., M. Sumiya, J. A. Summerfield, R. Booy, and M. Levin. 1999. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet 353:1049-1053. [DOI] [PubMed] [Google Scholar]
- 20.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Hoiby, H. Nokleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 21.Jack, D. L., A. W. Dodds, N. Anwar, C. A. Ison, A. Law, M. Frosch, M. W. Turner, and N. J. Klein. 1998. Activation of complement by mannose-binding lectin on isogenic mutants of Neisseria meningitidis serogroup B. J. Immunol. 160:1346-1353. [PubMed] [Google Scholar]
- 22.Jack, D. L., N. J. Klein, and M. W. Turner. 2001. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180:86-99. [DOI] [PubMed] [Google Scholar]
- 23.Jack, D. L., R. C. Read, A. J. Tenner, M. Frosch, M. W. Turner, and N. J. Klein. 2001. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J. Infect. Dis. 184:1152-1162. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis, G. A., and N. A. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson, G., L. Truedsson, G. Sturfelt, V. A. Oxelius, J. H. Braconier, and A. G. Sjoholm. 2005. Hereditary C2 deficiency in Sweden: frequent occurrence of invasive infection, atherosclerosis, and rheumatic disease. Medicine 84:23-34. [DOI] [PubMed] [Google Scholar]
- 26.Kemper, C., and D. E. Hourcade. 2008. Properdin: new roles in pattern recognition and target clearance. Mol. Immunol. 45:4048-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knutzen Steuer, K. L., L. B. Sloan, T. J. Oglesby, T. C. Farries, M. W. Nickells, P. Densen, J. B. Harley, and J. P. Atkinson. 1989. Lysis of sensitized sheep erythrocytes in human sera deficient in the second component of complement. J. Immunol. 143:2256-2261. [PubMed] [Google Scholar]
- 28.Kugelberg, E., B. Gollan, and C. M. Tang. 2008. Mechanisms in Neisseria meningitidis for resistance against complement-mediated killing. Vaccine 26(Suppl. 8):I34-I39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lappegard, K. T., D. Christiansen, A. Pharo, E. B. Thorgersen, B. C. Hellerud, J. Lindstad, E. W. Nielsen, G. Bergseth, D. Fadnes, T. G. Abrahamsen, E. A. Hoiby, L. Schejbel, P. Garred, J. D. Lambris, M. Harboe, and T. E. Mollnes. 2009. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc. Natl. Acad. Sci. U. S. A. 106:15861-15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mollnes, T. E., O. L. Brekke, M. Fung, H. Fure, D. Christiansen, G. Bergseth, V. Videm, K. T. Lappegard, J. Kohl, and J. D. Lambris. 2002. Essential role of the C5a receptor in Escherichia coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100:1869-1877. [PubMed] [Google Scholar]
- 31.Mollnes, T. E., P. Garred, and G. Bergseth. 1988. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin. Exp. Immunol. 73:484-488. [PMC free article] [PubMed] [Google Scholar]
- 32.Ovstebo, R., P. Brandtzaeg, B. Brusletto, K. B. Haug, K. Lande, E. A. Hoiby, and P. Kierulf. 2004. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J. Clin. Microbiol. 42:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platonov, A. E., I. V. Vershinina, E. J. Kuijper, R. Borrow, and H. Kayhty. 2003. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 21:4437-4447. [DOI] [PubMed] [Google Scholar]
- 34.Plested, J. S., and D. M. Granoff. 2008. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin. Vaccine Immunol. 15:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 37.Sandbu, S., B. Feiring, P. Oster, O. S. Helland, H. S. Bakke, L. M. Naess, A. Aase, I. S. Aaberge, A. C. Kristoffersen, K. M. Rydland, S. Tilman, H. Nokleby, and E. Rosenqvist. 2007. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin. Vaccine Immunol. 14:1062-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 176:7566-7575. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, M. C., R. M. Exley, S. Ram, R. B. Sim, and C. M. Tang. 2007. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 15:233-240. [DOI] [PubMed] [Google Scholar]
- 40.Selander, B., U. Martensson, A. Weintraub, E. Holmstrom, M. Matsushita, S. Thiel, J. C. Jensenius, L. Truedsson, and A. G. Sjoholm. 2006. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J. Clin. Invest. 116:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjoholm, A. G., G. Jonsson, J. H. Braconier, G. Sturfelt, and L. Truedsson. 2006. Complement deficiency and disease: an update. Mol. Immunol. 43:78-85. [DOI] [PubMed] [Google Scholar]
- 42.Sprong, T., P. Brandtzaeg, M. Fung, A. M. Pharo, E. A. Hoiby, T. E. Michaelsen, A. Aase, J. W. van der Meer, M. van Deuren, and T. E. Mollnes. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102:3702-3710. [DOI] [PubMed] [Google Scholar]
- 43.Sprong, T., D. Roos, C. Weemaes, C. Neeleman, C. L. Geesing, T. E. Mollnes, and M. van Deuren. 2006. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood 107:4865-4870. [DOI] [PubMed] [Google Scholar]
- 44.Stephens, D. S., B. Greenwood, and P. Brandtzaeg. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196-2210. [DOI] [PubMed] [Google Scholar]
- 45.Tully, J., R. M. Viner, P. G. Coen, J. M. Stuart, M. Zambon, C. Peckham, C. Booth, N. Klein, E. Kaczmarski, and R. Booy. 2006. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. BMJ 332:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Emmerik, L. C., E. J. Kuijper, C. A. Fijen, J. Dankert, and S. Thiel. 1994. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin. Exp. Immunol. 97:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsch, J. A., and D. Granoff. 2007. Immunity to Neisseria meningitidis group B in adults despite lack of serum bactericidal antibody. Clin. Vaccine Immunol. 14:1596-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welsch, J. A., S. Ram, O. Koeberling, and D. M. Granoff. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 197:1053-1061. [DOI] [PubMed] [Google Scholar]