Abstract
Fasciola hepatica is a helminth pathogen that drives Th2/Treg immune responses in its mammalian host. The parasite releases a large number of molecules that are critical to inducing this type of immune response. Here we have selected recombinant forms of two major F. hepatica secreted molecules, the protease cathepsin L (rFhCL1) and an antioxidant, sigma class glutathione transferase (rFhGST-si), to examine their interactions with dendritic cells (DCs). Despite enzymatic and functional differences between these molecules, both induced interleukin-6 (IL-6), IL-12p40, and macrophage inflammatory protein 2 (MIP-2) secretion from DCs and enhanced CD40 expression. While this induction was mediated by Toll-like receptor 4 (TLR4), the subsequent intracellular signaling pathways differed; rFhCL1 signaled through p38, and rFhGST-si mediated its effect via c-Jun N-terminal kinase (JNK), p38, p-NF-κBp65, and IRF5. Neither rFhCL1 nor rFhGST-si enhanced DC phagocytosis or induced Th2 immune responses in vivo. However, DCs matured in the presence of either enzyme attenuated IL-17 production from OVA peptide-specific T cells in vivo. In addition, DCs exposed to either antigen secreted reduced levels of IL-23. Therefore, both F. hepatica FhCL1 and FhGST-si modulate host immunity by suppressing responses associated with chronic inflammation—an immune modulatory mechanism that may benefit the parasite's survival within the host.
Dendritic cells (DCs) have a central role among innate immune cells in presenting antigen and priming naïve T cells to differentiate into Th1/Th17 and Th2/Treg subsets. In response to pathogen-associated molecular patterns (PAMPs) that bind to pattern recognition receptors (PPRs), DCs express surface molecules and produce cytokines that modulate the effector functions of responding T cells (29). While much is known of how DCs respond to bacterial and viral pathogens that drive Th1/Th17 subsets (13), comparatively little is understood about how these cells respond to and influence the Th2/Treg adaptive immune response to helminth parasites (33).
Gene expression and proteomic analyses of DCs have revealed that remarkably few genes are induced following stimulation with helminth antigens (8, 17). DCs activated and matured in the presence of helminth antigens lack the classical markers, such as high levels of proinflammatory cytokines (interleukin-12 p70 [IL-12p70], tumor necrosis factor alpha [TNF-α], and nitric oxide) and expression of costimulatory markers (CD80 and CD86), observed in DCs matured with Toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS) (41). Additionally, TLR-mediated activation of DCs can be inhibited significantly if they are first exposed to helminths or helminth-derived products (24, 29).
Despite their limited maturation, helminth-primed DCs can nevertheless activate naïve T cells (35). Dendritic cells exposed to a soluble preparation of Schistosoma mansoni egg antigen (SEA) and either cocultured with naive T cells in vitro or injected into mice can polarize T-cell responses toward a Th2 phenotype (34). Similar findings have been reported for S. mansoni larval antigens (26, 27) and excretory-secretory (ES) material from Nippostrongylus brasiliensis (35), Acanthocheilonema viteae (52), and Echinococcus granulosus (43). However, Segura et al. found that adoptive transfer of DCs treated with ES from Heligmosomoides polygyrus resulted in suppression of both Th1 and Th2 responses in recipient mice (46). The same DCs promoted the differentiation of T cells with a regulatory phenotype and an ability to suppress effector CD4+ cell proliferation and cytokine secretion (46). Therefore, a diverse range of DC phenotypes can be induced by using complex mixtures of helminth antigens. For this reason, it is important to investigate the interactions of defined helminth-derived molecules with DCs and to elucidate the mechanism by which they alter DC function.
The liver fluke Fasciola hepatica is an important global helminth of humans and livestock (36). During infection, this pathogen induces potent polarized Th2/Treg immune responses coincident with a suppression of Th1 cytokines (15, 16, 18, 39, 40). Furthermore, infection also results in the bystander suppression of Th1 responses to a concurrent bacterial infection or to immunization with a Th1-inducing bacterial vaccine (6, 19). We previously demonstrated that ES molecules of F. hepatica can mimic the immunomodulatory effect observed with active infection. One of the predominant secreted products, a cathepsin L1 cysteine protease (FhCL1), suppressed the onset of protective Th1 immune responses to bacterial infections in mice and prevented the development of a Th1 response to vaccination (11, 22). Another major antigen, comprising 4% of F. hepatica ES material, is the antioxidant glutathione transferase (FhGST) (30), which in dimeric form significantly inhibited the proliferation of rat spleen cells in response to concanavalin A (ConA) stimulation in vitro (7).
Using recombinant forms of FhCL1 and FhGST, we show that both molecules partially activate DCs, via the PRR TLR4. However, despite activating DCs via different intracellular signaling pathways, both rFhCL1- and rFhGST-treated DCs suppressed the development of Th17 cells and did not induce the differentiation of Th2 cells. Our data suggest that helminth parasites secrete multiple molecules possessing a unique mechanism of modulation which suppresses inflammatory Th1/Th17 responses with redundancy, thus permitting the uninhibited development of Th2/Treg cells in response to other secretory molecules.
MATERIALS AND METHODS
Animals and Ags.
C57BL/6 or BALB/c mice, aged 6 to 8 weeks old, were purchased from Harlan Ltd. (United Kingdom), and DO11.10 mice (on a BALB/c background) were purchased from Charles River Laboratories (United Kingdom). C3H/HeJ and C3H/HeN bone marrow cells were a gift from Christine Loscher (Dublin City University, Ireland), and TLR4KO (on a C57BL/6 background) bone marrow cells were a gift from Padraic Fallon (Trinity College Dublin, Ireland). All mice were maintained according to guidelines of the Irish Department of Children and Health.
Active Fasciola hepatica cathepsin L antigen (rFhCL1; FHU62288) and variant inactive rvFhCL1 were produced in the Pichia pastoris expression system as previously described (11). F. hepatica GST class sigma (rFhGST-si; ABI79450) and mu (rFhGST-mu; ACF59730) were produced using Escherichia coli recombinant expression systems as previously described (29). All enzymes except rvFhCL were enzymatically active, as determined using specific assays as previously described (11; E. J. LaCourse, S. Perally, J. V. Moxon, G. Chemale, D. J. Dowling, S. M. O'Neill, and P. M. Brophy, unpublished data). Furthermore, all enzymes in this study showed significant activity within the culture medium. Protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (Pierce). Endotoxin levels were measured using the Pyrogene endotoxin detection system (Cambrex). All antigens were revealed to have endotoxin levels similar to those of background and complete RPMI 1640 medium (supplemented with 5% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol), so they were taken to be endotoxin free (data not shown).
Isolation, maturation, and characterization of bone marrow-derived DCs.
Bone marrow-derived immature DCs were prepared by culturing bone marrow cells isolated from the femurs and tibiae of mice in complete RPMI 1640 with recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (20 ng/ml; R&D Systems) at 37°C. On days 3 and 6 of culture, fresh medium with GM-CSF (20 ng/ml) was added to the cells (24). On day 8, cells were harvested, counted, and stained with CD11c (Caltag Laboratories) for analysis by flow cytometry to determine purity (>90%). For experiments, DCs were seeded into 24-well plates (Nunc) at 106/ml in complete RPMI 1640 plus 5 ng/ml GM-CSF. DCs were treated with medium only, F. hepatica recombinant antigens (0 to 10 μg), LPS (100 ng/ml; Alexa), or zymosan A (5 μg/ml; Sigma-Aldrich) alone for 18 h or were stimulated for 2.5 h with recombinant antigens prior to maturation with LPS.
Supernatants from cultured DCs were tested for the production of IL-12p40, IL-12p70, IL-6, IL-10, IL-23, TNF-α, macrophage inflammatory protein 1α (MIP-1α), and MIP-2 by sandwich enzyme-linked immunosorbent assay (ELISA) (BD OptEIA ELISA sets; BD Biosciences), and nitrite was measured using the Griess reagent system (Promega). Expression of cell surface markers on DCs was quantified by four-color flow cytometry, using phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, Cy5.5-, and allophycocyanin-conjugated antibodies specific for CD80, CD86, CD40 (BD Biosciences), and CD11c (Caltag Laboratories). Appropriately labeled isotype-matched antibodies were used as controls. Acquisition was performed using a FACSCalibur flow cytometer (BD Biosciences), and analysis of results was performed using FlowJo software (Tree Star).
Protein extraction and Western blot analysis.
Total protein was extracted from cell lysates by use of RIPA buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease and phosphatase inhibitor cocktails (Sigma-Aldrich) (22). Cells were incubated in the extraction buffer on ice for 5 min before being centrifuged at 8,000 × g for 10 min at 4°C. Supernatants were transferred to clean tubes, and protein concentrations were determined using a BCA protein assay kit (Pierce). Protein samples (10 to 40 μg) and prestained protein markers (Precision Plus protein standards; Bio-Rad) were separated by SDS-PAGE and blotted onto 0.45-μm Immobilon-P polyvinylidene difluoride membranes (Sigma-Aldrich). Membranes were blocked for 1 h at room temperature in 5% nonfat dried milk in phosphate-buffered saline (PBS) and incubated overnight at 4°C with anti-phospho-p38 (1:1,000; Cell Signaling Technology), anti-total p38 (1:1,000; Cell Signaling Technology), anti-phospho-extracellular signal-regulated kinase (anti-phospho-ERK), anti-total ERK, anti-phospho-c-Jun N-terminal kinase (anti-phospho-JNK), anti-total JNK, anti-phospho-NF-κBp65, or anti-interferon regulatory factor 5 (anti-IRF5) (1:1,000; Santa Cruz). Membranes were washed in PBS with 0.05% Tween 20 (PBS-T) and incubated for 1 h at room temperature with peroxide-conjugated anti-rabbit IgG (1:2,000; Sigma-Aldrich). After further washing, proteins were visualized with a Super Signal kit (Pierce), exposed to film for 10 to 30 min, and processed using an FPM 100A processor (Fuji Film). Protein bands were quantified using GeneSnap acquisition and GeneTools analysis software (GeneGenius gel documentation and analysis system; Syngene). Levels of phospho-JNK, -ERK, and -p38 were normalized to total JNK, ERK, and p38 and expressed in arbitrary units as percent increases over the medium control levels. Levels of p-NF-κBp65 and -IRF5 were expressed in arbitrary units as percent increases over medium control levels.
HEK293 cells and NF-κB activation.
rFhCL1 or rFhGST-si (10 μg/ml)-induced stimulation of TLR4 was tested in HEK293 cells expressing the TLR4 protein linked to an NF-κB promoter (Invivogen). NF-κB activation was assessed by measuring the luciferase emitted from the cells.
MTS and MTT assays.
Cell viability (MTS) and growth (MTT) assays during experimental conditions were performed by seeding DCs into 96-well plates (5 × 104 cells/ml and 1 × 106 cells/ml, respectively) and treating them with all antigens (0 to 10 μg/ml) for 18 h. Control cells were treated with medium or LPS alone. Following incubation, cell viability was assessed by adding 40 μl MTS solution {3-[4,5-dimethylthiazol-2-yl]-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium plus phenazine ethosulfate (PES)} to each well. Following an incubation of 3 h at 37°C, the absorbance of each well was read at 450 nm. Cell growth was assessed by adding a volume of MTT solution (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) equivalent to 10% of the culture medium to each well and incubating the cells for 3 h at 37°C. Culture medium was then removed, the resulting MTT formazan crystals were dissolved by adding MTT solvent (equal to the original culture volume), and absorbance was read at 562 nm.
Cell viability was also measured using an annexin V-FITC apoptosis detection kit I (BD Biosciences). Briefly, 1 × 106 cells/ml were seeded into a 24-well plate and treated with recombinant antigen (10 μg/ml) and each TLR ligand, either separately or together, or with medium alone. Cells were harvested 24 h later, washed twice in cold PBS, and incubated in binding buffer (0.1 M HEPES-NaOH, 1.4 M NaCl, 25 mM CaCl2) with annexin V-FITC and propidium iodide (PI) for 15 min. Following the addition of more binding buffer, cells were immediately analyzed by flow cytometry.
In vivo T-cell assays and cytokine measurements.
For assays of in vivo T-cell priming, DCs isolated from BALB/c mice were stimulated with rFhCL1 or rFhGST-si (10 μg/ml) prior to incubating the cells in the presence of OVA peptide (323-ISQAVHAAHAEINEAGR-329) (100 nM; GenScript Corp.) for 24 h. Following a wash step with sterile endotoxin-free PBS, DCs (3 × 105) were delivered over the sternums of naive DO11.10 mice by subcutaneous injection. After 7 days, skin draining lymph nodes (sdLN) were removed, and a single-cell suspension of cells was plated with medium, OVA peptide (500 nM), or phorbol myristate acetate (PMA) (25 ng/ml; Sigma-Aldrich) and anti-CD3ɛ MAb (1 μg/ml) (clone 145-2C11; BD Biosciences). After 72 h, supernatants were removed for measurement of IL-4, gamma interferon (IFN-γ), IL-10, and IL-17 by commercial assay (R&D Systems).
Phagocytosis assay.
The phagocytic ability of DCs was measured using the CytoSelect 96-well phagocytosis assay (Cell Biolabs Inc.) per the manufacturer's instructions. Briefly, DCs from C57BL/6 mice were plated at 0.5 × 106 cells/ml in complete RPMI and incubated overnight at 37°C to allow adherence to the plate. DCs were then cultured with rFhCL1, rFhGST-si (10 μg/ml), or LPS (100 ng/ml) for 2.5 h before the addition of sheep erythrocytes opsonized by IgG at a ratio of 1:50. After 1 h, phagocytosis was stopped with the removal of supernatant and adherent cells were washed with sterile PBS to remove nonphagocytosed erythrocytes. Adherent DCs were then lysed, substrate was added, and the number of engulfed erythrocytes was determined by colorimetric assay at an absorbance of 610 nm. Negative control cells were treated with 2 μM cytochalasin D to block phagocytosis.
Statistics.
All data were analyzed for normality prior to statistical testing. Where multiple group comparisons were made, data were analyzed using one-way analysis of variance (ANOVA). For comparisons between two groups, Student's t test was used. In all tests, P values of <0.05 were deemed significant.
RESULTS
rFhCL1 and rFhGST-si, but not rvFhCL1 or rFhGST-mu, induce partial activation of DCs.
To assess the immunomodulatory properties of F. hepatica secreted enzymes, functionally active recombinant sigma class GST (rFhGST-si), mu class GST (rFhGST-mu), and cathepsin L1 (rFhCL1) were synthesized (11; LaCourse et al., unpublished data). In addition, a conformationally intact proteolytically inactive variant of cathepsin L1 (rvFhCL1), which has the active site cysteine replaced with a glycine, was also produced (11). All recombinant preparations were deemed to be endotoxin free, as assessed using the Pyrogene endotoxin detection system (Cambrex).
Initially, a series of dose-response analyses were completed to determine the optimum quantity of recombinant protein for subsequent analyses. A concentration of 10 μg/ml for each antigen was selected, since DC viability or growth was not affected and this concentration stimulated optimum cellular responses (data not shown).
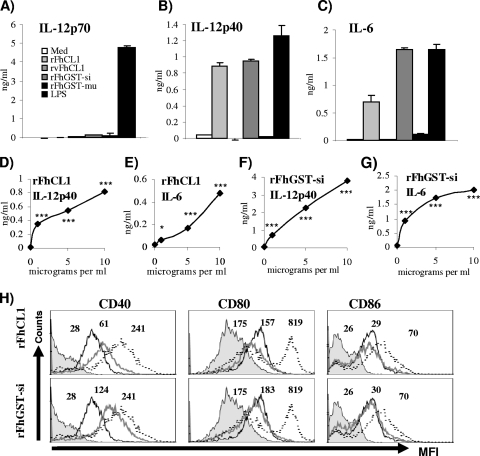
Dendritic cells stimulated with rFhCL1 secreted significant levels of IL-12p40 and IL-6, but not IL-12p70, TNF-α, nitric oxide, IL-23, or IL-10 (Fig. 1A to C and data not shown), compared to unstimulated cells. This activation of DCs was dependent upon the dose of rFhCL1 delivered, with increasing doses showing correspondingly increased secretion of IL-12p40 and IL-6 (Fig. 1D and E), indicating a requirement for enzymatic activity. Consistent with this was the observation that stimulation of DCs with the enzymatically inactive variant rvFhCL1 did not induce the secretion of any cytokines measured (Fig. 1A to C and data not shown).
FIG. 1.
Exposure of DCs to rFhCL1 and rFhGST-si, but not rvFhCL1 and rFhGST-mu, induces partial activation of DCs. (A to C) DCs from C57BL/6 mice were cultured with medium (Med), rFhCL1 (10 μg/ml), rvFhCL1 (10 μg/ml), rFhGST-si (10 μg/ml), rFhGST-mu (10 μg/ml), or LPS (100 ng/ml) for 24 h, and the production of IL-12p40, IL-6, and IL-12p70 in supernatants was determined by ELISA. DCs from C57BL/6 mice were cultured with a range of concentrations of rFhCL1 (D and E) or rFhGST-si (F and G) for 24 h, and the levels of IL-12p40 and IL-6 were measured by ELISA. Data are presented as means (± standard errors of the means [SEM]) and are representative of three experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (compared to unstimulated cells) (not shown). (H) Cells were harvested and analyzed by four-color flow cytometry for CD40, CD80, and CD86. Cells were gated on CD11c+ cells. The histograms show data for the isotype control (filled histograms), unstimulated cells (black lines), rFhCL1- or rFhGST-si-stimulated cells (gray lines), and LPS-stimulated cells (dotted lines). Numbers represent mean fluorescence intensities (MFI) for five different experiments.
Treatment of DCs with rFhGST-si induced an identical cytokine secretion profile to that with rFhCL1 (Fig. 1A to C), although the quantity of IL-6 measured was far greater than that seen following exposure to rFhCL1. Similar to the case with rFhCL1, the secretion of these cytokines was also dose dependent (Fig. 1F and G). However, rFhGST-mu did not induce the secretion of any cytokines (Fig. 1A to C and data not shown), suggesting that the enzyme activity required for DC activation by Fasciola GST fusion proteins may be specific to distinct subclasses.
We also determined if these enzymes could induce elevated expression of the costimulatory markers CD40, CD86, CD80, and major histocompatibility complex class II (MHC II) on DCs. Both rFhCL1 and rFhGST-si stimulated a significant increase in CD40 expression compared to the isotype control (Fig. 1H). The expression of CD80, CD86 (Fig. 1H), and MHC II (data not shown) was not altered following exposure to these recombinants. Both rvFhCL1 and rFhGST-mu failed to alter the expression levels of all cell surface markers measured (data not shown).
We have given due consideration to the possibility that residual endotoxin (whose major component is LPS) in the antigen preparations contributed to the activation of DCs described here. However, all of the data indicate that the effects seen were due to the activity of the recombinant proteins, not the presence of endotoxin. For example, the phenotype of DC activation described for rFhCL1 and rFh-GST-si was different from that induced by LPS (Fig. 1A to C and H). These enzymes were produced from the same expression systems as rvFhCL1 and rFhGST-mu, respectively, both of which had no measurable effect on the activation status of DCs, suggesting that any effect seen was unlikely to be due to endotoxin contamination. Finally, the effects of rFhCL1 and rFhGST-si on DC activation were shown to be heat labile, since they were no longer observed following boiling (data not shown), which contraindicates the involvement of endotoxin.
The partial activation of DCs by rFhCL1 and rFhGST-si is TLR4 dependent.
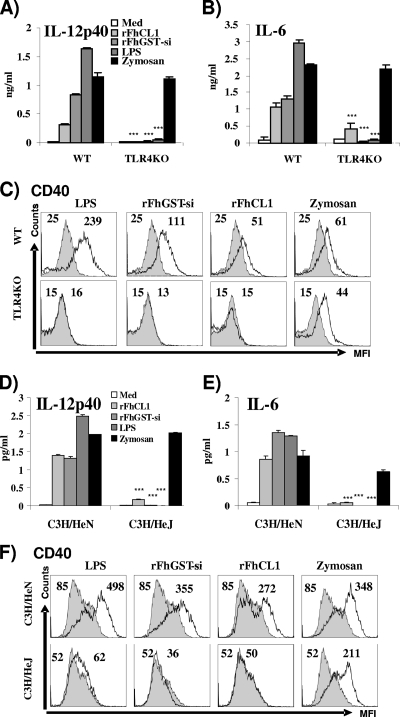
Since previous reports have shown that helminth antigens can induce partial maturation of DCs through TLR4 activation (22, 26), we went on to determine if the activation of DCs by rFhCL1 and rFhGST-si was dependent upon the TLR4 pathway. The secretion of IL-12p40 and IL-6 by rFhCL1 and rFhGST-si was significantly reduced in the absence of the TLR4 receptor (TLR4KO) (Fig. 2A and B). While a low level of IL-6 secretion was measured in response to rFhCL1 in the TLR4-deficient DCs, this was not significant compared to cells stimulated with culture medium. Similar to the cytokine results, rFhCL1, rFhGST-si, and LPS failed to induce any alteration in CD40 expression in the absence of TLR4 (Fig. 2C). As expected, TLR4-deficient DCs failed to respond to LPS. In addition, zymosan A, a TLR4-independent, TLR2-dependent agonist, induced the secretion of comparable quantities of IL-12p40 and IL-6 in both wild-type and TLR4-deficient strains of mice, confirming the integrity of TLR signaling in the knockout DCs. These results confirm that activation of DCs by rFhCL1 and rFhGST-si is dependent upon the presence of TLR4.
FIG. 2.
The partial activation of DCs by rFhCL1 and rFhGST-si is TLR4 dependent. DCs from C57BL/6 (background strain; WT), TLR4-deficient (TLR4KO), C3H/HeN, and C3H/HeJ mice were cultured with medium (Med), rFhCL1 (10 μg/ml), rFhGST-si (10 μg/ml), zymosan (5 μg/ml), or LPS (100 ng/ml) for 24 h, and the quantities of IL-12p40 (A and D) and IL-6 (B and E) in supernatants were determined by ELISA. Data are presented as means (± SEM) and are representative of three experiments. ***, P ≤ 0.001 compared to control mice. (C and F) Dendritic cells were harvested and analyzed by four-color flow cytometry, gated on CD11c+ cells, for the expression of CD40. In each histogram, the stimulated cells are represented by the black line and the isotype control by the filled histogram. Numbers represent mean fluorescence intensities (MFI) for three separate experiments.
C3H/HeJ mice exhibit a functional point mutation within the Pro712 residue of the TIR domain of TLR4, preventing the production of cytokines from DCs in response to bacterial LPS (42). However, the TLR4-dependent response of these cells to helminth-secreted ES-62 remained intact. In contrast, stimulation of DCs isolated from C3H/HeJ mice with either rFhCL1 or rFhGST-si did not increase the expression of CD40 or the secretion of IL-6 or IL-12p40 (Fig. 2D to F). The background wild-type mice, C3H/HeN, which possess a fully functional TLR4 protein, had increased CD40 expression and secreted IL-6 or IL-12p40 (Fig. 2D to F), as expected, in response to both antigens.
rFhCL1 and rFhGST-si induce the secretion of chemokines from DCs.
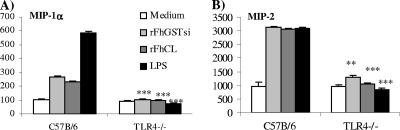
Recently, it was shown that Th1-promoting DCs selectively express elevated MIP-1α and promote recruitment of Th1 cells (20, 32), while MIP-2, a chemokine associated with helminth infection (38), promotes the recruitment of Th2 cells. Given the role played by these chemokines in the selective homing of immune cells, we sought to characterize the profiles of MIP-1α (CCL3) and MIP-2 (CXCL2) chemokines produced by DCs following treatment with rFhCL1 and rFhGST-si. rFhCL1 and rFhGST-si induced both MIP-1α and MIP-2 from DCs (Fig. 3A and B). However, these enzymes again showed a different profile from that for LPS with both enzymes, inducing significantly less MIP-1α than LPS but comparable levels of MIP-2. The polarized secretion of MIP-2 by these enzymes could influence a Th2 microenvironment by directly recruiting Th2 cells to the site of infection. As shown in Fig. 3A and B, medium controls produced endogenous levels of both chemokines.
FIG. 3.
Chemokine secretion is observed in DCs matured with rFhCL1 and rFhGST-si. DCs from C57BL/6 (wild-type background strain) and TLR4-deficient mice were cultured with medium, rFhCL1 (10 μg/ml), rFhGST-si (10 μg/ml), or LPS (100 ng/ml) for 24 h, and the production of MIP-1α (A) and MIP-2 (B) in supernatants was determined by ELISA. Data are presented as means ± SEM and are representative of three experiments. **, P ≤ 0.01; ***, P ≤ 0.001 (compared to wild-type mice).
The secretion of MIP-1α was TLR4 dependent, since the production of this chemokine was absent in DCs isolated from TLR4-deficient mice. In contrast, the secretion of MIP-2 appeared to be partially dependent upon TLR4 (Fig. 3A and B), suggesting the involvement of a coreceptor. Further studies are required to identify this receptor and also to characterize a role for MIP-2 in Fasciola infection.
rFhCL1 and rFhGST-si differentially activate MAPKs (p38, ERK, and JNK), NF-κB, and IRF5 in DCs compared to LPS.
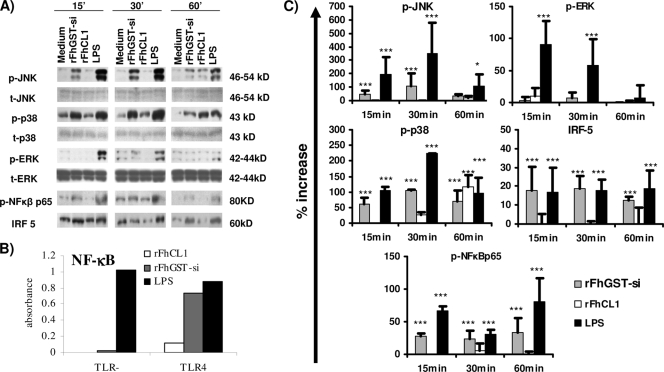
Since the partial activation of DCs by rFhCL1 and rFhGST-si is mediated through TLR4, we investigated whether these antigens activated intracellular mitogen-activated protein kinases (MAPKs) associated with the TLR4 signaling pathway. To address this question, the levels of phosphorylation of ERK, p38, and JNK in DCs following treatment with rFhCL1 and rFhGST-si were determined by Western blotting, using LPS as a positive control.
As expected, LPS caused the activation of all three signal transducers in comparison to medium, with distinct kinetics similar to previously published data (Fig. 4A and C) (3, 4). In contrast, only an increase in the activation of p38 was detected following treatment with rFhCL1. In addition, this was observed at a much later time point (60 min) than that seen for LPS activation. Stimulation of DCs with rFhGST-si induced the phosphorylation of JNK and p38, but not ERK, at most time points tested (Fig. 4A and C). While the kinetic profile of JNK and p38 activation was similar to that for LPS, peaking at 30 min, the level of phosphorylation activated by rFhGST-si was significantly lower than that with LPS.
FIG. 4.
Differential activation of MAPKs, NF-κB, and IRF5 in DCs treated with rFhCL1 and rFhGST-si compared to that in DCs treated with LPS. (A) DCs from C57BL/6 mice were cultured with culture medium, rFhGST-si (10 μg/ml), rFhCL1 (10 μg/ml), or LPS (100 ng/ml). At 15, 30, and 60 min, cells were lysed and total cellular protein was extracted. Protein (10 μg/sample) was separated in a 10% SDS-PAGE gel, transferred to a polyvinylidene difluoride (PVDF) membrane, and sequentially probed for phosphorylated JNK (p-JNK), p-p38, p-ERK, total JNK (t-JNK), total p38, total ERK, p-NF-κBp65, and IRF5. Representative blots from three experiments are shown. (B) Recombinant HEK-293 cells functionally expressing TLR4 protein linked to an NF-κB luciferase reporter gene were stimulated with LPS, rFhGST-si (10 μg/ml), or rFheCL1 (10 μg/ml) for 24 h, and representative data for two experiments are shown. (C) Densitometric analysis was performed on all immunoblots. p-JNK, p-p38, and p-ERK values were normalized to total JNK, ERK, or p38, and all values are expressed in arbitrary units as percent increases over the medium control group value. *, P ≤ 0.05; ***, P ≤ 0.001 (compared with medium control group).
The transcription factor NF-κB is primarily associated with TLR innate immune cell activation, typically DC maturation, and is involved in regulating the expression of a large number of inflammatory mediators (25). The phosphorylation of the DNA binding subunit p65 is an indicator of NF-κB activation (25). As expected, LPS stimulation of DCs led to an increase in the presence of phosphorylated NF-κBp65 (p-NF-κBp65) at 15, 30, and 60 min (Fig. 4A and C). Similarly, rFhGST-si induced a significant increase in the level of p-NF-κBp65 at 15, 30, and 60 min. However, the extents of phosphorylation measured at 15 and 60 min were less than those seen for LPS (Fig. 4C). Activation of NF-κBp65 was not detected in DCs stimulated by rFhCL1 at any time point examined. To confirm these findings, human recombinant HEK293 cells, functionally expressing TLR4 linked to an NF-κB reporter gene, were stimulated with rFhGST-si, rFhCL1, and LPS (Fig. 4B). Consistent with the Western blot analyses, the data indicated that rFhGST-si but not rFhCL1 activated NF-κB and demonstrated that this was dependent upon TLR4.
Since no phosphorylation of NF-κBp65 was detected following treatment of DCs with rFhCL1, we investigated whether this enzyme activated the IRF5 transcription factor. Following activation of TLR4, IRF5 directly interacts with the TLR4 adaptor protein MyD88 and functions as a key transcription factor for TLR4-dependent induction of IL-6 and IL-12p40, independent of NF-κB and the MAPKs (50). Since we have demonstrated that rFhCL1-induced secretion of IL-6 and IL-12p40 is dependent upon the presence of TLR4 (Fig. 2B), we measured the levels of IRF5 in DCs activated with rFhCL1 and rFhGST-si by Western blotting (Fig. 4A and C). However, only rFhGST-si, not rFhCL1, significantly enhanced the activation of IRF5 compared to that in unstimulated cells at 15 and 30 min.
rFhCL1- and rFhGST-si-treated DCs do not phagocytose antigen.
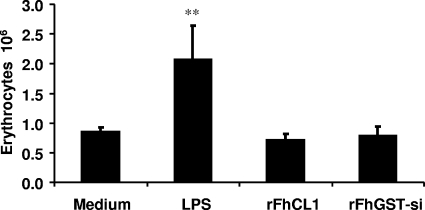
The functional hallmark of DC maturation is the ability to phagocytose antigens in combination with MHC II. Considering that both enzymes failed to induce the expression of MHC II or to fully activate DCs, we investigated the ability of Fasciola enzyme-activated DCs to phagocytose opsonized sheep erythrocytes. DCs fully matured by LPS stimulation demonstrated an ability to efficiently phagocytose antigen, with a significant increase (P ≤ 0.01) in the number of engulfed erythrocytes compared with medium controls (Fig. 5). In contrast, rFhCL1- and rFhGST-si-treated DCs engulfed the same number of erythrocytes as immature unstimulated DCs, indicating an inability to phagocytose antigen (Fig. 5).
FIG. 5.
rFhCL1- and rFhGST-si-treated DCs do not phagocytose antigen. DCs from C57BL/6 mice were cultured with medium, LPS (100 ng/ml), rFhCL1, or rFhGST-si (10 μg/ml) for 2.5 h before the addition of opsonized sheep erythrocytes at a ratio of 1:50. After 1 h, the extent of phagocytosis was assessed by measuring the number of engulfed erythrocytes, using a colorimetric assay. Data are presented as means (± SEM) and are representative of three experiments. **, P ≤ 0.01 compared to untreated medium control group.
rFhCL1 and rFhGST-si bias the specific immune response by attenuating Th17 immune responses by OVA-specific T cells.
Following phagocytosis, the role of fully matured DCs is to present antigen to T cells, thus inducing antigen-specific immune responses. We examined the ability of subcutaneously injected differentially activated DCs pulsed with OVA to prime T-helper-cell responses in the skin draining lymph nodes of DO11.10 transgenic mice, a strain expressing a T-cell receptor specific for OVA peptide (26).
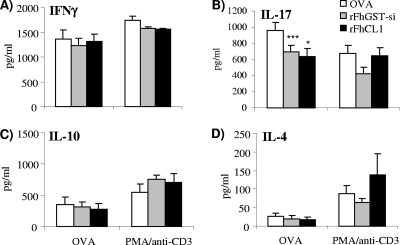
Skin draining lymph node cells isolated from mice which received DCs secreted antigen-specific IL-4, IL-10, IFN-γ, and IL-17 (Fig. 6A to D) in response to OVA stimulation ex vivo. However, lymph node cells from mice that received DCs cultured with either rFhCL1 or rFhGST-si and subsequently matured in the presence of OVA had significantly reduced antigen-specific IL-17 production in response to stimulation with OVA (Fig. 6B). No significant differences were observed in the antigen-specific production of IL-4, IL-10, and IFN-γ (Fig. 6A, C, and D).
FIG. 6.
DCs activated with rFhCL1 and rFhGST-si attenuate Th17 immune responses. DCs were cultured with OVA (100 nM) following exposure to medium, rFhGST-si (10 μg/ml), or rFheCL1 (10 μg/ml) overnight at 37°C. (A to D) Stimulated DCs were subcutaneously injected over the sternums of naïve DO11.10 mice. After 7 days, local skin draining lymph nodes were removed for restimulation in vitro with OVA (500 ng/ml) or PMA/anti-CD3 (25 ng/ml and 1 μg/ml). After 72 h, supernatants were analyzed by ELISA for the presence of IFN-γ, IL-17, IL-10, and IL-4. Data are means (± SEM) for three individual wells for four individual mice and are representative of three experiments. *, P ≤ 0.05; ***, P ≤ 0.001 (compared with OVA group).
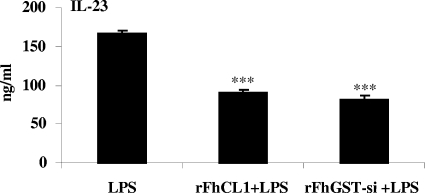
Since IL-23 from DCs is important in driving the secretion of IL-17 from CD4+ T cells (1), we determined whether DCs exposed to rFhCL1 or rFhGST-si were less capable of producing IL-23. DCs were exposed to rFhCL1 and rFhGST-si 2.5 h before activation with LPS. After 18 h of incubation in the presence of LPS, the quantity of IL-23 in the supernatant was measured. Concurrent with the ability to suppress IL-17-specific responses, treatment of DCs with rFhCL1 and rFhGST-si prior to LPS stimulation resulted in significant suppression of IL-23 production (Fig. 7).
FIG. 7.
rFhGST-si and rFhCL1 suppress the ability of DCs to secrete IL-23 in response to LPS. DCs from C57BL/6 mice were cultured with medium, rFhCL1, or rFhGST-si (10 μg/ml) 2.5 h prior to LPS stimulation (100 ng/ml). After 18 h, IL-23 in the supernatants was measured by sandwich ELISA. Data are presented as means (± SEM) and are representative of three experiments. ***, P ≤ 0.001 compared with LPS group.
DISCUSSION
We have previously demonstrated that F. hepatica ES modulates T-cell responses in mice (15). Here we demonstrate that two major components of ES, cathepsin L and GST-si, alter the function of DCs and, consequently, the ensuing T-cell response. Both antigens partially activate DCs to secrete IL-12p40, IL-6, and MIP-2 and to express CD40 in a TLR4-dependent manner. While the partial activation of DCs and the development of Th2 responses are typical of helminth infections (41), we found that rFhCL1- or rFhGST-si-stimulated DCs did not induce the differentiation of Th2 cells. In contrast, these antigens attenuated the development of a Th17 immune response, a function never previously described for helminth-activated DCs. Therefore, despite sharing some phenotypic characteristics of other helminth antigen-activated DCs, rFhCL1- and rFhGST-si-activated DCs exhibited a different activation state and effector function.
A strong correlation exists between Th1/Th17 immune responses and the development of severe inflammation-mediated pathology in helminth-infected mice (9, 44). Therefore, regulation of IL-17 is critical to the control of inflammatory pathology associated with helminth infection. It has been suggested previously that the strong polarized Th2 environment promoted by helminths prevents the development of Th1/Th17 immune responses (5). However, our data indicate that Fasciola secreted molecules actually suppress the differentiation of Th17 cells, independently of Th2 cells, by altering the function of DCs. Consistent with this hypothesis was the observation that Fasciola antigen-activated DCs secreted reduced levels of IL-23, a cytokine important in the expansion and survival of Th17 cells (31).
The attenuation of Th17 development could also be explained by the presence of the IL-12p40 homodimer, IL-12p80. Under a number of inflammatory conditions, the secretion of IL-12p40 was closely associated with increased levels of IL-12p80 (12, 21, 53). Recent studies have reported a bioactive role for IL-12p80 in preventing the biological activity of IL-23 by blocking its binding to IL-12rβ1, one of the two chains in the IL-23 receptor complex (12, 21, 53). We know that the immunological assay employed in this study to detect IL-12p40 levels cross-reacts with IL-12p80, making it possible that this antagonistic cytokine is secreted by DCs in response to these molecules. rFhCL1 or rFhGST-si also stimulated DCs to secrete IL-6, whose role in IL-17 attenuation is less clear given its involvement in the generation of Th17 cells. However, IL-6 may be important in the differentiation and growth of other T-cell subsets during infection (37).
The demonstration that two biochemically distinct enzymes inhibited Th17 immune responses would suggest that this immunological outcome is critical for the success of F. hepatica infection. This is supported by the fact that these molecules utilize alternative pathways ensuring redundancy in the process of DC-mediated suppression of T-cell responses. Indeed, other Fasciola antigens induce a DC phenotype, independent of p38, ERK, JNK, or NF-κB, that suppresses Th1 immune responses (24). Helminth-activated DCs that have a capacity to promote Th2 responses appear to be dependent upon the phosphorylation of ERK (1, 2, 51) and display a rapid but transient increase in the nuclear translocation of NF-κB (51). In contrast, neither rFhCL1 nor rFhGST-si activated ERK and rFhGST-si induced a more persistent activation of NF-κB in DCs than that observed for other helminths. Such distinct activation patterns may explain the functional capacity of Fasciola-treated DCs to suppress Th17 cells rather than to promote Th2 cells. In addition, IRF5 deregulation can impact the development of Th17-mediated autoimmune diseases (23). This study is the first to report IRF5 activation by helminth antigens, and IRF5 activation by rFhGST-si may be required for the induction of IL-6 and IL-12p40 or may be involved in the subsequent polarization of T-cell responses by rFhGST-si-activated DCs.
Both rFhCL1 and rFhGST-si induced a suppressor DC phenotype that was dependent upon the presence of the TLR4 receptor, thus identifying these two enzymes as possible novel helminth PAMPs. TLRs, while implicated in the recognition of helminth parasite products by DCs, may not be critical in the development of Th2 immune responses, since helminth-associated Th2 responses are not abrogated in the absence of TLRs (28). Instead, TLR ligation by helminth-derived antigens is recognized as a mechanism to limit the development of Th1 cytokine-mediated inflammation. For example, the filarial nematode ES product ES-62 inhibits IL-12 secretion from DCs in response to classic stimulators, such as LPS/IFN-γ (22), in a TLR4-dependent manner. Similarly, both rFhCL1 and rFhGST-si inhibit IL-23 secretion from LPS-matured DCs. F. hepatica secreted products therefore induce a phenotype of DCs, via interaction with TLR4, that is hyporesponsive to classical stimulation. However, unlike the case in other helminth studies, the consequence of this for Fasciola is a reduction in the development of inflammatory Th17 responses.
Considering that a diverse range of helminth products are recognized by TLR4, it is not surprising that these molecules utilize the same receptor. However, the variation in transcriptional activation of DCs by rFhCL1 and rFhGST may reflect differences in their enzyme activity. At least seven species-independent classes of cytosolic GST are known and may be distinguished from each other on the basis of substrate specificity and inhibitor sensitivity (45, 47). Three GST classes, representing at least six distinct enzymes, have been identified in F. hepatica (10, 45). We have not determined if enzyme activity is important for the activation of DCs described here. However, the sigma but not mu class induced the suppressor DC phenotype, which indicates that enzyme activity may be important. Given the dearth of knowledge on pathogen-derived GST interactions with DCs, it is not possible to speculate on a mechanism at this stage.
We have shown that the enzyme activity of rFhCL1, a member of the papain-like family of cysteine proteases (48, 49), is important for its immunomodulatory function. Similar proteases from this family, such as gingipain, a protease from Prevotella intermedia, and bromelain, a protease extracted from pineapple, both cleave CD14 from the surfaces of monocytes, rendering them hyporesponsive to LPS (14). However, we have shown that CD14 surface expression in response to rFhCL1 is unaltered (data not shown). While we do not know how rFhCL1 activates different TLR4 signaling, it is feasible that it may cleave peptides from the TLR4 receptor which, upon release, could disrupt subsequent binding of coreceptors, ultimately leading to the inactivation of signaling pathways.
The demonstration that two biologically distinct molecules, using different pathways, activate DCs with the same capacity to modulate developing adaptive responses supports the view that helminth parasites induce a heterogenous population of innate immune cells to control the outcome of infection. The majority of research investigating this phenomenon has used preparations of parasite secretions, which represent a composite of many different proteins, lipids, and sugars. A number of these molecules, including CL1 and GST, are universally expressed by all helminth parasites and suggest the existence of a common mechanism(s) of modulation. Understanding how each individual antigen from helminth antigen preparations affects DC maturation and function may shed light on the importance of discrete immunomodulatory mechanisms that collectively lead to the immune responses associated with helminth infection.
Acknowledgments
This work was supported by the Dublin City University Faculty of Science and Health Targeted Research Development Fund, the European Union (DELIVER; grant FOOD-CT-2005-023025), and the BBSRC (grant BB/C503638/2).
We thank Carolyn Wilson (DCU) for technical support.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 16 November 2009.
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal, A., S. Dillon, T. L. Denning, and B. Pulendran. 2006. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 176:5788-5796. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. [DOI] [PubMed] [Google Scholar]
- 4.Arrighi, J. F., M. Rebsamen, F. Rousset, V. Kindler, and C. Hauser. 2001. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 166:3837-3845. [DOI] [PubMed] [Google Scholar]
- 5.Bazzone, L. E., P. M. Smith, L. I. Rutitzky, M. G. Shainheit, J. F. Urban, T. Setiawan, A. M. Blum, J. V. Weinstock, and M. J. Stadecker. 2008. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis. Infect. Immun. 76:5164-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady, M. T., S. M. O'Neill, J. P. Dalton, and K. H. Mills. 1999. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 67:5372-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervi, L., G. Rossi, and D. T. Masih. 1999. Potential role for excretory-secretory forms of glutathione-S-transferase (GST) in Fasciola hepatica. Parasitology 119:627-633. [DOI] [PubMed] [Google Scholar]
- 8.Chaussabel, D., R. T. Semnani, M. A. McDowell, D. Sacks, A. Sher, and T. B. Nutman. 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102:672-681. [DOI] [PubMed] [Google Scholar]
- 9.Cheever, A. W., R. H. Duvall, T. A. Hallack, Jr., R. G. Minker, J. D. Malley, and K. G. Malley. 1987. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am. J. Trop. Med. Hyg. 37:85-97. [DOI] [PubMed] [Google Scholar]
- 10.Chemale, G., R. Morphew, J. V. Moxon, A. L. Morassuti, E. J. LaCourse, J. Barrett, D. Johnston, and P. M. Brophy. 2006. Proteomic analysis of glutathione transferases from the liver fluke parasite Fasciola hepatica. Proteomics 23:6263-6273. [DOI] [PubMed] [Google Scholar]
- 11.Collins, P. R., C. M. Stack, S. M. O'Neill, S. Doyle, T. Ryan, G. P. Brennan, A. Mousley, M. Stewart, A. G. Maule, J. P. Dalton, and S. Donnelly. 2004. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propeptide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J. Biol. Chem. 279:17038-17046. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, A. M., and S. A. Khader. 2006. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 1:33-38. [DOI] [PubMed] [Google Scholar]
- 13.Curtis, M. M., and S. S. Way. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschner, J., A. Singhal, P. Long, C. C. Liu, N. Piesco, and S. Agarwal. 2003. Cleavage of CD14 and LBP by a protease from Prevotella intermedia. Arch. Microbiol. 179:430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly, S., S. M. O'Neill, M. Sekiya, G. Mulcahy, and J. P. Dalton. 2005. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly, S., C. M. Stack, S. M. O'Neill, A. A. Sayed, D. L. Williams, and J. P. Dalton. 2008. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 22:4022-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferret-Bernard, S., R. S. Curwen, and A. P. Mountford. 2008. Proteomic profiling reveals that Th2-inducing dendritic cells stimulated with helminth antigens have a ‘limited maturation’ phenotype. Proteomics 5:980-993. [DOI] [PubMed] [Google Scholar]
- 18.Flynn, R. J., C. Mannion, O. Golden, O. Hacariz, and G. Mulcahy. 2007. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect. Immun. 75:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn, R. J., and G. Mulcahy. 2008. The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimental Fasciola hepatica infection. Int. J. Parasitol. 38:1673-1680. [DOI] [PubMed] [Google Scholar]
- 20.Gafa, V., M. E. Remoli, E. Giacomini, M. C. Gagliardi, R. Lande, M. Severa, R. Grillot, and E. M. Coccia. 2007. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microbes Infect. 8:971-980. [DOI] [PubMed] [Google Scholar]
- 21.Gillessen, S., D. Carvajal, P. Ling, F. J. Podlaski, D. L. Stremlo, P. C. Familletti, U. Gubler, D. H. Presky, A. S. Stern, and M. K. Gately. 1995. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 1:200-206. [DOI] [PubMed] [Google Scholar]
- 22.Goodridge, H. S., F. A. Marshall, K. J. Else, K. M. Houston, C. Egan, L. Al-Riyami, F. Y. Liew, W. Harnett, and M. M. Harnett. 2005. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J. Immunol. 174:284-293. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez-Roelens, I., and B. R. Lauwerys. 2008. Genetic susceptibility to autoimmune disorders: clues from gene association and gene expression studies. Curr. Mol. Med. 6:551-561. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton, C. M., D. J. Dowling, C. E. Loscher, R. M. Morphew, P. M. Brophy, and S. M. O'Neill. 2009. Fasciola hepatica tegumental antigen suppresses dendritic cell maturation and function. Infect. Immun. 6:2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayden, M. S., and S. Ghosh. 2008. Shared principles in NF-kappaB signaling. Cell 132:344-362. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins, S. J., J. P. Hewitson, S. Ferret-Bernard, and A. P. Mountford. 2005. Schistosome larvae stimulate macrophage cytokine production through TLR4 dependent and -independent pathways. Int. Immunol. 17:1409-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins, S. J., and A. P. Mountford. 2005. Dendritic cells activated with product released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect. Immun. 73:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kane, C. M., E. Jung, and E. J. Pearce. 2008. Schistosoma mansoni egg antigen-mediated modulation of Toll-like receptor (TLR)-induced activation occurs independently of TLR2, TLR4, and MyD88. Infect. Immun. 12:5754-5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapsenberg, M. L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984-993. [DOI] [PubMed] [Google Scholar]
- 30.LaCourse, E. J., M. Hernandez-Viadel, J. R. Jefferies, C. Svendsen, D. J. Spurgeon, J. Barrett, P. Kille, and P. M. Brophy. 2009. Glutathione transferase (GST) as a candidate molecular based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus. Environ. Pollut. 157:2459-2469. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Lebre, M. C., T. Burwell, P. L. Vieira, J. Lora, A. J. Coyle, M. L. Kapsenberg, B. E. Clausen, and E. C. De Jong. 2005. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol. Cell Biol. 5:525-535. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald, A. S., and R. M. Maizels. 2008. Alarming dendritic cells for Th2 induction. J. Exp. Med. 205:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald, A. S., A. D. Straw, B. Bauman, and E. J. Pearce. 2001. CD8− dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 167:1982-1988. [DOI] [PubMed] [Google Scholar]
- 35.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89-116. [DOI] [PubMed] [Google Scholar]
- 36.McManus, D. P., and J. P. Dalton. 2006. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology 133(Suppl.):S43-S61. [DOI] [PubMed] [Google Scholar]
- 37.Mudter, J., and M. F. Neurath. 2007. IL-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 8:1016-1023. [DOI] [PubMed] [Google Scholar]
- 38.Müller, K., S. Bischof, F. Sommer, M. Lohoff, W. Solbach, and T. Laskay. 2003. Differential production of macrophage inflammatory protein 1gamma (MIP-1gamma), lymphotactin, and MIP-2 by CD4(+) Th subsets polarized in vitro and in vivo. Infect. Immun. 11:6178-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Neill, S. M., M. T. Brady, J. J. Callanan, G. Mulcahy, P. Joyce, K. H. Mills, and J. P. Dalton. 2000. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 22:147-155. [DOI] [PubMed] [Google Scholar]
- 40.O'Neill, S. M., K. H. Mills, and J. P. Dalton. 2001. Fasciola hepatica cathepsin L cysteine proteinase suppresses Bordetella pertussis-specific interferon-gamma production in vivo. Parasite Immunol. 23:541-547. [DOI] [PubMed] [Google Scholar]
- 41.Perrigoue, J. G., F. A. Marshall, and D. Artis. 2008. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell. Microbiol. 9:1757-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 43.Riganò, R., B. Buttari, E. Profumo, E. Ortona, F. Delunardo, P. Margutti, V. Mattei, A. Teggi, M. Sorice, and A. Siracusano. 2007. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect. Immun. 4:1667-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutitzky, L. I., L. Bazzone, M. G. Shainheit, B. Joyce-Shaikh, D. J. Cua, and M. J. Stadecker. 2008. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J. Immunol. 180:2486-2495. [DOI] [PubMed] [Google Scholar]
- 45.Salvatore, L., G. Wijffels, J. L. Sexton, M. Panaccio, S. Mailer, I. McCauley, and T. W. Spithill. 1995. Biochemical analysis of recombinant glutathione S-transferase of Fasciola hepatica. Mol. Biochem. Parasitol. 69:281-288. [DOI] [PubMed] [Google Scholar]
- 46.Segura, M., Z. Su, C. Piccirillo, and M. M. Stevenson. 2007. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 37:1887-1904. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan, D., G. Meade, V. M. Foley, and C. A. Dowd. 2001. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 360:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stack, C. M., S. Donnelly, J. Lowther, W. Xu, P. R. Collins, L. S. Brinen, and J. P. Dalton. 2007. The major secreted cathepsin L1 protease of the liver fluke, Fasciola hepatica: a Leu-12 to Pro-12 replacement in the nonconserved C-terminal region of the prosegment prevents complete enzyme autoactivation and allows definition of the molecular events in prosegment removal. J. Biol. Chem. 22:16532-16543. [DOI] [PubMed] [Google Scholar]
- 49.Stack, C. M., C. R. Caffrey, S. M. Donnelly, A. Seshaadri, J. Lowther, T. F. Tort, P. R. Collins, M. W. Robinson, W. Xu, J. H. McKerrow, C. S. Craik, S. R. Geiger, R. Marion, L. S. Brinen, and J. P. Dalton. 2008. Structural and functional relationships in the virulence-associated cathepsin L proteases of the parasitic liver fluke, Fasciola hepatica. J. Biol. Chem. 283:9896-9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takaoka, A., H. Yanai, S. Kondo, G. Duncan, H. Negishi, T. Mizutani, S. Kano, K. Honda, Y. Ohba, T. W. Mak, and T. Taniguchi. 2005. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434:243-249. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, P. G., M. R. Carter, A. A. Da'dara, T. M. DeSimone, D. A. Harn. 2005. A helminth glycan induces APC maturation via alternative NF-kappa B activation independent of I kappa B alpha degradation. J. Immunol. 175:2082-2090. [DOI] [PubMed] [Google Scholar]
- 52.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 12:6453-6460. [DOI] [PubMed] [Google Scholar]
- 53.Wysocka, M., M. Kubin, L. Q. Vieira, L. Ozmen, G. Garotta, P. Scott, and G. Trinchieri. 1995. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 3:672-676. [DOI] [PubMed] [Google Scholar]