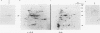Abstract
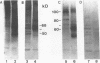
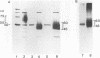
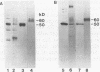
Mannoproteins of approximately 50 kDa from blastoconidia and 60 kDa from hyphae of Candida albicans reacted in Western blots (immunoblots) with either a polyclonal rabbit antiserum (CA-7) or a monoclonal antibody (CA-A) to the C. albicans C3d-binding protein (complement receptor type 2). The glycosylated nature of these proteins was demonstrated by their reactivity with concanavalin A and by selective labeling with the biotin-hydrazide reagent following periodate oxidation. Differences in the oligosaccharides of these proteins were observed in regard to their reactivity with lectin-peroxidase reagents and sensitivity to glycosidases such as N-glycanase or endoglycosidase F (but not endoglycosidase H). The 60-kDa mannoprotein reacted with wheat germ agglutinin, while the 50-kDa mannoprotein did not. Treatment of the 60-kDa mannoprotein with the glycosidases mentioned above resulted in its conversion into a species of 40 to 45 kDa. Enzyme treatment had no obvious effect on the electrophoretic mobility of the 50-kDa species from blastoconidia. Both the 50- and 60-kDa glycoproteins remained immunoreactive after treatment with the glycosidases. Reactivities of the two mannoproteins to neuraminidase also differed. Finally, the 50-kDa (blastoconidia) and the 60-kDa (hyphae) mannoproteins were purified by using ion-exchange chromatography and electroelution. The purified proteins differed in net charge, the 60-kDa species having a more acidic pI. Functional activity of the purified mannoproteins was demonstrated, as each inhibited the rosetting of antibody-sensitized sheep erythrocytes conjugated with iC3b or C3d by hyphae. Thus, an epitope(s) common to both a mycelial and blastoconidial mannoprotein is associated with a structurally different oligosaccharide for each growth form.
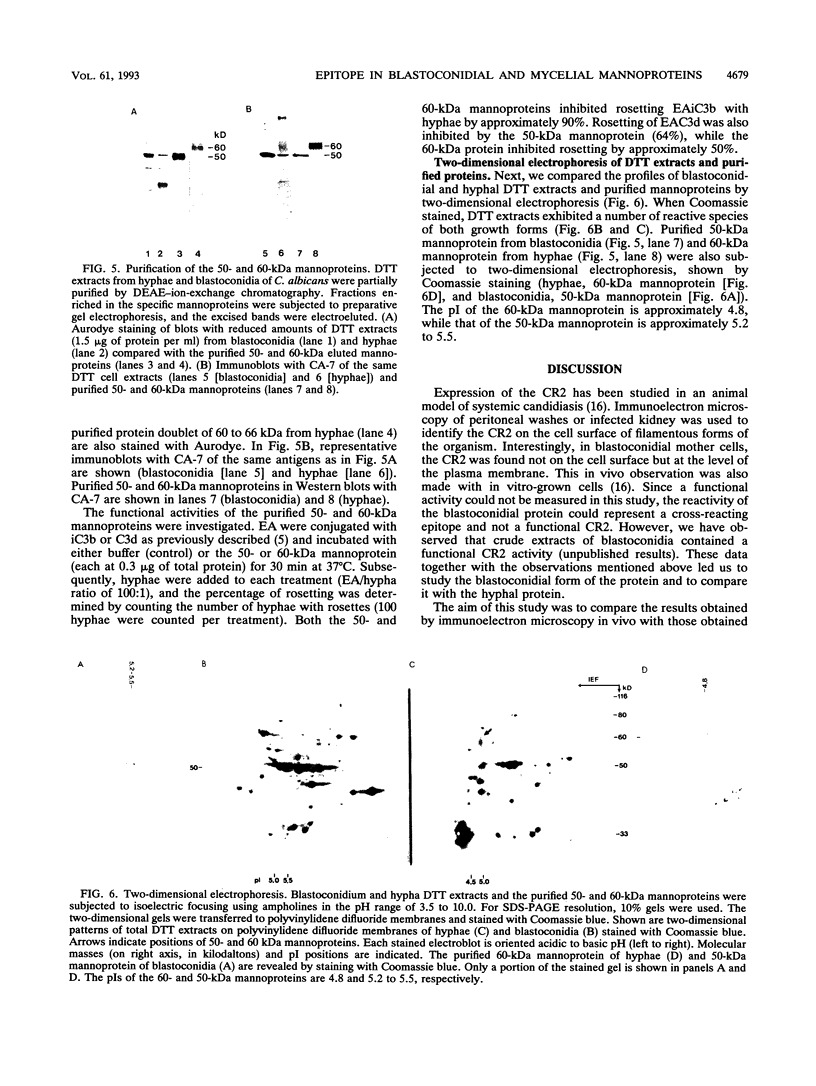
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alaei S., Larcher C., Ebenbichler C., Prodinger W. M., Janatova J., Dierich M. P. Isolation and biochemical characterization of the iC3b receptor of Candida albicans. Infect Immun. 1993 Apr;61(4):1395–1399. doi: 10.1128/iai.61.4.1395-1399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Characterization of binding of human fibrinogen to the surface of germ-tubes and mycelium of candida albicans. J Gen Microbiol. 1987 Mar;133(3):545–551. doi: 10.1099/00221287-133-3-545. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Chaffin W. L. Cell wall glycoproteins of Candida albicans as released by different methods. J Gen Microbiol. 1991 May;137(5):1045–1051. doi: 10.1099/00221287-137-5-1045. [DOI] [PubMed] [Google Scholar]
- Casanova M., Gil M. L., Cardeñoso L., Martinez J. P., Sentandreu R. Identification of wall-specific antigens synthesized during germ tube formation by Candida albicans. Infect Immun. 1989 Jan;57(1):262–271. doi: 10.1128/iai.57.1.262-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987 Mar;133(3):629–636. doi: 10.1099/00221287-133-3-629. [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Marcilla A., Sentandreu R. Wall mannoproteins of the yeast and mycelial cells of Candida albicans: nature of the glycosidic bonds and polydispersity of their mannan moieties. J Gen Microbiol. 1988 Aug;134(8):2393–2403. doi: 10.1099/00221287-134-8-2393. [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Murgui A., Sentandreu R. Dimorphism in Candida albicans: contribution of mannoproteins to the architecture of yeast and mycelial cell walls. J Gen Microbiol. 1985 Sep;131(9):2209–2216. doi: 10.1099/00221287-131-9-2209. [DOI] [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem. 1985 Aug 15;149(1):218–224. doi: 10.1016/0003-2697(85)90498-1. [DOI] [PubMed] [Google Scholar]
- Fukayama M., Calderone R. A. Adherence of cell surface mutants of Candida albicans to buccal epithelial cells and analyses of the cell surface proteins of the mutants. Infect Immun. 1991 Apr;59(4):1341–1345. doi: 10.1128/iai.59.4.1341-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Kanbe T., Li R. K., Wadsworth E., Calderone R. A., Cutler J. E. Evidence for expression of the C3d receptor of Candida albicans in vitro and in vivo obtained by immunofluorescence and immunoelectron microscopy. Infect Immun. 1991 May;59(5):1832–1838. doi: 10.1128/iai.59.5.1832-1838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijimoto-Ochiai S., Katagiri Y. U., Ochiai H. Analysis of N-linked oligosaccharide chains of glycoproteins on nitrocellulose sheets using lectin-peroxidase reagents. Anal Biochem. 1985 May 15;147(1):222–229. doi: 10.1016/0003-2697(85)90031-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Shannessy D. J., Voorstad P. J., Quarles R. H. Quantitation of glycoproteins on electroblots using the biotin-streptavidin complex. Anal Biochem. 1987 May 15;163(1):204–209. doi: 10.1016/0003-2697(87)90114-x. [DOI] [PubMed] [Google Scholar]
- Ollert M. W., Calderone R. A. A monoclonal antibody that defines a surface antigen on Candida albicans hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990 Mar;58(3):625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Analysis of cell wall extracts of Candida albicans by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot techniques. Infect Immun. 1986 Sep;53(3):565–572. doi: 10.1128/iai.53.3.565-572.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S. C., Dritschilo A. High-resolution two-dimensional electrophoresis of nuclear proteins: a comparison of HeLa nuclei prepared by three different methods. Anal Biochem. 1992 Nov 15;207(1):121–128. doi: 10.1016/0003-2697(92)90512-6. [DOI] [PubMed] [Google Scholar]
- Roffman E., Meromsky L., Ben-Hur H., Bayer E. A., Wilchek M. Selective labeling of functional groups on membrane proteins or glycoproteins using reactive biotin derivatives and 125I-streptavidin. Biochem Biophys Res Commun. 1986 Apr 14;136(1):80–85. doi: 10.1016/0006-291x(86)90879-x. [DOI] [PubMed] [Google Scholar]
- Saxena A., Calderone R. Purification and characterization of the extracellular C3d-binding protein of Candida albicans. Infect Immun. 1990 Feb;58(2):309–314. doi: 10.1128/iai.58.2.309-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Fukasawa S., Kobayashi H., Tojo M., Yonezu T., Ambo A., Ohkubo Y., Suzuki S. Structural analysis of phospho-D-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr Res. 1989 Apr 15;187(2):239–253. doi: 10.1016/0008-6215(89)80006-0. [DOI] [PubMed] [Google Scholar]
- Shibata N., Kobayashi H., Tojo M., Suzuki S. Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase-100T. Arch Biochem Biophys. 1986 Dec;251(2):697–708. doi: 10.1016/0003-9861(86)90379-6. [DOI] [PubMed] [Google Scholar]
- Smail E. H., Jones J. M. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect Immun. 1984 Jul;45(1):74–81. doi: 10.1128/iai.45.1.74-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Nichols E. J., Kenny G. E. Antigenic differences between mannoproteins of germ tubes and blastospores of Candida albicans. Infect Immun. 1987 Mar;55(3):616–620. doi: 10.1128/iai.55.3.616-620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Tam M. R., Nichols E. J., Kenny G. E. Antigenic differences in the surface mannoproteins of Candida albicans as revealed by monoclonal antibodies. Infect Immun. 1988 Mar;56(3):601–606. doi: 10.1128/iai.56.3.601-606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh F. D., Douglas L. J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect Immun. 1992 Nov;60(11):4734–4739. doi: 10.1128/iai.60.11.4734-4739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989 Dec;50(2):285–290. [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R., Senet J. M. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect Immun. 1988 Aug;56(8):1987–1993. doi: 10.1128/iai.56.8.1987-1993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]